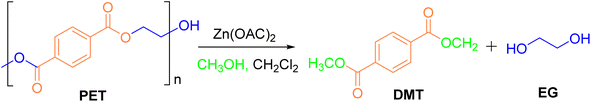Open Access Article
Open Access ArticleChemical degradation and recycling of polyethylene terephthalate (PET): a review
Zhiqiang
Guo
 *ab,
Jin
Wu
c and
Junhong
Wang
*b
*ab,
Jin
Wu
c and
Junhong
Wang
*b
aScientific Instrument Center, Shanxi University, Taiyuan, 030006, P.R. China. E-mail: gzq@sxu.edu.cn
bInstitute of Applied Chemistry, Shanxi University, Taiyuan, 030006, P.R. China
cSchool of Chemistry and Chemical Engineering, Shanxi University, Taiyuan, 030006, P.R. China
First published on 25th February 2025
Abstract
Polyethylene terephthalate (PET) is one of the most common plastics, which is mainly used in food packaging and textiles. In recent years, the massive use of PET has led to the destruction of the ecological environment, and it is necessary to develop green, low-cost, and efficient recycling technologies to alleviate such problems. In this paper, we summarized the advantages and disadvantages of chemical degradation of PET in the past decade, including alcoholysis, hydrolysis, aminolysis and pyrolysis. Among them, several new catalysts have been applied to the depolymerization of plastics, such as ionic liquids, eutectic solvents, metal–organic frameworks and polyoxometalate, which not only shorten the reaction time, but also increase the yield of the product and the conversion of PET. This review emphatically introduced the conversion of PET and the yield of the product under different parameters, and clarified the direction of future research on the chemical degradation of PET.
Sustainability spotlightIn this paper, we summarized the advantages and disadvantages on chemical degradation of PET in the past decade. And this review emphatically introduced the conversion rate of PET and the yield of the product under different parameters, clarified the direction of future research on the chemical degradation of PET. |
1 Introduction
Plastic is a composite material predominantly composed of synthetic polymers. Its widespread adoption over traditional materials can be attributed to its remarkable properties, such as resistance to corrosion, low density, cost-effectiveness, and excellent chemical and physical stability.1 Plastics have become integral to various sectors, including textiles, medical devices, food packaging, construction, and transportation, among others.2 Since the 1940s, certain countries began leveraging petroleum-based feedstocks for plastic production. Over time, as industrialization progressed and global populations expanded, the demand for plastics surged. Statistical data reveals that the global production of plastics reached 234 million tons in 2000 and 461 million tons in 2019, representing an approximate 40% increase over the two-decade period.3,4 If this trend persists, global plastic production is anticipated to surpass 1 billion tons per year by 2025.While plastics exhibit exceptional durability and stability, they are highly resistant to natural degradation processes, with degradation potentially taking hundreds of millions of years. During this prolonged degradation, a substantial release of toxic compounds occurs, significantly contributing to environmental pollution.5 Numerous studies highlight the pervasive presence of plastic waste in marine environments, where it either floats on the ocean surface or accumulates on the seabed, gradually releasing pollutants into the ecosystem. This ongoing process poses severe risks to marine biodiversity and ecological balance.6–10 Currently, approximately 9% of plastic waste is recycled, 12% is incinerated, and the remaining 79% accumulates in landfills or disperses into natural habitats.4,11 Incineration of plastics results in the generation of hazardous air pollutants, including dioxins, furans, mercury, sulfur oxides, and polychlorinated biphenyls (PCBs), as well as residual ash that contaminates both water and soil.12,13 Landfilling, on the other hand, involves the deposition of plastic waste into soil or underground voids, where it can leach into groundwater and adversely affect water quality and soil integrity.14 Consequently, the development of efficient and sustainable recycling methodologies for plastic waste has become an urgent and critical focus in contemporary scientific research, particularly in the context of environmental chemistry and material science.
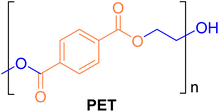
The main types of plastic are polyethylene terephthalate (PET), polyethylene (PE), polyvinyl chloride (PVC), polypropylene (PP), polystyrene (PS), polylactic acid (PLA), polycarbonate (PC), polyoxymethylene (POM), etc.3 The most commonly used plastic is PET,15 as shown in Fig. 1. It is a polyester formed by condensation of multiple ethylene glycol (EG) and terephthalic acid (TPA) through ester bonds, and is a semi-crystalline thermoplastic material,16,17 usually identified by the “1” in various plastics.18 There are generally three ways of synthesising PET: (1) esterification of terephthalic acid with ethylene glycol; (2) transesterification reaction of dimethyl terephthalate with ethylene glycol; (3) direct polycondensation reaction of bis(2-hydroxyethyl) terephthalate.19,20
The primary application of PET is in food packaging, owing to its excellent transparency and corrosion resistance.21,22 The most prevalent use is in the production of beverage bottles. As of January 2023, China's total PET bottle production has reached nearly 13 million tons, with a significant globally increase from over 41 million tons in 2014 to approximately 56 million tons by 2016. Alongside this growth in PET production, tens of millions of tons of PET plastic waste are generated annually, posing numerous environmental challenges. The accumulation of such waste has serious negative impacts on both the human environment and ecological systems.23,24 Thus, there is an urgent need to develop environmentally friendly, cost-effective, and efficient recycling technologies to mitigate these adverse effects.
Currently, PET recycling methods are categorized into four types: primary, secondary, tertiary, and quaternary recycling.25 Primary and secondary recycling are collectively referred to as mechanical recycling. The difference between the two types lies in the recycling process: primary recycling involves separation, sorting and purification, and there is minimal treatment of the material, it is re-used in its original form and for the same purpose, such a plastic bottle remains a plastic bottle after primary recycling. Secondary recycling, on the other hand, involves reprocessing the materials with a new purpose that reduces their properties. For example, plastic bottles get textiles after secondary recycling.10 Tertiary recycling, or chemical recycling, involves the degradation of polymers into monomers or other polymeric structures via chemical reactions in the presence of reagents.26 Quaternary recycling, or energy recovery, refers to the incineration of plastics to recover energy.27,28
There are generally three strategies for converting polymers into high-value chemicals: (1) depolymerization of polymers into monomers, oligomers, or other derivatives, followed by their recycling into high-value chemicals in a closed-loop system; (2) degradation of polymers into small molecules such as CO2, CH4, or CH3OH, which are then upgraded into high-value chemicals; (3) direct conversion of polymers into high-value chemicals.29 Recycling not only serves as a cornerstone of a circular economy but is also a critical requirement for environmental protection.
2 Chemical degradation of PET
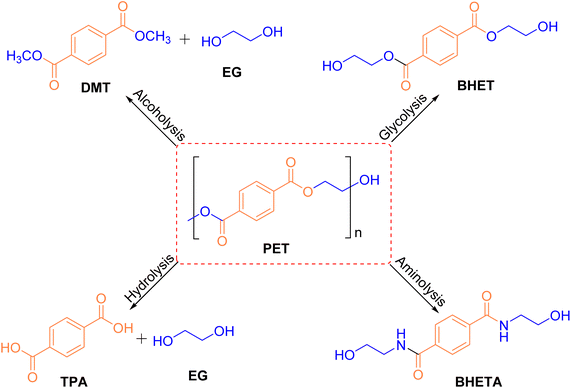
To date, several chemical methods for the degradation of PET have been reported. As shown in Fig. 2, these methods include hydrolysis, alcoholysis, glycolysis, pyrolysis, aminolysis, and hydrogenolysis.30–34 While these techniques have significantly enhanced the efficiency of PET degradation, the development of low-temperature, low-energy, cost-effective, and highly efficient processes that can completely convert PET into high-value chemicals remains a critical challenge.352.1 Alcoholysis
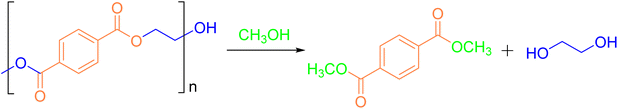
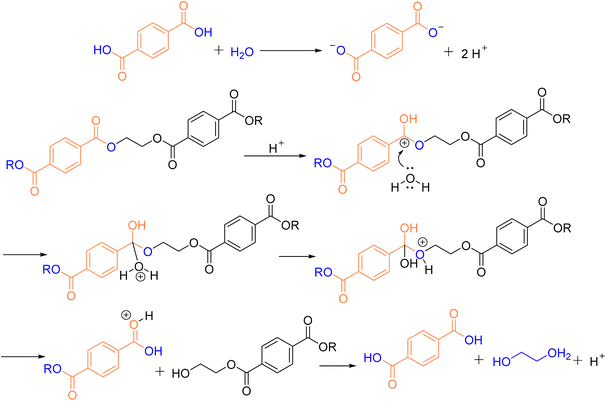
Alcoholysis is considered a reliable and effective chemical method for PET degradation.36 The solvents commonly employed in this process include alcohols such as methanol, ethylene glycol, and isooctanol. The primary products of alcoholysis are typically dimethyl terephthalate (DMT) and EG,37,38 which can subsequently be converted into bis(hydroxyethyl) terephthalate (BHET) monomers. The reaction mechanism for methanolysis is depicted in Fig. 3.| Entry | Catalyst loading [mol%] | MeOH [equiv.] | CH2Cl2 [equiv.] | T [°C] | t [min] | Yield of DMT [%] |
|---|---|---|---|---|---|---|
| 1 | 1 | 46.2 | 17.4 | 160 | 20 | 98 |
| 2 | 1 | 46.2 | 17.4 | 160 | 15 | 81 |
| 3 | 1 | 46.2 | 17.4 | 160 | 10 | 75 |
| 4 | 1 | 46.2 | 17.4 | 140 | 20 | <1 |
| 5 | 1 | 46.2 | 17.4 | 140 | 60 | 92 |
| 6 | 1 | 46.2 | 17.4 | 120 | 20 | <1 |
| 7 | 1 | 69.4 | 17.4 | 160 | 20 | <1 |
| 8 | 1 | 92.5 | 8.7 | 160 | 20 | <1 |
| 9 | 1 | 46.2 | 26.2 | 160 | 20 | 98 |
| 10 | 0.75 | 46.2 | 17.4 | 160 | 20 | 76 |
| 11 | 0.5 | 46.2 | 17.4 | 160 | 20 | <1 |
| 12 | — | 46.2 | 17.4 | 160 | 20 | <1 |
The Wang group40 dispersed ZnO nanoparticles, prepared from Zn(Ac)2·2H2O, into methanol and EG solutions to obtain methanol and EG dispersions of ZnO nanoparticles. These dispersions were utilized as pseudo-homogeneous catalysts in the methanol hydrolysis process. The effects of reaction parameters, such as the mass ratio of methanol to PET, reaction time, and temperature, on PET conversion and DMT yield were systematically investigated. It was observed that both dispersions exhibited excellent stability and transparency. Experimental results indicated that a PET conversion of 97% and a DMT yield of 95% were achieved after 15 minutes at 170 °C. Cho41 and colleagues employed a strategy to convert waste PET into DMT at relatively low temperatures. By controlling moisture content and using K2CO3 as a catalyst, they facilitated PET decomposition with only methanol, achieving a PET conversion of 4.5% and a DMT yield of 3.6%. The process also generated by-products such as 1-(2-hydroxyethyl)-4-terephthalate methyl ester (HEMT) and monomethyl terephthalate (MMT). The catalytic efficiency was significantly enhanced by the addition of the non-polar proton co-solvent dimethyl carbonate (DMC). Optimal conditions involved molar ratios of catalyst, methanol, and moisture to PET repeat units of 0.2, 50, and 0.4, respectively, as well as a DMC to PET repeat unit ratio of 50 at 25 °C for 24 hours. The use of chlorinated methane solvents, such as dichloromethane (DCM) and chloroform, reduced by-product formation and improved DMT selectivity. Zhang42 introduced aromatic boronic acids to facilitate the in situ capture of EG during the methanolysis of waste PET, yielding pure DMT and five-membered arylboronic esters (ABE) (Fig. 4). Arylboronic esters are valuable organoboron compounds that serve as intermediates for the synthesis of medicinal materials and natural products via coupling reactions. Without a catalyst, yields of DMT and ABE were 8% and 10%, respectively, after 2 hours at 180 °C. To enhance yield, they adjusted the calcination temperature, catalyst loading, and the Mg/Al ratio in magnesium–aluminum layered double oxide (Mg–Al-LDO) catalysts, thereby introducing weak alkaline sites. Under optimal conditions, the conversion of PET reached 100%, with ABE and DMT yields of 96% and 99%, respectively. Furthermore, it was demonstrated that the Mg4–Al1-LDO catalyst could be recycled, highlighting its potential for sustainable chemical processes.
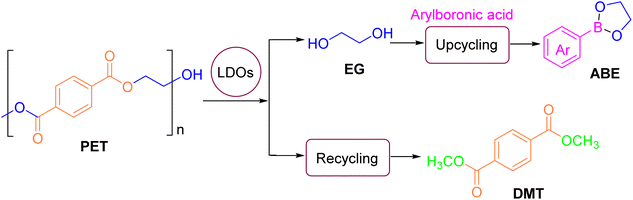 | ||
| Fig. 4 Waste PET uncycling by in situ capturing EG with arylboronic acid in the methanolysis process. Reproduced with permission.42 Copyright 2024, Elsevier Ltd. | ||
Lv43 utilized low-cost, environmentally friendly sodium silicate (Na2SiO3·9H2O) as an alkaline catalyst for the hydrolysis of PET to DMT. The reaction was conducted in an autoclave, with reaction parameters such as methanol quantity, reaction temperature, and catalyst loading systematically optimized. Catalyst activity was found to be related to its alkalinity, which could be modulated through calcination. The highest alkalinity was achieved at 300 °C for 2 hours, but maximum catalyst activity was observed after calcination at 400 °C. The optimal conditions for complete PET degradation were a 5 wt% catalyst, a methanol-to-PET mass ratio of 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
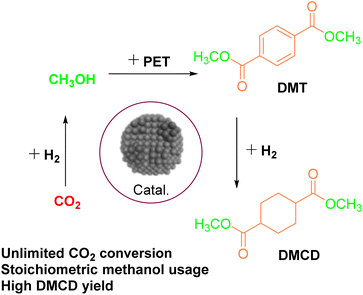
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, and a reaction time of 30 minutes at 200 °C, yielding a DMT yield of 95%. To address the limitations of conventional PET degradation methods, Wang44 proposed a one-pot catalytic system that combined CO2 hydrogenation, PET methanol hydrolysis, and DMT hydrogenation. The system (Fig. 5) employed a Cu4Fe1Cr1 catalyst to promote CO2 hydrogenation and DMT conversion. It was observed that the presence of PET enhanced CO2 hydrogenation, while H2 generated from CO2 reduction facilitated PET methanolysis. This process yielded valuable degradation products, including DMT, dimethyl cyclohexanedicarboxylate (DMCD), and paraxylene (PX). The yield of EG increased from 12.1% to approximately 88% when CO2 and H2 were used instead of excess methanol, demonstrating the synergistic effect of CO2 hydrogenation and PET methanolysis. The use of a single hydrogenation catalyst for multiple reactions enhances efficiency and sustainability, converting waste PET and CO2 into high-value chemicals. It is noteworthy that PET undergoes transesterification with excess methanol in the presence of an appropriate catalyst to yield DMT and EG. Both of these products can be reutilized in industrial polymerization processes. DMT, as a valuable chemical intermediate, can be repolymerized into new PET, thereby supporting sustainable production and the circular economy of polymer materials.
1, and a reaction time of 30 minutes at 200 °C, yielding a DMT yield of 95%. To address the limitations of conventional PET degradation methods, Wang44 proposed a one-pot catalytic system that combined CO2 hydrogenation, PET methanol hydrolysis, and DMT hydrogenation. The system (Fig. 5) employed a Cu4Fe1Cr1 catalyst to promote CO2 hydrogenation and DMT conversion. It was observed that the presence of PET enhanced CO2 hydrogenation, while H2 generated from CO2 reduction facilitated PET methanolysis. This process yielded valuable degradation products, including DMT, dimethyl cyclohexanedicarboxylate (DMCD), and paraxylene (PX). The yield of EG increased from 12.1% to approximately 88% when CO2 and H2 were used instead of excess methanol, demonstrating the synergistic effect of CO2 hydrogenation and PET methanolysis. The use of a single hydrogenation catalyst for multiple reactions enhances efficiency and sustainability, converting waste PET and CO2 into high-value chemicals. It is noteworthy that PET undergoes transesterification with excess methanol in the presence of an appropriate catalyst to yield DMT and EG. Both of these products can be reutilized in industrial polymerization processes. DMT, as a valuable chemical intermediate, can be repolymerized into new PET, thereby supporting sustainable production and the circular economy of polymer materials.
 | ||
| Fig. 5 One-pot catalytic system. Reproduced with permission.44 Copyright 2021, Wiley-VCH GmbH. | ||
The Yu group45 developed a CO2–H2-PET system for one-pot methanol decomposition by coupling CO2 hydrogenation to methanol (CH3OH) with PET methanolysis to DMT. In this system, Cu/ZnO served as the catalyst, and tetrahydrofuran (THF) was used as the solvent to dissolve PET particles. To enhance the yield of methanol from CO2 hydrogenation, various alcohols, including ethanol (EtOH), n-propanol (n-PrOH), n-butanol (n-BuOH), and isopropanol (i-PrOH), were introduced. The results revealed that the ability of these alcohols to promote PET conversion followed the order: i-PrOH < n-BuOH < n-PrOH < EtOH. Although EtOH improved methanol yield, it also produced more by-products, resulting in a lower DMT yield. Among the tested alcohols, i-PrOH was found to be the most effective, as it enhanced the yields of both methanol and DMT while minimizing by-product formation. Under optimal conditions, the PET degradation rate increased from 31.9% to 63.1%, and the DMT monomer yield reached 92.7%. Yin46 explored the use of supercritical methanol to depolymerize PET into its monomers, DMT and EG. However, the harsh reaction conditions posed challenges for industrial application. To address this, the researchers introduced CO2 to facilitate the supercritical methanolysis of PET and examined the effects of the methanol-to-PET weight ratio, reaction time, and initial CO2 pressure on the DMT yield. Their findings demonstrated that increasing the methanol-to-PET ratio and prolonging the reaction time both led to higher DMT yields. It was further confirmed that the presence of CO2 enhanced the supercritical methanolysis of PET by accelerating its depolymerization. This was attributed to the insertion of CO2 into the PET molecular chain, which weakened intermolecular forces, thereby promoting chain cleavage. Under optimal reaction conditions (270 °C, methanol-to-PET mass ratio of 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, and 40 minutes reaction time), a DMT yield of 95% was achieved. Tanaka47 proposed a novel method for PET degradation by capturing EG with DMC as a trapping agent to produce DMT at room temperature (Fig. 6). The method utilized the principle of transesterification as a reversible reaction, where the equilibrium was shifted toward the product side, thus increasing the DMT yield (Fig. 6a). During the reaction, ethylene carbonate (EC) was formed from EG and DMC. As a five-membered cyclic compound, EC exhibited high stability, which hindered the reverse reaction to DMC and EG (Fig. 6b). Simultaneously, methanol produced during the process further reacted with PET, facilitating PET decomposition through methanolysis (Fig. 6c). Initially, Tanaka's team ground post-consumer PET into powder and mixed it with 10 mol% lithium tert-butoxide (LiOtBu) at 65 °C for 5 hours. However, this approach yielded only 18% DMT and 22% EG. The introduction of THF increased the yields, but they remained below 50%. Subsequent experiments revealed that substituting THF with DMC significantly improved the DMT yield, reaching 86%. This breakthrough enabled a deeper understanding of the depolymerization mechanism and prompted investigations into the effects of alkali metal alkoxide catalysts, the dosage of MeOH and DMC, and reaction time on DMT yield. Under optimized conditions, the DMT yield exceeded 90%, demonstrating the effectiveness of the strategy.
1, and 40 minutes reaction time), a DMT yield of 95% was achieved. Tanaka47 proposed a novel method for PET degradation by capturing EG with DMC as a trapping agent to produce DMT at room temperature (Fig. 6). The method utilized the principle of transesterification as a reversible reaction, where the equilibrium was shifted toward the product side, thus increasing the DMT yield (Fig. 6a). During the reaction, ethylene carbonate (EC) was formed from EG and DMC. As a five-membered cyclic compound, EC exhibited high stability, which hindered the reverse reaction to DMC and EG (Fig. 6b). Simultaneously, methanol produced during the process further reacted with PET, facilitating PET decomposition through methanolysis (Fig. 6c). Initially, Tanaka's team ground post-consumer PET into powder and mixed it with 10 mol% lithium tert-butoxide (LiOtBu) at 65 °C for 5 hours. However, this approach yielded only 18% DMT and 22% EG. The introduction of THF increased the yields, but they remained below 50%. Subsequent experiments revealed that substituting THF with DMC significantly improved the DMT yield, reaching 86%. This breakthrough enabled a deeper understanding of the depolymerization mechanism and prompted investigations into the effects of alkali metal alkoxide catalysts, the dosage of MeOH and DMC, and reaction time on DMT yield. Under optimized conditions, the DMT yield exceeded 90%, demonstrating the effectiveness of the strategy.
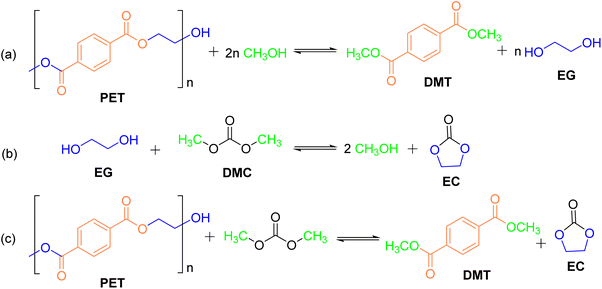 | ||
| Fig. 6 General process of depolymerization of polyethylene terephthalate, (a) The products of the alcoholysis of PET are the compounds DMT and EG. (b) EC was formed from EG and DMC. (c) The methanol formed in situ further reacts with PET, and facilitates the overall PET methanolysis. Reproduced with permission.47 Copyright 2021, The Royal Society of Chemistry. | ||
To develop an efficient and environmentally friendly system for PET methanolysis, Yan48 synthesized a series of non-metallic deep eutectic solvent49,50 (DES) catalysts composed of a hydrogen bond acceptor (HBA) and a hydrogen bond donor (HBD). Among the synthesized catalysts, the 1,5-diazabicyclo[4.3.0]-5-nonene (DBN)/phenol catalyst exhibited the best catalytic performance with the added advantage of leaving no metal residues in the final product. FT-IR spectra revealed the formation of N–H–O hydrogen bonds between DBN and phenol, where the N–H group interacted with the carbonyl oxygen of PET, while the oxygen atom of phenol activated the hydroxyl (–OH) group in methanol. During the methanolysis of PET, one of the key products is EG. It was observed that when the reaction temperature exceeded 130 °C, the reaction time was extended to 90 minutes, or the catalyst loading was increased, the concentration of EG in the solvent rose. This shift in equilibrium favored the formation of by-products, specifically 2-hydroxyethyl methyl terephthalate (MHET) and BHET, thereby reducing the yield of DMT. To achieve optimal reaction conditions, the researchers identified that conducting the reaction at 130 °C for 1 hour, with a catalyst mass fraction of 5 wt% and a methanol-to-PET mass ratio of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, resulted in complete conversion of PET. Under these conditions, a high DMT yield of 95.3% was achieved, demonstrating the efficiency of the DBN/phenol DES catalyst in PET methanolysis.
1, resulted in complete conversion of PET. Under these conditions, a high DMT yield of 95.3% was achieved, demonstrating the efficiency of the DBN/phenol DES catalyst in PET methanolysis.
2.1.2.1 Heterogeneous catalysts. MOFs are porous crystalline materials that have gained widespread application in various fields due to their biomimetic catalysis and biocompatibility.60 General PET depolymerization is a process in which degradation occurs layer by layer, from the outermost layer to the innermost. Notably, Xia61 proposed a strategy to achieve rapid depolymerization by concurrently degrading both the outer and inner layers of PET. Traditional PET degradation proceeds from the outer layer to the inner layer, where the surface chains are depolymerized into shorter chains, which subsequently detach from the PET substrate to form oligomers. As the inner layer begins to degrade, oligomers from the outer layer gradually convert to monomers. The simultaneous degradation of the outer and inner layers transforms the PET particles from a dense structure to a flocculent one under high temperature and micro pressure, resulting in a multi-layer porous structure that significantly increases the contact area between PET and reactants, thereby enhancing the yield. In the course of research, two “Dawson”-type polyoxometalates (POMs) were synthesized. The first is α2-K8P2W17O61X(H2O)·16H2O (X = Zn, Mn, Co, Ni, Cu), prepared in four steps with transition metal-substituted POM framework structures. Among these, the Zn- and Ni-substituted POM catalysts exhibited the best oligomer performance, although the Ni-substituted POM catalysts showed the lowest activity. Steric hindrance and catalyst activity are critical factors in PET degradation. The hydrolysis process is divided into three stages: the first stage (from the start to 3 minutes) is characterized by the expansion of PET and an increase in chain spacing, significantly enhancing the contact area between PET, the catalyst, and the solvent. In the second stage (approximately 10 minutes), BHET and oligomers begin to form. In the third stage (10–15 minutes), oligomers disappear and are converted to BHET. Under optimal conditions, PET is completely degraded, yielding a product with a 72.1% yield. The controlled degradation of PET has potential applications in the production process of PET–PLA copolymers, enabling sustainable recycling technology. The second catalyst is K10[M4(H2O)2(PW9O34)2]·H2O (M = Zn, Mn, Cu, Ni, Co).62 In this case, the transition metal active sites attack the C–O bond in PET, and the catalyst forms numerous hydrogen bonds between W–O and EG, facilitating rapid PET degradation. Characterization results of the catalyst and PET alcoholysis products revealed two distinct weight losses between 30 °C and 800 °C, attributed to the presence of crystal water in the catalyst and the mass loss resulting from structural collapse. In the exploration of optimal conditions (Table 2), when the temperature ranged from 210 °C to 230 °C, the conversion and BHET yield reached 100% and 63.7%, respectively, with the BHET yield increasing to 92.8% upon further increasing the temperature to 240 °C. Within 3–10 minutes, PET was completely degraded at 5 minutes, and the BHET yield reached its maximum (92.8%) at 8 minutes. The amount of catalyst also significantly influenced the degradation yield; within the range of 0.5–4.0 wt%, the BHET yield first increased and then decreased, reaching its maximum value at a catalyst dosage of 2.0 wt%. Mechanistic studies on controlled alcoholysis revealed that the formation of the flocculent structure in PET is a key factor in achieving controlled degradation.
| Catalyst | Cat/PET (wt%) | PET![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EG (w/w) EG (w/w) |
Temp (°C) | Time (min) | C PET (%) | Y BHET (%) |
|---|---|---|---|---|---|---|
| a Reaction conditions: PET (1.0 g), EG (4.0 g), cat (2.0 wt%), 240 °C, 8 minutes. | ||||||
| Na12[WZn3(H2O)2(ZnW9O34)2] | 0.5 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
190 | 40 | 100 | 84.5 |
| SiW11Zn | 2.0 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
185 | 30 | 100 | 84.1 |
| K2CO3/EG | 4.2 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
180 | 120 | 100 | 88.0 |
| [amim][ZnCl3] | 10.0 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
175 | 75 | 100 | 80.1 |
| 1,3-DMU/Zn(OAc)2 | 5.0 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
190 | 20 | 100 | 82.0 |
| [Dmin][Zn(OAc)3] | 16.7 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
180 | 90 | 100 | 67.1 |
| [Bmim]2[CoCl4] | 16.7 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11.7 11.7 |
175 | 90 | 100 | 81.1 |
| Urea | 10.0 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
180 | 180 | 100 | 77.7 |
| K10[Co4(H2O)2(PW9O34)2]·H2O | 2.0 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
240 | 8 | 100 | 39.0 |
| K10[Ni4(H2O)2(PW9O34)2]·H2O | 2.0 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
240 | 8 | 100 | 48.9 |
| K10[Cu4(H2O)2(PW9O34)2]·H2O | 2.0 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
240 | 8 | 100 | 58.5 |
| K10[Mn4(H2O)2(PW9O34)2]·H2O | 2.0 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
240 | 8 | 100 | 86.0 |
| K10[Zn4(H2O)2(PW9O34)2]·H2O | 2.0 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
240 | 8 | 100 | 92.8 |
Suo63 synthesized metal–organic framework (MOF) catalysts ZIF-8, ZIF-67, and MOF-5 using Zn(NO3)2·6H2O, Co(NO3)2·6H2O, and 2-methylimidazole (Hmim) as raw materials, which exhibited high catalytic activity. Among these, ZIF-8 demonstrated higher activity than ZIF-67 and MOF-5, likely due to differences in the molar ratio of Hmim to metal in the catalyst. When the molar ratio was 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, PET could not be fully converted, and the yield of BHET reached only 47.73%. However, when the molar ratio increased to 4
1, PET could not be fully converted, and the yield of BHET reached only 47.73%. However, when the molar ratio increased to 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, both the conversion of PET and the yield of BHET improved significantly. Beyond a molar ratio of 4
1, both the conversion of PET and the yield of BHET improved significantly. Beyond a molar ratio of 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, the cavity of ZIF-8 became increasingly obstructed by Hmim, leading to a decrease in both conversion and yield. In the presence of ZIF-8 and EG, complete depolymerization of PET was achieved by reacting at 197 °C for 1.5 hours, resulting in a BHET yield of 76.75%. The depolymerization process is illustrated in Fig. 7.
1, the cavity of ZIF-8 became increasingly obstructed by Hmim, leading to a decrease in both conversion and yield. In the presence of ZIF-8 and EG, complete depolymerization of PET was achieved by reacting at 197 °C for 1.5 hours, resulting in a BHET yield of 76.75%. The depolymerization process is illustrated in Fig. 7.
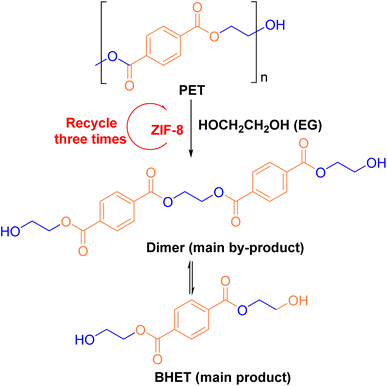 | ||
| Fig. 7 ZIF-8 catalyzes the depolymerization process of PET. Reproduced with permission.63 Copyright 2016, Springer Science, Business Media New York. | ||
Nguyen64et al. also utilized the metal–organic framework M-BDC (M = Cu, Co, Ni, and Zn) as catalysts for PET glycolysis. These frameworks, composed of benzene dicarboxylic acid (BDC) and the metals Cu, Co, Ni, and Zn, showed varying catalytic performances, with Zn-BDC exhibiting the best catalytic activity. The researchers further developed a deep neural network (DNN) model to optimize the parameters for PET depolymerization, finding that the error between experimental results and DNN model predictions was minimal (<1%). Under optimal conditions, the catalyst could be recycled three times, and the product BHET could also be converted into TPA. Maihom65 demonstrated that PET glycolysis could yield MHET and BHET monomers using a Zn-supported MOF-808 metal–organic framework. Additionally, catalysts based on three tetravalent metals—Zn–Hf-MOF-808, Zn–Zr-MOF-808, and Zn–Ti-MOF-808—were synthesized, with Zn–Ti-MOF-808 exhibiting the lowest catalytic activity among the three. Sardon66 reported that the yield of BHET could reach 88% when 1,3-dioxane was used as a co-solvent, EG as the solvent, and triazacyclodecene (TBD) as the catalyst, under reaction conditions of 65 °C for 1 hour. The study also demonstrated that a heterogeneous depolymerization process could be converted into a homogeneous one with the addition of an effective co-solvent. In recent years, nanomaterials have gained significant attention in various industries, including PET glycolysis. Numerous studies have shown that nanostructured heterogeneous catalysts can enhance the yield of BHET while maintaining the ability to be recycled. Kim67 synthesized an Fe2O3@MoS2 0D/2D nanocomposite catalyst for PET glycolysis, designed to strip MoS2 and uniformly precipitate it on Fe2O3 nanoparticles within a Taylor–Couette flow reactor. The reaction mechanism is shown in Fig. 8. After optimizing reaction conditions, the conversion of PET and the yield of BHET reached 97% and 90%, respectively, at a temperature of 225 °C for 3 hours.
 | ||
| Fig. 8 Glycolysis mechanism of PET using Fe2O3 nanoparticles as catalysts. Reproduced with permission.63 Copyright 2021, the Royal Society of Chemistry. | ||
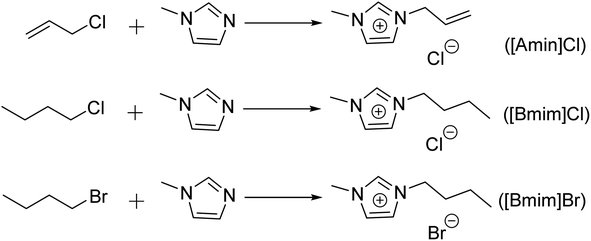
The Choi group68 developed a method to rapidly insert the alkali cation K+ into MnO2 to prepare a catalyst for PET glycolysis, resulting in an ultra-thin manganese dioxide nanosheet (e-MON) with a two-dimensional structure, which is also recyclable. In the study, reaction conditions were optimized by varying the reaction time, temperature, and catalyst amount. Ultimately, a catalyst concentration of 0.01 wt% was used to achieve complete depolymerization of PET via continuous reaction at 200 °C for 30 minutes, resulting in a 100% yield of BHET. Wang69 coordinated the KH550 modifier with CeO2 to form defect-rich CeO2 nanoparticles (NPs) with a very small size (2.7 nm). The cerium ions in CeO2 exist in two valence states, Ce3+ and Ce4+, with Ce4+ being reduced to Ce3+ during the glycolysis process, thereby enhancing the degradation efficiency of PET. The CeO2 NPs catalyst achieved 98.6% PET conversion and 90.3% BHET yield after a 15 minutes reaction at 196 °C. Glycolysis can also be applied to PET-containing textiles. For instance, Yu70 synthesized a new heterogeneous catalyst, Zn-MCM-41-25, by using Si/Al with a Zn to molar ratio of 25. Zn-MCM-41-25 demonstrated good catalytic activity in the depolymerization of waste PET textiles. Under optimal conditions, with 8% Zn loading, 5% catalyst dosage, an EG/PET mass ratio of 6, and a reaction temperature of 200 °C for 1 hour and 45 minutes, 100% PET conversion and an 81.4% BHET yield were achieved. Hydrogen bonding and coordination were identified as key factors for rapid alcoholysis. Shen71 developed a green, new catalyst—titanium phthalate (Ti-PA)—by gradually adding Ti(OiPr)4 to phthalic acid over 12 hours at 90–120 °C, followed by washing with ethanol and drying to obtain a white powder. Ti-PA can decompose into TiO2, CO2, and H2O at high temperatures. Used as a catalyst in EG for the hydrolysis of waste PET, Ti-PA demonstrated good catalytic activity. Response surface methodology72 indicated optimal conditions at 0.86% Ti-PA dosage, 13.7 mL EG, and a reaction time of 3.98 hours at 191 °C. The Yu group73 synthesized an orderly octahedral titanium benzoate (Ti-BA) catalyst by using benzoic acid (BA) and titanium ester via a hydrothermal method. The Ti-BA catalyst achieved a BHET yield of 90.01% at 217 °C for 3.3 hours, with an EG/PET mass ratio of 3.59 and a catalyst dosage of 2% by weight. The efficient degradation of PET was attributed to the synergistic effect of titanium ions and EG in the catalyst, enhancing its catalytic performance. Titanium was chosen for its biocompatibility and catalytic activity. Ti-BA exhibited good hydrolysis resistance and was easily recoverable. The Zhai group74 synthesized acetylacetone titanium oxide [TiO(acac)2] by reacting acetylacetone and isopropyl titanate in a three-necked flask. The catalyst, a pale yellow powder, demonstrated good stability, as evidenced by FT-IR analysis showing the chelation of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond in acetylacetone with titanium atoms. The hydrolysis of waste PET was conducted using [TiO(acac)2] as a catalyst and EG as a solvent under optimal conditions: 3 g of PET, 0.467% catalyst, 13.5 mL EG, and a 3.77 hours reaction at 200.5 °C, yielding 96.41% BHET. Lu75 designed and synthesized the novel bisidazole cationic zinc eutectic solvent catalyst [C2(Mim)2][OAc]2-2Zn(OAc)2 to catalyze the degradation of PET in EG. The catalyst demonstrated good catalytic activity, with a synergistic effect between [C2(Mim)2][OAc]2 and Zn(OAc)2. The catalyst led to 100% PET conversion and 85.2% BHET yield. Fang76 investigated the use of neopentyl glycol (NPG), dipropylene glycol (DPG), and polypropylene glycol (PPG) for PET degradation to obtain a series of alcohololytic products. It was found that the alcoholysis activity of DPG was lower than that of NPG and EG. The study also observed that small molecule alcohols could convert PET into oligomers, dimers, and trimers, with higher temperatures enhancing alcoholysis activity. Thus, glycolysis can serve as an environmentally friendly method for PET degradation and contribute to the synthesis of waterborne polyurethane. Shaver's research77 focused on the sustainable recycling of plastic cards, commonly made from thermoplastic composite materials containing PET. Using ethylene glycol-modified polyethyl terephthalate (PET-G) as an alternative thermoplastic, Shaver's group developed a strategy for converting polyester components in plastic cards into monomer units and then polymerizing them to form complex laminate materials, preserving the performance of the plastic cards. This strategy promotes the recycling of plastic cards and supports the circular economy. In addition to diols, polyols can also serve as solvents for PET degradation. Chen78 used the ionic liquid [Bmim]Cl and isooctanol (2-EH) to catalyze the degradation and conversion of PET into dioctyl terephthalate (DOTP). The preparation strategy for the ionic liquid is shown in Fig. 9.
O bond in acetylacetone with titanium atoms. The hydrolysis of waste PET was conducted using [TiO(acac)2] as a catalyst and EG as a solvent under optimal conditions: 3 g of PET, 0.467% catalyst, 13.5 mL EG, and a 3.77 hours reaction at 200.5 °C, yielding 96.41% BHET. Lu75 designed and synthesized the novel bisidazole cationic zinc eutectic solvent catalyst [C2(Mim)2][OAc]2-2Zn(OAc)2 to catalyze the degradation of PET in EG. The catalyst demonstrated good catalytic activity, with a synergistic effect between [C2(Mim)2][OAc]2 and Zn(OAc)2. The catalyst led to 100% PET conversion and 85.2% BHET yield. Fang76 investigated the use of neopentyl glycol (NPG), dipropylene glycol (DPG), and polypropylene glycol (PPG) for PET degradation to obtain a series of alcohololytic products. It was found that the alcoholysis activity of DPG was lower than that of NPG and EG. The study also observed that small molecule alcohols could convert PET into oligomers, dimers, and trimers, with higher temperatures enhancing alcoholysis activity. Thus, glycolysis can serve as an environmentally friendly method for PET degradation and contribute to the synthesis of waterborne polyurethane. Shaver's research77 focused on the sustainable recycling of plastic cards, commonly made from thermoplastic composite materials containing PET. Using ethylene glycol-modified polyethyl terephthalate (PET-G) as an alternative thermoplastic, Shaver's group developed a strategy for converting polyester components in plastic cards into monomer units and then polymerizing them to form complex laminate materials, preserving the performance of the plastic cards. This strategy promotes the recycling of plastic cards and supports the circular economy. In addition to diols, polyols can also serve as solvents for PET degradation. Chen78 used the ionic liquid [Bmim]Cl and isooctanol (2-EH) to catalyze the degradation and conversion of PET into dioctyl terephthalate (DOTP). The preparation strategy for the ionic liquid is shown in Fig. 9.
 | ||
| Fig. 9 Preparation strategies for ILs. Reproduced with permission.78 Copyright 2014, Elsevier Ltd. | ||
The alcoholysis of PET in various ionic liquids (ILs) is presented in Table 3. Zinc acetate (ZA), a solid that is poorly soluble in alcohols and ionic liquids and easily separable, was used as the catalyst in this experiment. The reaction conditions included a reaction time of 5 hours, an IL![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2-EH
2-EH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PET mass ratio of 2
PET mass ratio of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, and a catalyst/PET mass ratio of 1.2%. PET was found to be completely depolymerized, with a yield of 93.1% for dioctyl terephthalate (DOTP). This method resulted in a shorter reaction time compared to traditional ionic-free liquid alcoholysis reactions, and the ionic liquids could also be reused. The alcoholysis of PET using methanol, polyol (mainly EG), and polyols as solvents leads to the main products of DMT and BHET. The addition of an appropriate amount of catalyst or co-solvent during the reaction can accelerate the reaction rate, improving the conversion of PET and the yield of the product. In recent years, the development of catalysts, such as ionic liquids,79 deep eutectic solvents,80,81 and metal oxides,60 has greatly facilitated the degradation of waste PET.
1, and a catalyst/PET mass ratio of 1.2%. PET was found to be completely depolymerized, with a yield of 93.1% for dioctyl terephthalate (DOTP). This method resulted in a shorter reaction time compared to traditional ionic-free liquid alcoholysis reactions, and the ionic liquids could also be reused. The alcoholysis of PET using methanol, polyol (mainly EG), and polyols as solvents leads to the main products of DMT and BHET. The addition of an appropriate amount of catalyst or co-solvent during the reaction can accelerate the reaction rate, improving the conversion of PET and the yield of the product. In recent years, the development of catalysts, such as ionic liquids,79 deep eutectic solvents,80,81 and metal oxides,60 has greatly facilitated the degradation of waste PET.
| IL | Degradation rate of PET (%) | Yield of DOTP (%) |
|---|---|---|
a Reaction conditions: reflux temperature, 4 h, weight ratio of ionic liquid![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2-EH 2-EH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PET 2 PET 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. 1.
|
||
| — | 1.7 | 1.2 |
| [Amim]Cl | 56.1 | 42.4 |
| [Bmim]Cl | 57.3 | 43.2 |
| [Bmim]Br | 46.2 | 37.3 |
| [Bmim]NO3 | 10.5 | 6.5 |
| [Hmim]CF3SO3 | 5.2 | 3.7 |
| [Bsmim]HSO4 | 28.6 | 20.5 |
| [Bmim]BF4 | 4.3 | 3.1 |
| [Bmim]PF6 | 3.7 | 2.2 |
2.1.2.2 Homogeneous catalysts. Homogeneous catalysts exhibit high depolymerization efficiency; however, they are challenging to recover after the reaction and separation.4,82 Llopis83 used a large amount of EG to perform glycolysis of solid PET at various times and temperatures, without the use of catalysts, and observed the depolymerization process. Initially, EG diffuses into the PET particles and attacks the amorphous regions, which is a heterogeneous process, followed by a homogeneous process, generating oligomers and the monomer BHET, with yields exceeding 97%. However, this process is slow and time-consuming, and the addition of a catalyst can accelerate the reaction rate. Compared to traditional catalysts, ionic liquids serve as green catalysts that minimize product residues. The Shuangjun group84 synthesized a series of Lewis acidic ionic liquid (LAILs) as catalysts, using 1-hexyl-3-methylimidazole chloride ([Hmim]Cl) and metal oxides (CoCl2, FeCl3, ZnCl2, CuCl2) in the presence of dichloromethane for 8 hours, followed by evaporation. Experimental results showed that [Hmim]CoCl3 catalyzed the complete conversion of PET in 4 hours, while [Hmim]ZnCl3 only required 1.5 hours, indicating its high reactivity. However, when the reaction time was 4 hours, the yield of BHET catalyzed by [Hmim]ZnCl3 was lower than that of [Hmim]CoCl3, due to the formation of by-products from the high activity of [Hmim]ZnCl3. Furthermore, the hybrid catalyst of [Hmim]ZnCl3 and [Hmim]CoCl3 achieved an 87.1% BHET yield from PET conversion, which was higher than the yield from using a single ionic liquid catalyst. Barikani85 and colleagues synthesized a cobalt based ionic liquid functionalized graphene (rGO[TESPMI]2CoCl4) and explored the effects of reaction temperature, time, catalyst dosage, and EG dosage on PET depolymerization. They found that when the catalyst dosage ranged from 0.05 to 0.15 g, the yield of BHET increased, but it decreased when the dosage exceeded 0.15 g. The yield of monomer gradually increased between 150 °C and 190 °C and stabilized around 180 °C. Reaction time also played a role in the conversion rate of PET, and the yield and selectivity of BHET increased between 1–3 hours, but began to decrease after 4 hours. The optimal reaction conditions were identified as follows: 1 g of PET, 0.15 g of catalyst, 14 g of ethylene glycol, and a reaction temperature of 190 °C for 3 hours. Under these conditions, PET conversion reached 100%, and the BHET yield was 95.22%. Furthermore, the catalyst maintained good activity after 5 consecutive uses. Zhang86 synthesized a TPA-based ionic liquid as a catalyst for PET glycolysis. The carboxyl group in TPA and the hydroxyl group in EG can form hydrogen bonds that promote transesterification. After screening various catalysts with different cations and anions (Table 4), the results showed that 1-ethyl-3-methylimidazole terephthalate ([EMIm]2TPA) exhibited excellent catalytic activity, which remained unchanged after 15 uses. After 106 minutes of reaction under the influence of the catalyst, PET was completely converted, and the BHET yield reached 83.6%. The repolymerization of BHET into recycled PET (r-PET) led to less yellowing, and [EMIm]2TPA significantly reduced by-products, improving the color quality of r-PET.
| Entry | Catalyst | Conversion of PET (%) | Yield of BHET (%) |
|---|---|---|---|
| a Reaction conditions: 5.0 g of PET, 20.0 g of EG, and 0.25 g of ILs, reacting for 120 min at 190 °C. | |||
| 1 | — | 3.3 | 0 |
| 2 | [EMIm]2TPA | 99.8 | 73.9 |
| 3 | [PMIm]2TPA | 97.8 | 71.8 |
| 4 | [BMIm]2TPA | 97.4 | 70.8 |
| 5 | [BMMIm]2TPA | 92.4 | 62.9 |
| 6 | [EMIm]2IPA | 98.4 | 70.5 |
| 7 | [EMIm]2PA | 96.6 | 69.0 |
He87 synthesized four ionic liquids containing organophosphorus groups, namely [P4444]For, [P4444]Ace, [P4444]Pro, and [P4444]But, using tetrabutyl phosphonium hydroxide and four carboxylic acids (formic acid, acetic acid, propionic acid, and butyric acid) as raw materials. Among these, [P4444]Ace exhibited good glycolytic activity on waste PET and demonstrated excellent thermal stability. After 120 minutes at 195 °C, PET conversion reached 100%, but the yield of BHET was relatively low, at only 37.8%. When the temperature was increased to 200 °C, the PET conversion remained stable, and the BHET yield improved to 40.8%. The optimal catalyst amount was found to be 15 wt%. The mechanism of [P4444]Ace-catalyzed PET depolymerization was further investigated, revealing that [P4444]Ace formed a new chemical bond with the hydroxyl group in EG, which accelerated PET depolymerization. Sert88 studied the glycolysis of PET using EG in the presence of five different DES catalysts. The best catalytic effect was observed with a DES synthesized from potassium carbonate and EG, which achieved an 88% yield of BHET at 180 °C, with an EG/DES ratio of 15 and a DES/PET ratio of 6. While many studies have reported on the use of homogeneous catalysts, several disadvantages persist, such as difficulty in catalyst separation, low selectivity, and low product purity.
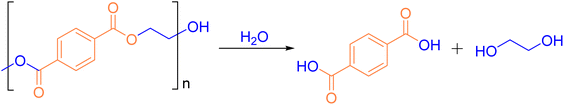
2.2 Hydrolysis
In addition to alcoholysis, hydrolysis can also be used for the chemical degradation of PET, and it is considered one of the most efficient methods for PET degradation.89 The reaction formula for PET hydrolysis is shown in Fig. 10. The general products of PET hydrolysis are TPA and EG, which are the basic monomers of synthetic PET. Since the resulting TPA is of high purity, it can be purified and then regenerated into PET, contributing to material recycling. PET hydrolysis is classified into three categories: acid hydrolysis, alkaline hydrolysis, and neutral hydrolysis.16 Several factors influence the hydrolysis process, including temperature, reaction time, concentration of acid or base, and the type and concentration of the catalyst.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O mass ratio of 1
H2O mass ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8, 2.5 g of PET, a reaction temperature of 220 °C, and a reaction time of 180 minutes, achieving 100% PET conversion and a 95.5% TPA yield. Abedsoltan91 investigated a series of hydrophobic aryl sulfonic acids as catalysts for PET hydrolysis. Compared to H2SO4, p-toluenesulfonic acid monohydrate (PTSA), 2-naphthalenesulfonic acid (2-NSA), and 1,5-naphthalenedisulfonic acid tetrahydrate (1,5-NDSA) exhibited higher catalytic activity. At a catalyst concentration of 4 M and 150 °C, these three catalysts required less time than H2SO4 to degrade PET to TPA, with yields exceeding 90%. The study also evaluated the contact angle of the catalyst solution on PET film, finding that the catalyst exhibited good wettability on the PET surface, which positively impacted the hydrolysis efficiency (Fig. 11).
8, 2.5 g of PET, a reaction temperature of 220 °C, and a reaction time of 180 minutes, achieving 100% PET conversion and a 95.5% TPA yield. Abedsoltan91 investigated a series of hydrophobic aryl sulfonic acids as catalysts for PET hydrolysis. Compared to H2SO4, p-toluenesulfonic acid monohydrate (PTSA), 2-naphthalenesulfonic acid (2-NSA), and 1,5-naphthalenedisulfonic acid tetrahydrate (1,5-NDSA) exhibited higher catalytic activity. At a catalyst concentration of 4 M and 150 °C, these three catalysts required less time than H2SO4 to degrade PET to TPA, with yields exceeding 90%. The study also evaluated the contact angle of the catalyst solution on PET film, finding that the catalyst exhibited good wettability on the PET surface, which positively impacted the hydrolysis efficiency (Fig. 11).
 | ||
| Fig. 11 PET hydrolysis mechanism with TPA as an acid catalyst. Reproduced with permission.89 Copyright 2021, Elsevier Ltd. | ||
The Neaţu group92 used sulfonic acid-modified Ti3C2-MXene as a solid acid catalyst for PET hydrolysis. MXene, a two-dimensional (2D) metal carbide–nitride material, was functionalized with –SO3H groups, which were immobilized between the MXene layers. Characterization revealed that the higher the initial concentration of sulfonic acid, the greater the number of sulfonating groups and the larger the interlayer spacing. Initially, at 180 °C for 4 hours, no PET conversion was observed, and the conversion was low at 12 hours. However, after 24 hours, nearly 100% of PET was hydrolyzed into TPA and EG, with the TPA yield reaching approximately 99%. In addition to these sulfonic acids, Alba-Rubio93 demonstrated that poly(4-styrenesulfonic acid) (PSSA), a reusable and recyclable acid catalyst, could also catalyze the hydrolysis of PET to TPA and EG. Their experiments measured the effects of parameters such as catalyst concentration, time, and temperature. They found that the activity of PSSA was higher than that of H2SO4, and the hydrophobic polystyrene backbone in PSSA improved its wettability, resulting in a smaller contact angle on the PET surface. This, in turn, increased the reaction rate and shortened the reaction time. However, the relatively high cost of PSSA is a concern, as it significantly increases the degradation cost. Hu94 used the recyclable and inexpensive strong acid p-toluenesulfonic acid (PTSA) as a catalyst for PET hydrolysis. The study revealed that the reaction system contained more hydrogen, which enhanced hydrolysis. Scanning electron microscopy (SEM) images showed morphological changes in PET during hydrolysis catalyzed by PTSA. The hydrolysis occurred on the surface of PET, and as the reaction progressed, small pores formed on the surface, allowing the solution to penetrate the material and accelerate the hydrolysis. X-ray diffraction (XRD) and NMR analysis detected no stray peaks in the TPA spectrum, confirming the high purity of the product. Under the conditions of 80% PTSA concentration and a mass ratio of 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to waste PET at 150 °C, nearly 100% PET was degraded to 96.2% TPA within 90 minutes. Moreover, after five consecutive cycles, the recovered PTSA maintained its hydrolysis catalytic efficiency. The recovered PTSA was in the same form as the fresh PTSA (a white powder), and its purity reached 96.6%. The yield of TPA obtained by R-PTSA catalysis remained in the range of 95.2% to 97.7%.
1 to waste PET at 150 °C, nearly 100% PET was degraded to 96.2% TPA within 90 minutes. Moreover, after five consecutive cycles, the recovered PTSA maintained its hydrolysis catalytic efficiency. The recovered PTSA was in the same form as the fresh PTSA (a white powder), and its purity reached 96.6%. The yield of TPA obtained by R-PTSA catalysis remained in the range of 95.2% to 97.7%.
| Run | Independent variable | Dependent variable | ||||
|---|---|---|---|---|---|---|
| X 1 (mL) | X 2 (s) | X 3 (wt%) | Y 1 (%) | Y 2 (%) | Y 3 (%) | |
| a X 1: volume of the DES, X2: MW irradiation time, X3: concentration of NaOH. Y1: percentage weight loss of PET, Y2: PET carbonyl index, Y3: PET crystallinity index. | ||||||
| 1 | 35 | 60 | 5.5 | 34.63 | 4.19 | 32.35 |
| 2 | 25 | 60 | 10.0 | 63.32 | 3.73 | 44.67 |
| 3 | 25 | 90 | 5.5 | 46.00 | 3.25 | 35.26 |
| 4 | 15 | 90 | 1.0 | 9.50 | 3.75 | 42.38 |
| 5 | 25 | 90 | 5.5 | 47.30 | 3.26 | 35.85 |
| 6 | 35 | 90 | 1.0 | 11.15 | 4.45 | 36.54 |
| 7 | 15 | 60 | 5.5 | 49.30 | 3.25 | 43.67 |
| 8 | 25 | 120 | 10.0 | 82.37 | 3.21 | 46.52 |
| 9 | 25 | 60 | 1.0 | 7.64 | 3.02 | 38.60 |
| 10 | 35 | 120 | 5.5 | 52.66 | 5.06 | 39.17 |
| 11 | 35 | 90 | 10.0 | 68.21 | 4.19 | 43.44 |
| 12 | 15 | 90 | 10.0 | 87.68 | 2.92 | 48.45 |
| 13 | 25 | 120 | 1.0 | 10.84 | 5.00 | 37.73 |
| 14 | 15 | 120 | 5.5 | 56.20 | 3.84 | 38.17 |
| 15 | 25 | 90 | 5.5 | 48.00 | 3.33 | 34.80 |
The Xu group97 selectively cleaved the C–O bonds in PET using a high-concentration 90 vol% ethanol aqueous solution, which resulted in the formation of small pores on the PET surface, thereby increasing the contact area for the reaction. Under low temperature and short reaction time conditions, KOH catalyzed the conversion of PET into dipotassium terephthalate (TPA-2K). TPA-2K is insoluble in the reaction system, facilitating separation, but it dissolves in water, forming a clear and transparent solution. TPA is obtained after neutralization with a small amount of acid. The concentration of KOH significantly influenced the degradation rate, as shown in Table 6. Additionally, the concentration of ethanol played a crucial role in the separation of the product. After 15 minutes of dehydrolysis at 80 °C, white powder and undepolymerized PET were observed. When the reaction time was extended to 1 hour, the degradation rate reached 73.87% with a KOH concentration of 0.02 g mL−1. The conversion of PET increased to 100% when the KOH concentration was raised to 0.2 g mL−1. Characterization analysis revealed that the tensile vibration of the C–O bond gradually decreased and eventually disappeared with the extended reaction time. This alcohol–water-based coupling system significantly accelerates the degradation of PET, and the separated TPA can be directly recovered without affecting its hydrolytic activity.
| Entry | KOH concentration (g mL−1) | Temperature (°C) | Time (min) | Degradation rate (%) |
|---|---|---|---|---|
| a Reaction solvent: ethanol aqueous solution with a concentration of 90 vol%. | ||||
| 1 | 0.02 | 80 | 60 | 73.87 |
| 2 | 0.05 | 80 | 60 | 95.31 |
| 3 | 0.05 | 80 | 180 | 99.37 |
| 4 | 0.1 | 80 | 60 | 98.32 |
| 5 | 0.2 | 80 | 60 | 100.00 |
| 6 | 0.2 | 70 | 60 | 98.59 |
| 7 | 0.2 | 60 | 60 | 96.22 |
| 8 | 0.2 | 50 | 60 | 78.05 |
| 9 | 0.2 | 80 | 90 | 100.00 |
| 10 | 0.2 | 80 | 30 | 97.61 |
| 11 | 0.2 | 80 | 15 | 87.85 |
| 12 | 0.2 | 80 | 5 | 46.07 |
Sun98 also used the alcohol–alkali hydrolysis method to produce TPA, using ethylene glycol and weak alkali sodium bicarbonate as the reaction media. First, they conducted optimization experiments by increasing the reaction temperature from 150 °C to 200 °C. The maximum TPA yield (94%) was achieved at 180 °C, while the TPA purity remained consistent at 98% throughout the process. The reaction temperature was maintained at 60–75 °C, and the mass ratio of NaHCO3 to PET was increased from 0.8 to 1.1, resulting in a yield of 90.9%. The final ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.1 was chosen because the excess NaHCO3 could neutralize the basic hydrolysate TPA. During the experiment, it was observed that when the VEG/mPET was 10/3, the yield of TPA remained stable as the amount of EG increased, the ratio of Vwater/mPET was tested in the range of 20/3 to 60/3, with the highest TPA yield occurring at 40/3, after which the yield began to decline. Finally, infrared and thermogravimetric analyses confirmed that the target product was pure TPA. Under optimal conditions, 98% of PET was successfully converted into TPA with a purity of 97%. This combination of alcoholysis and hydrolysis offers a promising method for degrading PET waste. Thielemans99 used methanol potassium hydroxide (KMH) solution to degrade cut PET flakes into TPA. Spectroscopic analysis (FTIR) revealed that alcohol did not act as a reactive substance but rather as a medium for the alkaline catalyst. Experimental results showed that the conversion of PET flakes reached 100% after 4 minutes at 100 °C, while complete conversion was achieved in just 1 minute at 140 °C. They also observed a temperature-dependant competitive relationship between methanolysis and hydrolysis: high temperatures favored hydrolysis, whereas low temperatures favored methanolysis.
1.1 was chosen because the excess NaHCO3 could neutralize the basic hydrolysate TPA. During the experiment, it was observed that when the VEG/mPET was 10/3, the yield of TPA remained stable as the amount of EG increased, the ratio of Vwater/mPET was tested in the range of 20/3 to 60/3, with the highest TPA yield occurring at 40/3, after which the yield began to decline. Finally, infrared and thermogravimetric analyses confirmed that the target product was pure TPA. Under optimal conditions, 98% of PET was successfully converted into TPA with a purity of 97%. This combination of alcoholysis and hydrolysis offers a promising method for degrading PET waste. Thielemans99 used methanol potassium hydroxide (KMH) solution to degrade cut PET flakes into TPA. Spectroscopic analysis (FTIR) revealed that alcohol did not act as a reactive substance but rather as a medium for the alkaline catalyst. Experimental results showed that the conversion of PET flakes reached 100% after 4 minutes at 100 °C, while complete conversion was achieved in just 1 minute at 140 °C. They also observed a temperature-dependant competitive relationship between methanolysis and hydrolysis: high temperatures favored hydrolysis, whereas low temperatures favored methanolysis.
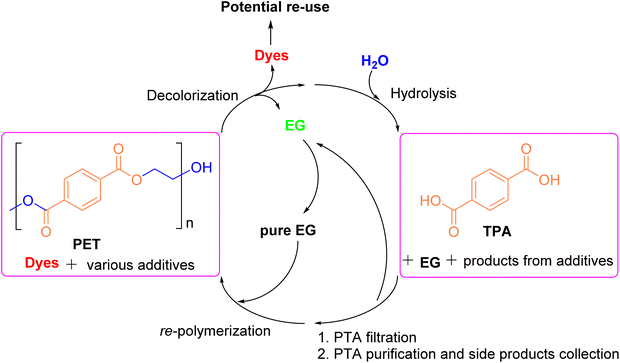 | ||
| Fig. 12 A closed-loop route for recycling waste polyester textiles that includes pre-decolorization, neutral hydrolysis, PTA purification, and chemicals recovery. Reproduced with permission.104 Copyright 2024, Elsevier Ltd. | ||
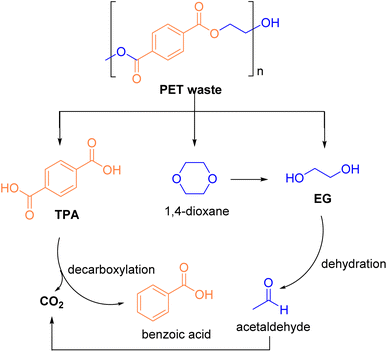
Currently, methods used for decolorizing non-ferrous waste textiles include the redox method, the electrochemical method, and the ozonation method, etc.95,105 However, the processing conditions for these methods are often harsh and negatively impact the final product. The decolorization method developed by the research group promotes the sustainable development of the textile industry, while neutral hydrolysis enables the closed-loop utilization of polyester resources within the industry. Many researchers have identified ZSM-5 zeolite as an effective catalyst for the degradation of PET.106 Cao107 employed supercritical carbon dioxide (SCCO2), which exhibits both swelling and crystallization effects on PET, in the presence of ZSM-5 zeolite to achieve efficient neutral hydrolysis. The effects of SCCO2 and ZSM-5 zeolite on PET depolymerization under varying pressures and temperatures were studied. It was observed that the swelling effect of SCCO2 facilitated PET hydrolysis, whereas its crystallization effects hindered hydrolysis. SCCO2 exhibited a dual role in the PET hydrolysis process, occurring in two stages: the swelling stage and the PET hydrolysis stage. Once the CO2 concentration reached a specific level, PET expanded to a loose state, after which hydrolysis proceeded efficiently. The Škerget100 group explored the use of subcritical and supercritical water in high-pressure, high-temperature batch reactors to degrade both colorless and colored PET waste. The pathways for PET waste treatment under subcritical and supercritical water conditions are illustrated in Fig. 13. Their results showed that TPA crystals obtained from colorless PET waste under subcritical conditions were light yellow, while those obtained under supercritical conditions appeared light brown. For colored PET waste, the presence of additives and dyes led to the formation of black solids in the final product. The degradation of both colorless and colored PET under subcritical conditions at 250 °C for 10 minutes revealed that more than half of the PET remained undegraded, resulting in the lowest TPA yield. However, when the reaction time was extended to 30 minutes and the temperature was increased to 300 °C, the degradation efficiency reached its maximum. At this point, the TPA yield for colorless PET and colored PET was 90.0% and 85.0%, respectively.
 | ||
| Fig. 13 Potential pathways for PET waste under sub- and supercritical water. Reproduced with permission.100 Copyright 2020, Elsevier Ltd. | ||
Researchers108 have also explored the use of marine components for neutral hydrolysis of PET, utilizing seawater as the reactant. In this approach, the salts naturally present in seawater (chlorides, bicarbonates, sulfates and bromides) act as catalyst. The final products are a mixture of solid TPA and sodium terephthalate dissolved in water, with all TPA precipitating upon the addition of H2SO4. Experimental results indicated that the depolymerization time and salt concentration in seawater had no significant effect on the reaction rate; temperature was identified as the primary factor controlling the depolymerization rate. The study also demonstrated that, in any seawater worldwide, PET would take approximately 162 years to fully depolymerize at a constant temperature of 30 °C. However, incorporating additional factors, such as microbial and physical degradation, could reduce the total degradation time of PET by 10–20%.
The above research demonstrates that each of the three hydrolysis methods (acid, alkaline, and neutral) has its own advantages and limitations. In acid and alkaline hydrolysis, the use of acid or alkali catalysts effectively increases the hydrolysis rate. Still, acid–base catalysts are difficult to recycle, leading to resource waste and higher production costs, while also generating significant amounts of strong acid or alkali wastewater. Neutral hydrolysis, on the other hand, avoids these environmental and economic issues. Currently, all three methods can achieve a PET conversion rate exceeding 90%. Future research should focus on developing more efficient and sustainable acid–base catalysts, as well as identifying optimal neutral conditions to further enhance the efficiency and sustainability of hydrolysis-based PET recovery.
2.3 Aminolysis
At present, studies on the aminolysis of PET remain limited in the literature. Generally, PET is depolymerized using ethanolamine as a solvent, producing bis(2-hydroxyethylene)terephthalic acid (BHETA). Tian109 employed ethanolamine to depolymerize PET sheets treated with acetic acid for swelling, resulting in the high-value product BHETA. The optimal swelling temperature was identified as 60 °C, achieving a degradation rate of 99% and an expansion rate exceeding 75% within 30 minutes. Further investigations on the effects of temperature and reaction time revealed that PET did not dissolve below 60 °C, whereas complete dissolution occurred at 70 °C, with the degradation rate reaching 99%. While shorter reaction times left undepolymerized PET in the product, excessively long reaction times increased experimental costs. Therefore, the optimal reaction time was determined to be 4 hours, yielding the highest PET conversion. This method offers a low-cost approach to PET depolymerization, as it does not require catalysts to produce valuable monomers. Ghorbantabar110 conducted experiments using monoethanolamine and PET at a ratio of 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 at 160 °C for 2 hours to obtain BHETA. Samples were analyzed at regular intervals using FTIR, differential scanning calorimetry (DSC), thermogravimetric analysis (TGA) and elemental analysis (CHN) to obtain the corresponding calibration curves and calculate the PET conversion. DSC results demonstrated that as BHETA production gradually increased, its intensity peak rose correspondingly while the PET peak diminished. TGA analysis conducted between 25 °C and 800 °C revealed PET weight loss in the 339 °C to 484 °C range, consistent with PET degradation. BHETA reached its maximum value at 40 minutes, after which the yield remaining relatively stable thereafter. These analyses confirmed the reliability of DSC and TGA for calculating conversion. Sivamurugan111 was continuously stirred with a mixture of Zn(NO3)2·6H2O and SnCl2·2H2O in 50 mL of distilled water for 15 minutes, and then ethylenediamine was added dropwise to obtain a white precipitate, and the catalyst Sn-doped ZnO nanoparticles were obtained after filtration, washing and drying. The treated PET was cut into small pieces, and the solvent ethanolamine and catalyst were added for degradation to obtain BHETA. The study examined the effects of different amounts of Sn in the catalyst and the ratio of PET and ethanolamine on the products were emphatically discussed. The yield of undoped Sn can only reach 83%, 0.5 mol%, 1.0 mol%, and 2.0 mol% of Sn doped in ZnO nanoparticles, and the yield gradually increases, and the maximum yield can exceed 95%. At a ratio of PET to ethanolamine was 1
1 at 160 °C for 2 hours to obtain BHETA. Samples were analyzed at regular intervals using FTIR, differential scanning calorimetry (DSC), thermogravimetric analysis (TGA) and elemental analysis (CHN) to obtain the corresponding calibration curves and calculate the PET conversion. DSC results demonstrated that as BHETA production gradually increased, its intensity peak rose correspondingly while the PET peak diminished. TGA analysis conducted between 25 °C and 800 °C revealed PET weight loss in the 339 °C to 484 °C range, consistent with PET degradation. BHETA reached its maximum value at 40 minutes, after which the yield remaining relatively stable thereafter. These analyses confirmed the reliability of DSC and TGA for calculating conversion. Sivamurugan111 was continuously stirred with a mixture of Zn(NO3)2·6H2O and SnCl2·2H2O in 50 mL of distilled water for 15 minutes, and then ethylenediamine was added dropwise to obtain a white precipitate, and the catalyst Sn-doped ZnO nanoparticles were obtained after filtration, washing and drying. The treated PET was cut into small pieces, and the solvent ethanolamine and catalyst were added for degradation to obtain BHETA. The study examined the effects of different amounts of Sn in the catalyst and the ratio of PET and ethanolamine on the products were emphatically discussed. The yield of undoped Sn can only reach 83%, 0.5 mol%, 1.0 mol%, and 2.0 mol% of Sn doped in ZnO nanoparticles, and the yield gradually increases, and the maximum yield can exceed 95%. At a ratio of PET to ethanolamine was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20, the BHETA yield reached the maximum of 94%, and the yield remained relatively unchanged when the ratio was further increased. Notably, the Sn-doped ZnO nanoparticle catalyst demonstrated recyclability, retaining its performance over seven cycles without yield loss. The poor hydrophilicity in the structure of PET fibers leads to static electricity the formation and low moisture absorption. In addition to PET, PVC is one of the most widely used plastics. More112 investigated the synthesis of PVC plasticizers using BHETA, an aminolysis product of PET. Ethanolamine served as the solvent, while zinc acetate and sodium acetate were used as catalysts for PET depolymerization to obtain BHETA. The study explored the effects of PET and ethanolamine, catalyst selection, concentration and reaction time on PET degradation efficiency. Results in Table 7 (1–5) showed that it can be concluded that the yield can reach approximately 69% when the ratio of PET to ethanolamine is 1
20, the BHETA yield reached the maximum of 94%, and the yield remained relatively unchanged when the ratio was further increased. Notably, the Sn-doped ZnO nanoparticle catalyst demonstrated recyclability, retaining its performance over seven cycles without yield loss. The poor hydrophilicity in the structure of PET fibers leads to static electricity the formation and low moisture absorption. In addition to PET, PVC is one of the most widely used plastics. More112 investigated the synthesis of PVC plasticizers using BHETA, an aminolysis product of PET. Ethanolamine served as the solvent, while zinc acetate and sodium acetate were used as catalysts for PET depolymerization to obtain BHETA. The study explored the effects of PET and ethanolamine, catalyst selection, concentration and reaction time on PET degradation efficiency. Results in Table 7 (1–5) showed that it can be concluded that the yield can reach approximately 69% when the ratio of PET to ethanolamine is 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4, and the influence of reaction time and catalyst selection is continued to be discussed under these conditions, Table 7 (6–9) finally determines the optimal conditions for PET. Subsequently, BHETA and heptanoic acid were catalyzed using 0.5% w/w sulfuric acid to synthesize a plasticizer (Fig. 14). Compared with DOP, a commonly used PVC plasticizer, the synthesized plasticizer exhibited superior compatibility and performance with PVC, offering enhanced application properties.
4, and the influence of reaction time and catalyst selection is continued to be discussed under these conditions, Table 7 (6–9) finally determines the optimal conditions for PET. Subsequently, BHETA and heptanoic acid were catalyzed using 0.5% w/w sulfuric acid to synthesize a plasticizer (Fig. 14). Compared with DOP, a commonly used PVC plasticizer, the synthesized plasticizer exhibited superior compatibility and performance with PVC, offering enhanced application properties.
| Entry | Catalyst | PET![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethanolamine ethanolamine |
Time/h | Yield/% |
|---|---|---|---|---|
| a Reaction conditions: temperature = 60 °C, catalyst = 1.5 wt%. | ||||
| 1 | Sodium acetate | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1 | 62.15 |
| 2 | Sodium acetate | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.5 2.5 |
1 | 65.23 |
| 3 | Sodium acetate | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
1 | 69.56 |
| 4 | Sodium acetate | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5.5 5.5 |
1 | 70.66 |
| 5 | Sodium acetate | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 7 |
1 | 71.19 |
| 6 | Sodium acetate | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
2 | 74.17 |
| 7 | Sodium acetate | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
3 | 74.93 |
| 8 | Sodium acetate | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
4 | 75.03 |
| 9 | Zinc acetate | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
3 | 81.16 |
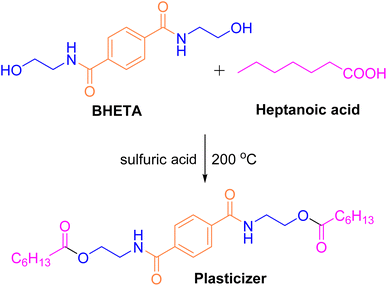 | ||
| Fig. 14 Synthesis of plasticizer from bis(2-hydroxyethyl)terephthalamide (BHETA). Reproduced with permission.112 Copyright 2013, Iran Polymer and Petrochemical Institute. | ||
In addition to ethanolamine, other amines like liquid ammonia and aliphatic polyamines, such as 1,3-diaminopropane (DAP), ethylenediamine, diethyltriamine (DETA), triethyltetramine (TETA), are also used for the aminolysis of PET. These polyamines from intermediates with PET that can react with other substances to produce valuable chemicals or industrial materials. Haque113 used PET fragments and DAP in a round-bottom flask, method A was reacted at 100 °C for 4 hours and then at room temperature for 20 hours, method B was reacted at 110 °C for 24 hours, and method C was reacted at 130 °C for 24 hours. Method C is a yellow-green liquid with the product of the monomer N1,N4-bis(2-(((E)-2-hydroxybenzylidene)amino)propyl)terephthalamide. Finally, the monomer N1,N4-bis(2-(((E)-2-hydroxybenzylidene)amino)propyl)terephthalamide generated by method C was condensed with salicylaldehyde in ethanol to obtain a yellow solid Schiff base and a yellow colloidal liquid condensation product, with yields of 25% and 62%, respectively, as shown in Fig. 15. Kang114 has developed a low-cost, low-energy, and environmentally friendly depolymerization method that does not require the addition of catalysts and solvents. In this method, the gas–solid reaction occurs under certain temperatures conditions, along with specific ratios of PET to anhydrous ammonia and stirring rates. The colorless PET undergoes ammonolysis to produce white or gray powder, which was identified as terephthalamide (TP) after characterization and analysis. Temperature was found to be the key factor influencing PET degradation. Within the temperature range of 40–110 °C, the yield and purity of TP increased, but when the temperature reached 120 °C, carbonization occurred, resulting in the conversion of TP powder into a black solid and negatively affecting both purity and yield of TP. Experimental verification based on the optimal conditions predicted by Response Surface Methodology (RSM) and Box-BBD (18.5 g PET, reaction at 115 °C for 7.5 hours) showed that the yield and purity of TP could reach 87.34% and 94.5%, respectively.
Zhang115 diluted and concentrated ethylenediamine to perform aminolysis with PET fibers, introducing NH2 groups into the PET molecules, which enhanced the hydrophilicity of the PET fibers. The hydrophilicity was assessed by measuring the absorption of water droplets by the PET fibers. The adsorption time decreased with increasing ethylenediamine concentration, and remained constant when the concentration exceeded 150 g L−1. While 70 °C was the intrinsic and optimal temperature for the reaction, temperatures above 70 °C led to the volatilization of ethylenediamine, causing a concentration effect. Due to the moderate reaction temperature and low ethylenediamine concentration, the treated PET fibers showed no significant degradation and retained their integrity. Zinchenko116 reported the reaction of PET with DETA and TETA, followed by crosslinking with ethylene glycol diglycidyl ether (EGDE) to produce hydrogel materials. The water solubility of the ammonolysis products of PET was critical for the preparation of hydrogels. The reaction was conducted at 190 °C, with a PET and DETA ration of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 for 30 minutes, resulting in the water-soluble product 13PD, which allowed for the complete depolymerization of PET. Mass spectrometry analysis revealed that 13PD was primarily a mixture of dimers composed of three DETA molecules and two terephthalic acid units. This product was then cross-linked with EGDE at 50 °C to form a transparent 13PD hydrogel, which exhibited a high degree of swelling compared to other hydrogels. This approach not only addresses environmental pollution concerns but also offers a promising solution for the industrial production of hydrogels.
3 for 30 minutes, resulting in the water-soluble product 13PD, which allowed for the complete depolymerization of PET. Mass spectrometry analysis revealed that 13PD was primarily a mixture of dimers composed of three DETA molecules and two terephthalic acid units. This product was then cross-linked with EGDE at 50 °C to form a transparent 13PD hydrogel, which exhibited a high degree of swelling compared to other hydrogels. This approach not only addresses environmental pollution concerns but also offers a promising solution for the industrial production of hydrogels.
2.4 Pyrolysis
Chemical degradation of PET can also be achieved through pyrolysis, a process where PET is depolymerized into valuable fuel products by heating at high temperatures in the presence of an inert gas. This method is one of the most effective ways to recover fuel products.117 The pyrolysis oil produced by pyrolysis includes various components such as alkanes, naphthenes, aromatics, etc., which are the primary components of petroleum.118 Other byproducts of pyrolysis include simple molecules like benzene dicarboxylic acid, benzoic acid, H2, CO, CO2, CH4, etc., which can be utilized in industrial applications. Kwon's group119 analyzed the gaseous and liquid products of PET pyrolysis in the presence of ultra-high purity CO2 and N2. From thermograms (TG) and differential thermograms (DTG) it was found that approximately 86% of the waste PET was decomposed within the temperature range of 380–670 °C. At 900 °C, about 14 wt% of PET remained, indicating that the mass decay and pyrolytic conversion of waste PET were consistent. Afterwards the group also investigated the concentration of pyrolysis products (H2, CH4, CO) as a function of temperature. Under N2 conditions they monitored that the concentration of H2 increased with temperature between 300–750 °C. However, above 750 °C, dehydrogenation occurred, causing the concentration of H2 to decrease. The concentration of CO2 remained unaffected by dehydrogenation, as evidenced by the similar concentration profiles of H2 and CH4 during pyrolysis. The mass balance indicated that liquid products accounted for 21.6 and 13.3% of the total pyrolysis products of waste PET under N2 and CO2 conditions, respectively, while gaseous products accounted for 63.8% and 71.5%, respectively. The study also demonstrated that CO2 facilitated the transfer of carbon from liquid (oil) to gas (natural gas). Pyrolysis oil consists of multiple components such as benzene derivatives, PAHs, linear hydrocarbons and acids. Further analysis reveals that CO2 inhibits cyclisation and aromatization during pyrolysis, notably with a decrease in benzoic acid content and a decrease in acidity. Overall, Eilhann's pyrolysis of waste PET to increased levels of gaseous products and inhibition of the acidic product benzoic acid using CO2 as the reaction medium provides a new environmentally friendly process for the degradation of waste PET. Shahi120 catalyzed the pyrolysis of PET using Lewis Brønsted acid-site catalysts, leading to the generation of solid, liquid and gaseous products. The effects of various parameters on the conversion rates were analyzed. The solid products primarily consisted of terephthalic acid and benzoic acid, while the gaseous products were mainly some alkane gases. The optimum conditions for the highest PET conversion were found to be 430 °C and a catalyst/polymer ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10. Increasing the catalyst amount resulted in a decrease in solid product content and an increase in gaseous products, with the color of the solid products changing from yellow to colorless. Higher temperatures also increased the yield of gaseous products, primarily CH4, C2H6 and C2H4. Diaz-Silvarrey121 employed an environmentally friendly SZ catalyst to catalyze PET pyrolysis. The catalyst, consisting of zirconium chloride octahydrate and ammonium sulphate, is strongly acidic. The results indicated that temperature had the greatest effect on PET conversion, with higher temperatures increasing the yield of gaseous products. In addition, the mass ratio of catalyst to PET also had an effect on the conversion of PET, with a more pronounced effect at higher temperatures. Increasing the mass ratio of catalyst to PET from 0 to 1
10. Increasing the catalyst amount resulted in a decrease in solid product content and an increase in gaseous products, with the color of the solid products changing from yellow to colorless. Higher temperatures also increased the yield of gaseous products, primarily CH4, C2H6 and C2H4. Diaz-Silvarrey121 employed an environmentally friendly SZ catalyst to catalyze PET pyrolysis. The catalyst, consisting of zirconium chloride octahydrate and ammonium sulphate, is strongly acidic. The results indicated that temperature had the greatest effect on PET conversion, with higher temperatures increasing the yield of gaseous products. In addition, the mass ratio of catalyst to PET also had an effect on the conversion of PET, with a more pronounced effect at higher temperatures. Increasing the mass ratio of catalyst to PET from 0 to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 at 450 °C increased the gas product content by 3%, whereas at 600 °C the gas product content increased by 29%. However, SZ catalyst deactivation occurred at temperatures above 400 °C, leading to a reduction in catalyst acidity and activity due to clogging of the pores. This deactivation occurred twice in the 100–700 °C interval, with the catalyst's active sites decreasing at 525 °C, necessitating increased loading to maintain degradation to light hydrocarbons. The optimal conditions for the pyrolysis of PET to obtain benzoic acid and gaseous products using SZ catalysts were found to be a catalyst loading of less than 10 wt% and a temperature below 525 °C. Straka's group122 used low-temperature slow pyrolysis to produce solid fuels with higher and lower calorific values as well as valuable chemicals like paraformaldehyde, ethylene glycol, benzoic acid and benzoates. The results showed that after activation at a heating rate of 40 °C min−1 up to 200 °C, the waste PET could be completely pyrolyzed into oils (paraformaldehyde, glycol, benzoic acid and benzoates) and gases (CO, CO2) by heating at a rate of 25 °C min−1 up to 400 °C. Olam's group123 used sodium borohydride (NaBH4) as a catalyst and hydrogen donor for PET pyrolysis and the experimental flow is shown in Fig. 16. As can be seen in Table 8. The total conversion increased from 15.2% to 55.3% and the liquid + gas yield rose from 6.9% to 28.6% as the reaction temperature increased from 325 °C to 400 °C in the presence of the catalyst. In the absence of the catalyst, the total conversion also increased substantially with higher temperatures, from 325 °C to 425 °C. Regardless of the presence of catalyst, total conversion and the yield of liquid + gas are also low at low temperatures, and high temperatures promote total conversion and the yield of liquid + gas. Overall, NaBH4 can increase both the total conversion and the yield of liquid + gas from PET during the pyrolysis process.
10 at 450 °C increased the gas product content by 3%, whereas at 600 °C the gas product content increased by 29%. However, SZ catalyst deactivation occurred at temperatures above 400 °C, leading to a reduction in catalyst acidity and activity due to clogging of the pores. This deactivation occurred twice in the 100–700 °C interval, with the catalyst's active sites decreasing at 525 °C, necessitating increased loading to maintain degradation to light hydrocarbons. The optimal conditions for the pyrolysis of PET to obtain benzoic acid and gaseous products using SZ catalysts were found to be a catalyst loading of less than 10 wt% and a temperature below 525 °C. Straka's group122 used low-temperature slow pyrolysis to produce solid fuels with higher and lower calorific values as well as valuable chemicals like paraformaldehyde, ethylene glycol, benzoic acid and benzoates. The results showed that after activation at a heating rate of 40 °C min−1 up to 200 °C, the waste PET could be completely pyrolyzed into oils (paraformaldehyde, glycol, benzoic acid and benzoates) and gases (CO, CO2) by heating at a rate of 25 °C min−1 up to 400 °C. Olam's group123 used sodium borohydride (NaBH4) as a catalyst and hydrogen donor for PET pyrolysis and the experimental flow is shown in Fig. 16. As can be seen in Table 8. The total conversion increased from 15.2% to 55.3% and the liquid + gas yield rose from 6.9% to 28.6% as the reaction temperature increased from 325 °C to 400 °C in the presence of the catalyst. In the absence of the catalyst, the total conversion also increased substantially with higher temperatures, from 325 °C to 425 °C. Regardless of the presence of catalyst, total conversion and the yield of liquid + gas are also low at low temperatures, and high temperatures promote total conversion and the yield of liquid + gas. Overall, NaBH4 can increase both the total conversion and the yield of liquid + gas from PET during the pyrolysis process.
 | ||
| Fig. 16 Flow chart of the pyrolysis experiments. Reproduced with permission.123 Copyright 2019, IOP Conference Series: Earth and Environmental Science. | ||
| Entry | Sample amount (wt as used) | Catalyst concentration (NaBH4, wt%) | Temperate (°C) | Char yield (wt%) | Total conversion (wt%) | Oil + gas (wt%) |
|---|---|---|---|---|---|---|
| 1 | 30.00 | 3 | 325 | 84.8 | 15.2 | 6.9 |
| 2 | 30.00 | 3 | 350 | 75.0 | 25.0 | 12.9 |
| 3 | 30.00 | 3 | 375 | 56.7 | 43.3 | 9.2 |
| 4 | 30.00 | 3 | 400 | 44.7 | 55.3 | 28.6 |
| 5 | 30.00 | 3 | 425 | 48.2 | 51.8 | 44.6 |
| 6 | 30.00 | — | 325 | 90.6 | 9.4 | 0.1 |
| 7 | 30.00 | — | 350 | 84.7 | 15.3 | 1.1 |
| 8 | 30.00 | — | 375 | 66.5 | 33.5 | 4.1 |
| 9 | 30.00 | — | 400 | 49.5 | 50.5 | 18.7 |
| 10 | 30.00 | — | 425 | 46.9 | 53.1 | 43.7 |
Ben106 investigated the pyrolysis of PET under N2 with ZSM-5 zeolite and NiCl2 as catalysts, focusing on the liquid phase products of PET pyrolysis as well as the effects of temperature and catalyst dosage on product yield. The yield of waxy products decreased from approximately 60 wt% to around 10 wt% while the gas yield increased from about 20 wt% to about 69 wt% with the gradual increase in ZSM-5 zeolite dosage at 450 °C. A similar trend was observed at 600 °C, indicating that ZSM-5 zeolite could enhance the gas yield and reduce the waxy product yield. Substituting NiCl2 as the catalyst also enhances the gas yield and reduces the waxy product yield. A comparison of the 13C NMR spectra of the waxy products with and without the ZSM-5 zeolite catalyst showed that ZSM-5 zeolite had the ability to deoxygenate the carbonyl groups and aliphatic C–O bonds in the products. Furthermore, using NiCl2 as a catalyst reduced the content of aliphatic C–O in the wax products and promoted the decomposition of alkyl, alkoxy and branched alkanes on the aromatic ring. Lee13 chose Pd/C, a stable and low-cost catalyst, to pyrolyze disposable plastic bottles primarily composed of PET. During the pyrolysis process, attention was given to the inhibition of harmful substances, such as polycyclic hydrocarbons and biphenyl derivatives, that could be produced. The analysis of the pyrolysis oil revealed that it consisted of polycyclic compounds, biphenyl derivatives and polycyclic amine compounds, with biphenyl derivatives including biphenyl-4-carboxylic acid, p-terphenyl, and terphenyl. They also examined the role of Pd catalyst to PET ratio in the PET pyrolysis process and found that there was no significant difference in the amounts of polycyclic hydrocarbons and biphenyl derivatives of the pyrolysis products between a Pd/PET ratio of 0.01 and non-catalytic conditions. However, the concentration of polycyclic hydrocarbons and biphenyl derivatives of the pyrolysis products decreased at 800 °C when the Pd/PET ratio was increased to 0.05. Small amounts of Pd did not significantly affect the concentrations of polycyclic hydrocarbons and biphenyl derivatives as pyrolysis products, but the concentrations increased at high temperatures or increased catalyst amounts because Pd catalyst promotes ring-opening reactions at the catalyst sites during the pyrolysis of PET, and high temperatures promote free radical reactions. It was also found that the production of amine compounds decreases with an increase in catalyst content. This not only inhibits the production of harmful substances, but also enhanced the yield of valuable compounds, providing a novel approach to the degradation of PET.
3 Summary and outlook
PET is one of the most widely used plastics, commonly found in everyday items such as water bottles, films, textiles, etc. Despite its widespread use, its special properties make it difficult to degrade, leading to significant plastic waste. Chemical recycling, on the other hand, provides an efficient method to address this issue by converting PET into valuable chemicals. This paper reviews various methods of chemical degradation, including alcoholysis (and glycolysis), hydrolysis, ammonolysis and pyrolysis. Among these methods, alcoholysis frequently uses methanol as a solvent, though other solvents such as ethanol and isooctanol, along with polyols, can also be used to produce DMT and EG, as well as BHET monomers. Glycolysis is a specialized form of alcoholysis that typically employs diols (such as ethylene glycol) as solvents, and can depolymerize PET to form BHET monomer using both homogeneous and heterogeneous catalysts. Hydrolysis involves both acid–base and neutral hydrolysis methods, which break down PET into TPA and EG. Neutral hydrolysis is considered simpler and more environmentally friendly compared to acid–base hydrolysis. Aminolysis, though less explored, is another promising method for chemical recycling, often using ethanolamine as a solvent to produce BHETA. Pyrolysis is a thermal degradation process in which PET is converted into simpler molecules by high-temperature heating. Key factors affecting the conversion of PET in pyrolysis include temperature and catalyst usage. The application of new technologies, such as ionic liquids, deep eutectic solvents and metal–organic frameworks, can accelerate the reaction rate and enhance the conversion and yield of PET degradation products. Other parameters, such as whether to add catalyst, different temperatures, times, solvents and their dosages, etc., also play a critical role in determining the products of PET degradation. Based on these published studies, significant potential remains for further advancing PET degradation technologies.In the future, efforts should focus on clarifying specific degradation goals and continuing to develop chemical degradation technologies. This includes the creation of greener solvents or catalysts for alcoholysis, further exploration of neutral hydrolysis to reduce the costs and waste associated with acid–base hydrolysis, and the use of a broader range of amine compounds for ammonolysis. Additionally, enhancing the yield of desired substances in pyrolysis through the discovery of new catalysts will be key. Ultimately, the goal is to achieve sustainable recycling of waste PET, contributing to the reduction of plastic pollution.
Data availability
This is a review article. All the data reported here can be found in the cited papers in the Reference section.Author contributions
Z. Q. Guo contributed to original draft preparation and writing—reviewing and editing. J. Wu contributed to conceptualization and writing—original draft preparation. J. H. Wang helped in conceptualization, writing—reviewing and editing. All the authors discussed and approved the current version of the manuscript.Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
Financial supports from the National Key Research and Development Program of China (Grant No. 2021YFD1901105-2), the Science and Technology Major Project of Shanxi Province (Grant No. 202101140601026), the Natural Science Foundation of Shanxi Province (Grant No. 201801D121041 and Grant No. 20210302123446) are gratefully acknowledged.References
- M. Han, S. Zhu, C. Xia and B. Yang, Photocatalytic upcycling of poly(ethylene terephthalate) plastic to high-value chemicals, Appl. Catal., B, 2022, 316, 121662, DOI:10.1016/j.apcatb.2022.121662.
- P. T. Benavides, J. B. Dunn, J. Han, M. Biddy and J. Markham, Exploring Comparative Energy and Environmental Benefits of Virgin, Recycled, and Bio-Derived PET Bottles, ACS Sustain. Chem. Eng., 2018, 6, 9725–9733, DOI:10.1021/acssuschemeng.8b00750.
- M. Wang, Y. Li, L. Zheng, T. Hu, M. Yan and C. Wu, Recycling and depolymerisation of poly(ethylene terephthalate): a review, Polym. Chem., 2024, 15, 585–608, 10.1039/d3py01218b.
- Y. Weng, C.-B. Hong, Y. Zhang and H. Liu, Catalytic depolymerization of polyester plastics toward closed-loop recycling and upcycling, Green Chem., 2024, 26, 571–592, 10.1039/d3gc04174c.
- A. C. Fernandes, Reductive depolymerization as an efficient methodology for the conversion of plastic waste into value-added compounds, Green Chem., 2021, 23, 7330–7360, 10.1039/d1gc01634b.
- N. Takahashi, Y. Miyanishi, R. Kato, T. Amimoto, Y. Iwamoto and K. Takeda, Migration of terephthalate from scraps of poly(ethylene terephthalate) (PET) in water and artificial seawater, Sci. Total Environ., 2022, 838, 156053, DOI:10.1016/j.scitotenv.2022.156053.
- S. Ahmaditabatabaei, G. Kyazze, H. M. N. Iqbal and T. Keshavarz, Fungal Enzymes as Catalytic Tools for Polyethylene Terephthalate (PET) Degradation, J. Fungi, 2021, 7, 931, DOI:10.3390/jof7110931.
- B. Nguyen, D. Claveau-Mallet, L. M. Hernandez, E. G. Xu, J. M. Farner and N. Tufenkji, Separation and Analysis of Microplastics and Nanoplastics in Complex Environmental Samples, Acc. Chem. Res., 2019, 52, 858–866, DOI:10.1021/acs.accounts.8b00602.
- L. Henderson and C. Green, Making sense of microplastics? Public understandings of plastic pollution, Mar. Pollut. Bull., 2020, 152, 110908, DOI:10.1016/j.marpolbul.2020.110908.
- T. N. Tsironi, S. M. Chatzidakis and N. G. Stoforos, The future of polyethylene terephthalate bottles: Challenges and sustainability, Packag. Technol. Sci., 2022, 35, 317–325, DOI:10.1002/pts.2632.
- K. Beydoun and J. Klankermayer, Efficient Plastic Waste Recycling to Value-Added Products by Integrated Biomass Processing, ChemSusChem, 2020, 13, 488–492, DOI:10.1002/cssc.201902880.
- H. Abedsoltan, A focused review on recycling and hydrolysis techniques of polyethylene terephthalate, Polym. Eng. Sci., 2023, 63, 2651–2674, DOI:10.1002/pen.26406.
- C. Park, S. Kim, Y. Kwon, C. Jeong, Y. Cho, C.-G. Lee, S. Jung, K.-Y. Choi and J. Lee, Pyrolysis of Polyethylene Terephthalate over Carbon-Supported Pd Catalyst, Catalysts, 2020, 10, 496, DOI:10.3390/catal10050496.
- L. Canopoli, B. Fidalgo, F. Coulon and S. T. Wagland, Physico-chemical properties of excavated plastic from landfill mining and current recycling routes, Waste Manage., 2018, 76, 55–67, DOI:10.1016/j.wasman.2018.03.043.
- A. C. Espinosa-López, C. A. Ávila-Orta, F. J. Medellín-Rodríguez, P. González-Morones, C. A. Gallardo-Vega, P. A. De León-Martínez, M. Navarro-Rosales and J. A. Valdez-Garza, Microwave-assisted esterification step of poly(ethylene terephthalate) (PET) synthesis through ethylene glycol and terephthalic acid, Polym. Bull., 2018, 76, 2931–2944, DOI:10.1007/s00289-018-2521-9.
- F. Cao, L. Wang, R. Zheng, L. Guo, Y. Chen and X. Qian, Research and progress of chemical depolymerization of waste PET and high-value application of its depolymerization products, RSC Adv., 2022, 12, 31564–31576, 10.1039/d2ra06499e.
- T. Ren, H. Zhan, H. Xu, L. Chen, W. Shen, Y. Xu, D. Zhao, Y. Shao and Y. Wang, Recycling and high-value utilization of polyethylene terephthalate wastes: A review, Environ. Res., 2024, 249, 118428, DOI:10.1016/j.envres.2024.118428.
- A. Rahimi and J. M. García, Chemical recycling of waste plastics for new materials production, Nat. Rev. Chem, 2017, 1, 0046, DOI:10.1038/s41570-017-0046.
- I. Flores, J. Demarteau, A. J. Müller, A. Etxeberria, L. Irusta, F. Bergman, C. Koning and H. Sardon, Screening of different organocatalysts for the sustainable synthesis of PET, Eur. Polym. J., 2018, 104, 170–176, DOI:10.1016/j.eurpolymj.2018.04.040.
- P. Švec, P. Hubená, Z. Růžičková, J. Holubová, M. Pouzar, J. Merna and A. Růžička, Poly(ethylene terephthalate) synthesis catalysed by chelated Sn, Zn and Mg complexes, Appl. Organomet. Chem., 2015, 30, 20–25, DOI:10.1002/aoc.3393.
- A. Jaime-Azuara, E. Longo, E. Boselli, M. Baratieri and T. H. Pedersen, Exploratory DSC investigation on the solvolytic depolymerization of PET in varied solvent systems and in the presence of model additives and contaminants, Polym. Degrad. Stab., 2024, 224, 110751, DOI:10.1016/j.polymdegradstab.2024.110751.
- M. Arhant, M. L. Gall and P.-Y. L. Gac, Non-Arrhenian hydrolysis of polyethylene terephthalate – A 5-year long aging study above and below the glass transition temperature, Polym. Degrad. Stab., 2023, 215, 110439, DOI:10.1016/j.polymdegradstab.2023.110439.
- R. V. Moharir and S. Kumar, Challenges associated with plastic waste disposal and allied microbial routes for its effective degradation: A comprehensive review, J. Cleaner Prod., 2019, 208, 65–76, DOI:10.1016/j.jclepro.2018.10.059.
- T. Sang, C. J. Wallis, G. Hill and G. J. P. Britovsek, Polyethylene terephthalate degradation under natural and accelerated weathering conditions, Eur. Polym. J., 2020, 136, 109873, DOI:10.1016/j.eurpolymj.2020.109873.
- Z. Jia, L. Gao, L. Qin and J. Yin, Chemical recycling of PET to value-added products, RSC Sustainability, 2023, 1, 2135–2147, 10.1039/d3su00311f.
- M. Muszyński, J. Nowicki, M. Zygadło and G. Dudek, Comparsion of Catalyst Effectiveness in Different Chemical Depolymerization Methods of Poly(ethylene terephthalate), Molecules, 2023, 28, 6385, DOI:10.3390/molecules28176385.
- E. Barnard, J. J. Rubio Arias and W. Thielemans, Chemolytic depolymerisation of PET: a review, Green Chem., 2021, 23, 3765–3789, 10.1039/d1gc00887k.
- S. C. Kosloski-Oh, Z. A. Wood, Y. Manjarrez, J. P. de los Rios and M. E. Fieser, Catalytic methods for chemical recycling or upcycling of commercial polymers, Mater. Horiz., 2021, 8, 1084–1129, 10.1039/d0mh01286f.
- M.-Q. Zhang, M. Wang, B. Sun, C. Hu, D. Xiao and D. Ma, Catalytic strategies for upvaluing plastic wastes, Chem, 2022, 8, 2912–2923, DOI:10.1016/j.chempr.2022.08.004.
- S. Joo, I. J. Cho, H. Seo, H. F. Son, H.-Y. Sagong, T. J. Shin, S. Y. Choi, S. Y. Lee and K.-J. Kim, Structural insight into molecular mechanism of poly(ethylene terephthalate) degradation, Nat. Commun., 2018, 9, 382, DOI:10.1038/s41467-018-02881-1.
- L. Zhou, X. Lu, Z. Ju, B. Liu, H. Yao, J. Xu, Q. Zhou, Y. Hu and S. Zhang, Alcoholysis of polyethylene terephthalate to produce dioctyl terephthalate using choline chloride-based deep eutectic solvents as efficient catalysts, Green Chem., 2019, 21, 897–906, 10.1039/c8gc03791d.
- F. Kawai, Emerging Strategies in Polyethylene Terephthalate Hydrolase Research for Biorecycling, ChemSusChem, 2021, 14, 4115–4122, DOI:10.1002/cssc.202100740.
- A. C. Fernandes, Reductive Depolymerization of Plastic Waste Catalyzed by Zn(OAc)2·2H2O, ChemSusChem, 2021, 14, 4228–4233, DOI:10.1002/cssc.202100130.
- M. H. Ghasemi, N. Neekzad, F. B. Ajdari, E. Kowsari and S. Ramakrishna, Mechanistic aspects of poly(ethylene terephthalate) recycling–toward enabling high quality sustainability decisions in waste management, Environ. Sci. Pollut. Res., 2021, 28, 43074–43101, DOI:10.1007/s11356-021-14925-z.
- A. Khan, M. Naveed, Z. Aayanifard and M. Rabnawaz, Efficient chemical recycling of waste polyethylene terephthalate, Resour. Conserv. Recycl., 2022, 187, 106639, DOI:10.1016/j.resconrec.2022.106639.
- D. M. Scremin, D. Y. Miyazaki, C. E. Lunelli, S. A. Silva and S. F. Zawadzki, PET Recycling by Alcoholysis Using a New Heterogeneous Catalyst: Study and its Use in Polyurethane Adhesives Preparation, Macromol. Symp., 2019, 383, 1800027, DOI:10.1002/masy.201800027.
- M. D. de Dios Caputto, R. Navarro, J. L. Valentín and Á. Marcos-Fernández, Chemical upcycling of poly(ethylene terephthalate) waste: Moving to a circular model, J. Polym. Sci., 2022, 60, 3269–3283, DOI:10.1002/pol.20220137.
- M. Ma, S. Wang, Y. Liu, H. Yu, S. Yu, C. Ji, H. Li, G. Nie and S. Liu, Insights into the depolymerization of polyethylene terephthalate in methanol, J. Appl. Polym. Sci., 2022, 139, e52814, DOI:10.1002/app.52814.
- M. Hofmann, J. Sundermeier, C. Alberti and S. Enthaler, Zinc(II) acetate Catalyzed Depolymerization of Poly(ethylene terephthalate), ChemistrySelect, 2020, 5, 10010–10014, DOI:10.1002/slct.202002260.
- J.-T. Du, Q. Sun, X.-F. Zeng, D. Wang, J.-X. Wang and J.-F. Chen, ZnO nanodispersion as pseudohomogeneous catalyst for alcoholysis of polyethylene terephthalate, Chem. Eng. Sci., 2020, 220, 115642, DOI:10.1016/j.ces.2020.115642.
- D. D. Pham and J. Cho, Low-energy catalytic methanolysis of poly(ethyleneterephthalate), Green Chem., 2021, 23, 511–525, 10.1039/d0gc03536j.
- M. Zhang, Y. Yu, B. Yan, X. Song, Y. Liu, Y. Feng, W. Wu, B. Chen, B. Han and Q. Mei, Full valorisation of waste PET into dimethyl terephthalate and cyclic arylboronic esters, Appl. Catal., B, 2024, 352, 124055, DOI:10.1016/j.apcatb.2024.124055.
- S. Tang, F. Li, J. Liu, B. Guo, Z. Tian and J. Lv, Calcined sodium silicate as solid base catalyst for alcoholysis of poly(ethylene terephthalate), J. Chem. Technol. Biotechnol., 2022, 97, 1305–1314, DOI:10.1002/jctb.7025.
- Y. Li, M. Wang, X. Liu, C. Hu, D. Xiao and D. Ma, Catalytic Transformation of PET and CO2 into High-Value Chemicals, Angew. Chem., Int. Ed., 2022, 61, e202117205, DOI:10.1002/anie.202117205.
- M. M. Lin, J. Tay Zheng and W.-Y. Yu, One-pot methanolysis of poly(ethylene terephthalate) enabled by isopropanol-assisted CO2 hydrogenation, J. Taiwan Inst. Chem. Eng., 2024, 158, 105069, DOI:10.1016/j.jtice.2023.105069.
- J. Liu and J. Yin, Carbon Dioxide Synergistic Enhancement of Supercritical Methanol on PET Depolymerization for Chemical Recovery, Ind. Eng. Chem. Res., 2022, 61, 6813–6819, DOI:10.1021/acs.iecr.2c00572.
- S. Tanaka, J. Sato and Y. Nakajima, Capturing ethylene glycol with dimethyl carbonate towards depolymerisation of polyethylene terephthalate at ambient temperature, Green Chem., 2021, 23, 9412–9416, 10.1039/d1gc02298a.
- J. Li, D. Yan, X. Cheng, C. Rong, J. Feng, X. Feng, J. Xin, Q. Zhou, Y. Li and J. Xu, et al., Efficient Methanolysis of PET Catalyzed by Nonmetallic Deep Eutectic Solvents, Ind. Eng. Chem. Res., 2024, 63, 12373–12384, DOI:10.1021/acs.iecr.4c00720.
- B. Gurkan, H. Squire and E. Pentzer, Metal-Free Deep Eutectic Solvents: Preparation, Physical Properties, and Significance, J. Phys. Chem. Lett., 2019, 10, 7956–7964, DOI:10.1021/acs.jpclett.9b01980.
- M. Li, W. Chen, W. Chen and S. Chen, Degradation of Poly(ethylene terephthalate) Using Metal-Free 1,8-Diazabicyclo[5.4.0]undec-7-ene-Based Deep Eutectic Solvents as Efficient Catalysts, Ind. Eng. Chem. Res., 2023, 62, 10040–10050, DOI:10.1021/acs.iecr.3c01071.
- E. M. Senra, A. E. F. A. da Silva, L. L. Y. Visconte, A. L. N. Silva and E. B. A. V. Pacheco, Influence of a Catalyst in Obtaining a Post-consumer Pet-Based Alkyd Resin that Meets Circular Economy Principles, J. Polym. Environ., 2022, 30, 3761–3778, DOI:10.1007/s10924-022-02471-9.
- A. Arunphacharawit, T. Poonsawat, T. Meechai, L. Chaicharoenwimolkul Chuaitammakit and E. Somsook, DFT study on the depolymerization of PET by Ca-catalyzed glycolysis reaction model, Heliyon, 2024, 10, e34666, DOI:10.1016/j.heliyon.2024.e34666.
- T. Textor, L. Derksen and J. S. Gutmann, Employing ionic liquids to deposit cellulose on PET fibers, Carbohydr. Polym., 2016, 146, 139–147, DOI:10.1016/j.carbpol.2016.03.053.
- T. Wang, C. Shen, G. Yu and X. Chen, Metal ions immobilized on polymer ionic liquid as novel efficient and facile recycled catalyst for glycolysis of PET, Polym. Degrad. Stab., 2021, 194, 109751, DOI:10.1016/j.polymdegradstab.2021.109751.
- K. Shi, L. Guo, R. Zheng, H. Wang and Y. Chen, Preparation of Diacid Comprising Ionic Liquid Catalyst and Its Application in Catalytic Degradation of PET, Catal. Lett., 2021, 152, 1182–1193, DOI:10.1007/s10562-021-03716-3.
- R. Wang, T. Wang, G. Yu and X. Chen, A new class of catalysts for the glycolysis of PET: Deep eutectic solvent@ZIF-8 composite, Polym. Degrad. Stab., 2021, 183, 109463, DOI:10.1016/j.polymdegradstab.2020.109463.
- D. Gangaraju, A. M. Shanmugharaj and V. Sridhar, Graphene Oxide Facilitates Transformation of Waste PET into MOF Nanorods in Ionic Liquids, Polymers, 2023, 15, 2479, DOI:10.3390/polym15112479.
- P. Anggo Krisbiantoro, Y.-W. Chiao, W. Liao, J.-P. Sun, D. Tsutsumi, H. Yamamoto, Y. Kamiya and C.-W. K. Wu, Catalytic glycolysis of polyethylene terephthalate (PET) by solvent-free mechanochemically synthesized MFe2O4 (M = Co, Ni, Cu and Zn) spinel, Chem. Eng. J., 2022, 450, 137926, DOI:10.1016/j.cej.2022.137926.
- Z. Fehér, J. Kiss, P. Kisszékelyi, J. Molnár, P. Huszthy, L. Kárpáti and J. Kupai, Optimisation of PET glycolysis by applying recyclable heterogeneous organocatalysts, Green Chem., 2022, 24, 8447–8459, 10.1039/d2gc02860c.
- Y.-J. Chen, X. Huang, Y. Chen, Y.-R. Wang, H. Zhang, C.-X. Li, L. Zhang, H. Zhu, R. Yang and Y.-H. Kan, et al., Polyoxometalate-Induced Efficient Recycling of Waste Polyester Plastics into Metal–Organic Frameworks, CCS Chem., 2019, 1, 561–570, DOI:10.31635/ccschem.019.20190028.
- P. Fang, X. Lu, Q. Zhou, D. Yan, J. Xin, J. Xu, C. Shi, Y. Zhou and S. Xia, Controlled alcoholysis of PET to obtain oligomers for the preparation of PET-PLA copolymer, Chem. Eng. J., 2023, 451, 138988, DOI:10.1016/j.cej.2022.138988.
- P. Fang, S. Xia and X. Lu, Rapid alcoholysis of PET enhanced by its swelling under high temperature, J. Environ. Chem. Eng., 2022, 10, 107823, DOI:10.1016/j.jece.2022.107823.
- Q. Suo, J. Zi, Z. Bai and S. Qi, The Glycolysis of Poly(ethylene terephthalate) Promoted by Metal Organic Framework (MOF) Catalysts, Catal. Lett., 2016, 147, 240–252, DOI:10.1007/s10562-016-1897-0.
- D. D. Pham, D.-N. Vo, M. T. Phong, H. H. Nguyen, T. Nguyen-Thoi, T.-P. T. Pham, D. Ha Le Phuong, L. K. H. Pham, D. H. Won and D. L. T. Nguyen, et al., A new circulation in glycolysis of polyethylene terephthalate using MOF-based catalysts for environmental sustainability of plastic, Chem. Eng. J., 2024, 490, 151667, DOI:10.1016/j.cej.2024.151667.
- K. Nilwanna, J. Sittiwong, P. Srifa, B. Boekfa, P. Treesukol, M. Probst, T. Maihom and J. Limtrakul, Theoretical insights into poly(ethylene terephthalate) glycolysis catalyzed by acid-base pairs in Zn-supported MOF-808 metal-organic framework, Chem. Phys. Lett., 2024, 836, 141034, DOI:10.1016/j.cplett.2023.141034.
- E. Luna, I. Olazabal, M. Roosen, A. Müller, C. Jehanno, M. Ximenis, S. de Meester and H. Sardon, Towards a better understanding of the cosolvent effect on the low-temperature glycolysis of Polyethylene Terephthalate (PET), Chem. Eng. J., 2024, 482, 148861, DOI:10.1016/j.cej.2024.148861.
- Y. Cha, Y.-J. Park and D. H. Kim, Hydrodynamic synthesis of Fe2O3@MoS2 0D/2D-nanocomposite material and its application as a catalyst in the glycolysis of polyethylene terephthalate, RSC Adv., 2021, 11, 16841–16848, 10.1039/d1ra02335g.
- S. G. Son, S. B. Jin, S. J. Kim, H. J. Park, J. Shin, T. Ryu, J.-M. Jeong and B. G. Choi, Exfoliated manganese oxide nanosheets as highly active catalysts for glycolysis of polyethylene terephthalate, FlatChem, 2022, 36, 100430, DOI:10.1016/j.flatc.2022.100430.
- L.-X. Yun, H. Wu, Z.-G. Shen, J.-W. Fu and J.-X. Wang, Ultrasmall CeO2 Nanoparticles with Rich Oxygen Defects as Novel Catalysts for Efficient Glycolysis of Polyethylene Terephthalate, ACS Sustain. Chem. Eng., 2022, 10, 5278–5287, DOI:10.1021/acssuschemeng.2c00480.
- C. Dai, Y. Liu, Z. Wang and G. Yu, Efficient glycolysis of waste polyethylene terephthalate textiles over Zn-MCM-41 catalysts, Catal. Today, 2024, 440, 114827, DOI:10.1016/j.cattod.2024.114827.
- R. Wen, G. Shen, Y. Yu, J. Zhu, S. Xu, J. Wei and Y. Huo, Optimization of Ti-PA efficiently catalytic the alcoholysis of waste PET using response surface methodology, Environ. Sci. Pollut. Res., 2024, 31, 33443–33453, DOI:10.1007/s11356-024-33371-1.
- F. E. P. Lapian, M. I. Ramli, M. Pasra and A. Arsyad, The Performance Modeling of Modified Asbuton and Polyethylene Terephthalate (PET) Mixture Using Response Surface Methodology (RSM), Appl. Sci., 2021, 11, 6144, DOI:10.3390/app11136144.
- R. Wen, G. Shen, Y. Yu, S. Xu, J. Wei, Y. Huo and S. Jiang, Optimization of Ti–BA efficiently for the catalytic alcoholysis of waste PET using response surface methodology, RSC Adv., 2023, 13, 17166–17178, 10.1039/d3ra01460f.
- R. Wen, G. Shen, J. Zhai, L. Meng and Y. Bai, Optimization of TiO(acac)2 for efficient catalytic alcoholysis of waste PET using response surface methodology, New J. Chem., 2023, 47, 14646–14655, 10.1039/d3nj02872k.
- X. Chen, J. Xu, X. Lu, J. Xin, Q. Zhou, D. Yan and K. Wang, Alcoholysis of PET catalyzed by bimidazolium cationic zinc acetate deep eutectic solvent, Sci. Sin.: Chim., 2021, 52, 768–778, DOI:10.1360/ssc-2021-0219.
- X. Zhou, C. Wang, C. Fang, R. Yu, Y. Li and W. Lei, Structure and thermal properties of various alcoholysis products from waste poly(ethylene terephthalate), Waste Manage., 2019, 85, 164–174, DOI:10.1016/j.wasman.2018.12.032.
- P. Huang, J. Pitcher, A. Mushing, F. Lourenço and M. P. Shaver, Chemical recycling of multi-materials from glycol-modified poly(ethylene terephthalate), Resour. Conserv. Recycl., 2023, 190, 106854, DOI:10.1016/j.resconrec.2022.106854.
- J. Chen, J. Lv, Y. Ji, J. Ding, X. Yang, M. Zou and L. Xing, Alcoholysis of PET to produce dioctyl terephthalate by isooctyl alcohol with ionic liquid as cosolvent, Polym. Degrad. Stab., 2014, 107, 178–183, DOI:10.1016/j.polymdegradstab.2014.05.013.
- Y. Liu, X. Yao, H. Yao, Q. Zhou, J. Xin, X. Lu and S. Zhang, Degradation of poly(ethylene terephthalate) catalyzed by metal-free choline-based ionic liquids, Green Chem., 2020, 22, 3122–3131, 10.1039/d0gc00327a.
- S. Ren, T. Mu and W. Wu, Advances in Deep Eutectic Solvents: New Green Solvents, Processes, 2023, 11, 1920, DOI:10.3390/pr11071920.
- Y. Wang, S. Ren, Y. Hou and W. Wu, Capture of Acidic Gases from Flue Gas by Deep Eutectic Solvents, Processes, 2021, 9, 1268, DOI:10.3390/pr9081268.
- A. M. Al-Sabagh, F. Z. Yehia, D. R. K. Harding, G. Eshaq and A. E. ElMetwally, Fe3O4-boosted MWCNT as an efficient sustainable catalyst for PET glycolysis, Green Chem., 2016, 18, 3997–4003, 10.1039/c6gc00534a.
- S. F. Llopis, E. Verdejo, O. Gil-Castell and A. Ribes-Greus, Partial glycolytic depolymerisation of poly(ethylene terephthalate) (PET) in the solid state: Modelling the contribution of time and temperature, Polym. Degrad. Stab., 2024, 221, 110695, DOI:10.1016/j.polymdegradstab.2024.110695.
- C. Shuangjun, S. Weihe, C. Haidong, Z. Hao, Z. Zhenwei and F. Chaonan, Glycolysis of poly(ethylene terephthalate) waste catalyzed by mixed Lewis acidic ionic liquids, J. Therm. Anal. Calorim., 2020, 143, 3489–3497, DOI:10.1007/s10973-020-10331-8.
- S. Najafi-Shoa, M. Barikani, M. Ehsani and M. Ghaffari, Cobalt-based ionic liquid grafted on graphene as a heterogeneous catalyst for poly (ethylene terephthalate) glycolysis, Polym. Degrad. Stab., 2021, 192, 109691, DOI:10.1016/j.polymdegradstab.2021.109691.
- R. Zhang, X. Zheng, X. Yao, K. Song, Q. Zhou, C. Shi, J. Xu, Y. Li, J. Xin and I. E.-T. El Sayed, et al., Light-Colored rPET Obtained by Nonmetallic TPA-Based Ionic Liquids Efficiently Recycle Waste PET, Ind. Eng. Chem. Res., 2023, 62, 11851–11861, DOI:10.1021/acs.iecr.3c01468.
- C. Zhang, H. He, Y. Shen, Q. Li and X. Ye, Green Catalytic Ionic Liquids Containing Organophosphorus for Efficient Glycolysis of Waste PET Bottle Flakes, Ind. Eng. Chem. Res., 2024, 63, 10903–10913, DOI:10.1021/acs.iecr.4c01549.
- E. Sert, E. Yılmaz and F. S. Atalay, Chemical Recycling of Polyethlylene Terephthalate by Glycolysis Using Deep Eutectic Solvents, J. Polym. Environ., 2019, 27, 2956–2962, DOI:10.1007/s10924-019-01578-w.
- W. Yang, R. Liu, C. Li, Y. Song and C. Hu, Hydrolysis of waste polyethylene terephthalate catalyzed by easily recyclable terephthalic acid, Waste Manage., 2021, 135, 267–274, DOI:10.1016/j.wasman.2021.09.009.
- M. S. Islam, Z. Islam, R. Hasan and A. H. M. S. Islam Molla Jamal, Acidic hydrolysis of recycled polyethylene terephthalate plastic for the production of its monomer terephthalic acid, Prog. Rubber Plast. Recycl. Technol., 2022, 39, 12–25, DOI:10.1177/14777606221128038.
- H. Abedsoltan and M. R. Coleman, Aryl sulfonic acid catalysts: Effect of pendant group structure on activity in hydrolysis of polyethylene terephthalate, J. Appl. Polym. Sci., 2022, 139, e52451, DOI:10.1002/app.52451.
- I. M. Chirica, A. G. Mirea, T. Şuteu, A. Kuncser, Ş. Neaţu, M. Florea, M. W. Barsoum and F. Neaţu, Acid-Modified, Ti3C2-Based MXene as Catalysts for Upcycling Polyethylene Terephthalate, ACS Sustain. Chem. Eng., 2024, 12, 9766–9776, DOI:10.1021/acssuschemeng.4c01920.
- H. Abedsoltan, I. S. Omodolor, A. C. Alba-Rubio and M. R. Coleman, Poly (4-styrenesulfonic acid): A recoverable and reusable catalyst for acid hydrolysis of polyethylene terephthalate, Polymer, 2021, 222, 123620, DOI:10.1016/j.polymer.2021.123620.
- W. Yang, J. Wang, L. Jiao, Y. Song, C. Li and C. Hu, Easily recoverable and reusable p-toluenesulfonic acid for faster hydrolysis of waste polyethylene terephthalate, Green Chem., 2022, 24, 1362–1372, 10.1039/d1gc04567a.
- S. Ügdüler, K. M. Van Geem, R. Denolf, M. Roosen, N. Mys, K. Ragaert and S. De Meester, Towards closed-loop recycling of multilayer and coloured PET plastic waste by alkaline hydrolysis, Green Chem., 2020, 2, 5376–5394, 10.1039/d0gc00894j.
- O. A. Attallah, A. Janssens, M. Azeem and M. B. Fournet, Fast, High Monomer Yield from Post-consumer Polyethylene Terephthalate via Combined Microwave and Deep Eutectic Solvent Hydrolytic Depolymerization, ACS Sustain. Chem. Eng., 2021, 9, 17174–17185, DOI:10.1021/acssuschemeng.1c07159.
- X.-L. Wang, W.-L. An, R. Du, F. Tian, Y. Yang, X. Zhao, S. Xu and Y.-Z. Wang, Rapid hydrolysis of PET in high-concentration alcohol aqueous solution by pore formation and spontaneous separation of terephthalate, J. Environ. Chem. Eng., 2023, 11, 109434, DOI:10.1016/j.jece.2023.109434.
- C.-H. Sun, X.-P. Chen, Q. Zhuo and T. Zhou, Recycling and depolymerization of waste polyethylene terephthalate bottles by alcohol alkali hydrolysis, J. Cent. South Univ., 2018, 25, 543–549, DOI:10.1007/s11771-018-3759-y.
- J. J. Rubio Arias and W. Thielemans, Instantaneous hydrolysis of PET bottles: an efficient pathway for the chemical recycling of condensation polymers, Green Chem., 2021, 23, 9945–9956, 10.1039/d1gc02896k.
- M. Čolnik, Ž. Knez and M. Škerget, Sub- and supercritical water for chemical recycling of polyethylene terephthalate waste, Chem. Eng. Sci., 2021, 233, 116389, DOI:10.1016/j.ces.2020.116389.
- P. Pereira, P. E. Savage and C. W. Pester, Neutral Hydrolysis of Post-Consumer Polyethylene Terephthalate Waste in Different Phases, ACS Sustain. Chem. Eng., 2023, 11, 7203–7209, DOI:10.1021/acssuschemeng.3c00946.
- F. Quartinello, S. Vajnhandl, J. Volmajer Valh, T. J. Farmer, B. Vončina, A. Lobnik, E. Herrero Acero, A. Pellis and G. M. Guebitz, Synergistic chemo-enzymatic hydrolysis of poly(ethylene terephthalate) from textile waste, Microb. Biotechnol., 2017, 10, 1376–1383, DOI:10.1111/1751-7915.12734.
- T. El Darai, A. Ter-Halle, M. Blanzat, G. Despras, V. Sartor, G. Bordeau, A. Lattes, S. Franceschi, S. Cassel and N. Chouini-Lalanne, et al., Chemical recycling of polyester textile wastes: shifting towards sustainability, Green Chem., 2024, 26, 6857–6885, 10.1039/d4gc00911h.
- H. Sun, Z. Chen, J. Zhou, L. Chen and W. Zuo, Recovery of high-quality terephthalic acid from waste polyester textiles via a neutral hydrolysis method, J. Environ. Chem. Eng., 2024, 12, 112558, DOI:10.1016/j.jece.2024.112558.
- J. Lou, L. Ren, J. Yuan, J. Xu, Z. Gu and X. Fan, Mechanism and application of ozone fading: Oxidative decolorisation of disperse dyes and waste-dyed polyester fabrics, Color. Technol., 2022, 139, 338–349, DOI:10.1111/cote.12654.
- H. Jia, H. Ben, Y. Luo and R. Wang, Catalytic Fast Pyrolysis of Poly (Ethylene Terephthalate) (PET) with Zeolite and Nickel Chloride, Polymers, 2020, 12, 705, DOI:10.3390/polym12030705.
- P. Gao, W.-H. Qiao, Z.-Y. Hu, B.-C. Yang, C.-Y. Cao, Y. Fu, Y. Xia, C.-X. Wang, G.-P. Cao and H. Lv, Hydrolysis of polyethylene terephthalate by ZSM-5 combined with supercritical carbon dioxide under neutral environment, Polym. Degrad. Stab., 2024, 219, 110590, DOI:10.1016/j.polymdegradstab.2023.110590.
- D. Stanica-Ezeanu and D. Matei, Natural depolymerization of waste poly(ethylene terephthalate) by neutral hydrolysis in marine water, Sci. Rep., 2021, 11, 4431, DOI:10.1038/s41598-021-83659-2.
- Y. Zhang, F. Tian, Z. Wu, X. Li, X. Liu and Y. He, Chemical conversion of waste PET to valued-added bis(2-hydroxyethyl) terephthalamide through aminolysis, Mater. Today Commun., 2022, 32, 104045, DOI:10.1016/j.mtcomm.2022.104045.
- S. Ghorbantabar, M. Ghiass, N. Yaghobi and H. Bouhendi, Investigation of conventional analytical methods for determining conversion of polyethylene terephthalate waste degradation via aminolysis process, J. Mater. Cycles Waste Manage., 2021, 23, 526–536, DOI:10.1007/s10163-020-01149-5.
- V. Vinitha, M. Preeyanghaa, M. Anbarasu, G. Jeya, B. Neppolian and V. Sivamurugan, Aminolytic Depolymerization of Polyethylene Terephthalate Wastes Using Sn-Doped ZnO Nanoparticles, J. Polym. Environ., 2022, 30, 3566–3581, DOI:10.1007/s10924-022-02455-9.
- A. P. More, R. A. Kute and S. T. Mhaske, Chemical conversion of PET waste using ethanolamine to bis(2-hydroxyethyl) terephthalamide (BHETA) through aminolysis and a novel plasticizer for PVC, Iran. Polym. J., 2013, 23, 59–67, DOI:10.1007/s13726-013-0200-0.
- A. A. A. Otaibi, A. K. D. Alsukaibi, M. A. Rahman, M. Mushtaque and A. Haque, From Waste to Schiff Base: Upcycling of Aminolysed Poly(ethylene terephthalate) Product, Polymers, 2022, 14, 1861, DOI:10.3390/polym14091861.
- J. Liang, J. Fu, H. Lin, J. Chen, S. Peng, Y. Sun, Y. Xu and S. Kang, Valorization of polyethylene terephthalate wastes to terephthalamide via catalyst-free ammonolysis, J. Ind. Eng. Chem., 2024, 132, 578–587, DOI:10.1016/j.jiec.2023.11.053.
- J. Zhou, M. Li, L. Zhong, F. Zhang and G. Zhang, Aminolysis of polyethylene terephthalate fabric by a method involving the gradual concentration of dilute ethylenediamine, Colloids Surf., A, 2017, 513, 146–152, DOI:10.1016/j.colsurfa.2016.11.016.
- K. Chan and A. Zinchenko, Conversion of waste bottles' PET to a hydrogel adsorbent via PET aminolysis, J. Environ. Chem. Eng., 2021, 9, 106129, DOI:10.1016/j.jece.2021.106129.
- Z. Alhulaybi and I. Dubdub, Comprehensive Kinetic Study of PET Pyrolysis Using TGA, Polymers, 2023, 15, 3010, DOI:10.3390/polym15143010.
- F. Nasution, H. Husin, M. Mahidin, F. Abnisa, F. Tirta Yani, L. Maulinda and A. Ahmadi, Conversion of pyrolysis vapors derived from non-biodegradable waste plastics (PET) into valuable fuels using nickel-impregnated HZSM5-70 catalysts, Energy Convers. Manage., 2022, 273, 116440, DOI:10.1016/j.enconman.2022.116440.
- J. Lee, T. Lee, Y. F. Tsang, J.-I. Oh and E. E. Kwon, Enhanced energy recovery from polyethylene terephthalate via pyrolysis in CO2 atmosphere while suppressing acidic chemical species, Energy Convers. Manage., 2017, 148, 456–460, DOI:10.1016/j.enconman.2017.06.026.
- A. Shahi, B. Roozbehani and M. Mirdrikvand, Catalytic pyrolysis of waste polyethylene terephthalate granules using a Lewis-Brønsted acid sites catalyst, Clean Technol. Environ. Policy, 2022, 24, 779–787, DOI:10.1007/s10098-021-02260-3.
- L. S. Diaz-Silvarrey, A. McMahon and A. N. Phan, Benzoic acid recovery via waste poly(ethylene terephthalate) (PET) catalytic pyrolysis using sulphated zirconia catalyst, J. Anal. Appl. Pyrolysis, 2018, 134, 621–631, DOI:10.1016/j.jaap.2018.08.014.
- P. Straka, O. Bičáková and M. Šupová, Slow pyrolysis of waste polyethylene terephthalate yielding paraldehyde, ethylene glycol, benzoic acid and clean fuel, Polym. Degrad. Stab., 2022, 198, 109900, DOI:10.1016/j.polymdegradstab.2022.109900.
- M. Olam and H. Karaca, Effect of sodium boron hydride (NaBH4) on waste polyethylene terephthalate pyrolysis, IOP Conference Series: Earth and Environmental Science, IOP Publishing, 2019, vol. 362, p. 012032, DOI:10.1088/1755-1315/362/1/012032.
| This journal is © The Royal Society of Chemistry 2025 |