Open Access Article
Open Access ArticleAddressing adoption barriers and accelerating market deployment of new technologies†
Greg Avery *a,
Arpit Bhatt
*a,
Arpit Bhatt *a,
Shubhankar Upasania,
Julien Walzberga,
Andre Fernandes Tomon Avelino
*a,
Shubhankar Upasania,
Julien Walzberga,
Andre Fernandes Tomon Avelino a,
Jason DesVeaux
a,
Jason DesVeaux a,
John Minh Quang Pham
a,
John Minh Quang Pham b and
Alberta Carpentera
b and
Alberta Carpentera
aNational Renewable Energy Laboratory, 15013 Denver West Parkway, Golden, CO 80401, USA. E-mail: Greg.Avery@nrel.gov; Arpit.Bhatt@nrel.gov; Shubhankar.Upasani@nrel.gov; Julien.Walzberg@nrel.gov; Andre.FernandesTomonAvelino@nrel.gov; Jason.Desveaux@nrel.gov; quangmp@uci.edu; Alberta.Carpenter@nrel.gov
bChemical and Biomolecular Engineering, University of California, Irvine, 92697, USA. E-mail: quangmp@uci.edu
First published on 25th February 2025
Abstract
Although established technologies are technically sound and have good commercialization records, they are not always sustainable. With companies aiming to develop and deploy more sustainable technologies to the market, there are often overlooked or unidentified social, economic, and environmental risks associated with the adoption of these new technologies. This paper evaluates a new method for assessing potential barriers to market adoption for developing technologies. As a case study, an example technology was selected in the enzymatic recycling of polyethylene terephthalate to produce recycled ethylene glycol and terephthalic acid. This technology was assessed for emissions to air, water, and waste streams; techno-economic viability; local economic impacts; life cycle; potential supply chain risks; and technology adoption rates using Bass diffusion curves. The framework can be used for evaluating the sustainability potential for the fast deployment of all technologies. It can also help decision makers such as investors, regulators, and manufacturers address the barriers associated with technology adoption and deployment to make informed decisions, as well as aid in technology transitions.
Sustainability spotlightIn a circular economy focused on sustainability, new technologies will be needed to reduce emissions, material waste, and energy use. The proposed framework estimates the adoption rate for such new technologies based on life cycle emissions, criteria pollutants and water discharges, environmental regulations, and economics. The example selected to demonstrate the framework is a recycling process that considers enzymatic depolymerization of poly(ethylene) terephthalate (PET), which follows the sustainability principles for materials to be reused to produce the same or new products rather than going into a landfill at their end-of-life. This work aligns with United Nations sustainable development goals 9 (industry, innovation and infrastructure) and 12 (responsible consumption and production). |
1. Introduction
For any new technology or business, there will be a significant number of barriers to entry in the current marketplace.1 These barriers can range from a lack of familiarity with the product or brand, the scale of operation, licenses or policies restricting operation, or high requirements for entry due to infrastructure and costs. Barriers to entry are different for larger firms, which might be seeking to simply enter a new geographic location while being established in others, and smaller companies, which might initially lack resources and recognition. Capital and supply chain buildout are two of the more important barriers,2 whereas other concerns, such as the need for a notable different product or regulation, are not typically seen as significant obstacles. Even if cost is a major factor when entering an established field,3 it is not the only barrier that determines the success of a new product. Environmental regulations have been known to influence economic activity and the overall market,4 even if the effect is not consistent. Recent work from the U.S. Environmental Protection Agency (EPA) found mixed effects from environmental regulations on the economy, depending on which type of model and study was used.5 The study noted that when accounting for individual health and behaviors as well as the negative effects of pollution, the increase in environmental quality has yielded positive impacts on the overall U.S. economy.The question of how new technologies can coexist with sustainability and environmental goals has been addressed before, in part to challenge the notion that meeting new regulations and standards would negatively impact businesses.6 This concept was further explored through the Porter hypothesis, which countered conventional wisdom by stating that environmental regulations can encourage efficiency and competition rather than hinder progress.7 One view is that regulations can impose limitations on business operations especially if expensive control technologies are required to meet the regulations. Previous studies have found agreement with these trends8 through a negative impact on factors such as productivity, employment, and trade, though the impacts last only a few years and are small compared to normal business operations. Dechezleprêtre and Sato8 found that an increase in innovation and efficiency is possible with the goal of meeting new environmental regulations, though the costs of these changes might need further technological advancements before they can break even. New technologies that aim to improve environmental performance for an existing product can yield improvements in multiple areas, from the desired environmental performance to the cost and use of resources.9,10
A technology adoption framework is not a new concept either, though the literature has often focused on the adoption rate by customers rather than manufacturers.11,12 These studies examine the psychology of why a technology may or may not be adopted and focus on behaviors that would either have to change through adaption or that individuals can engage in upon introduction of the new technology. Technology adoption as seen from the user perspective looks at resistance to change, unfamiliarity, and attitudes, which can be examined through surveys, interviews, and addressing mental states.13 Modeling productivity from new technology also introduces barriers that are not user-centric, but relative to the market and the business that would operate a given technology. The barriers can be represented numerically when modeling the productivity change from a new technology,14–16 which shows the effect that barriers to entry have for a new technology but does not always address what contributes to a barrier-representative value or what might be done to address these issues before deployment.
Listing individual barriers related to a new technology can show not only what areas can be improved upon, but allows for categorization of the barriers to address underlying causes.17,18 These include technical and competence barriers, behavioral and psychological barriers, economic and market-based barriers, and barriers specific to a certain region or materials used in the process. In addressing the barriers that make up these categories, a framework can be constructed that applies rankings to each category and demonstrates the relative importance of each category through weighting of the barrier strength. Previous work on vehicle pollution17 and the proposed use of environmentally sound technologies in nine countries across four industrial sub-sectors19 are some examples of this. Acting alongside the barriers to implementing new technology are enablers, which are positive influences that act opposite to barriers and can be applied to advanced manufacturing technologies20,21 or sustainable technologies.22 These often are displayed in the form of a framework that identifies categories of barriers and enablers for a type of technology, which factors are newly identified within the literature23 or are connected in new ways that improve previous understanding of the listed barriers and enablers.20 Design methods for new technologies are also examined within the context of designing a new technology with existing barriers and enablers to technology adoption in mind.22
There is an opportunity to use several specific and measurable barriers to entry for new manufacturing technologies as inputs for estimating the adoption rate of that new technology, which is new in technology adoption literature. Although the attitudes toward and acceptance of a new product can be estimated through surveys and market reports, there is no standardized method for determining the rate of acceptance against competing products. Risks such as environmental regulations and costs can be reasonably estimated before production begins, and they can demonstrate whether a new product has cleared those barriers to entry; therefore, the goal of this work is to develop a general framework to examine several specific barriers to entry and test it through a case study for a new technology. Ultimately, the goal is to help decision makers for a new technology by estimating a rate of acceptance for a new manufacturing process, and identifying which barriers can be addressed to improve market acceptance.
2. Methodology
We consider an approach to aid new technologies for existing products that are at approximately the midpoint of the technology readiness level24 scale in capturing an increased market share. Because a new technology has not been fully tested at the commercial scale, there are several roadblocks that the business needs to overcome across social (e.g., jobs), economic (e.g., production costs), and environmental (e.g., permits and emissions) metrics to compete in the existing market. We examined a wide range of facility-level and product-related barriers that can potentially affect any business creating a new physical product; these barriers would exist for all new manufacturers, whether or not a new or emerging technology is employed. These barriers include:• Air pollutant emissions: all new sources of air pollutants must be evaluated for emissions of criteria and hazardous air pollutants, such as emission restrictions dictated by federal regulations for on-site unit operations or the type of facility for each product.
• Wastewater and solid wastes: wastewater and solid waste streams must be examined for potential barriers via federal and state regulations.
• Production cost: this includes measures such as the rate of return, capital and operating costs, and sensitivity of costs to outside factors.
• Economic impacts: these include the economic impacts as a result of introducing a new manufacturing facility, as measured in dollars of local economic impacts and jobs created.
• Life cycle environmental impacts: these include quantifications of environmental impacts to account for emissions upstream and downstream of the production facility, covering all stages of a product's life cycle while comparing it with conventional processes.
• Supply chain risks: these include assessing the relative risk for future accessibility and the scarcity of raw materials used in the process.
Numerical values are assigned to each of these barriers based on comparing the evaluation of each barrier to relevant markers, such as permitting requirements, emission totals, costs or economic impacts relative to existing methods, and raw material availability. The values representing each barrier are entered into a modeling framework that can estimate the relative adoption rate of a new technology based on the strength of each barrier. There are two models used to evaluate relative technology adoption, each of which depend on the strength of the barriers as well as other aspects of a new technology. The results of the two models along with supply chain risk analysis combine to arrive at a relative adoption level for a selected technology. Depending on the adoption rate (low, medium, high) that manufacturing technology falls under, we provide recommendations to improve the rate based on barriers which are a roadblock to adoption.
As a case study, we consider an enzymatic process to depolymerize polyethylene terephthalate (PET) to recycled terephthalic acid (rTPA) and ethylene glycol (EG)25 (Fig. 1). We consider two plant scales for each barrier analysis, at 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 and 100
000 and 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 metric tons (MT) year−1, unless otherwise noted, based on the current or planned production capacities of operational facilities that use plastic waste as feedstocks.26–28 The motivation behind selecting enzymatic depolymerization technology is to (a) increase the PET recycling rate in the U.S. and (b) enable PET circularity as PET is currently synthesized via polycondensation of ethylene glycol and TPA; this new technology has favorable economic and sustainability impacts.25
000 metric tons (MT) year−1, unless otherwise noted, based on the current or planned production capacities of operational facilities that use plastic waste as feedstocks.26–28 The motivation behind selecting enzymatic depolymerization technology is to (a) increase the PET recycling rate in the U.S. and (b) enable PET circularity as PET is currently synthesized via polycondensation of ethylene glycol and TPA; this new technology has favorable economic and sustainability impacts.25
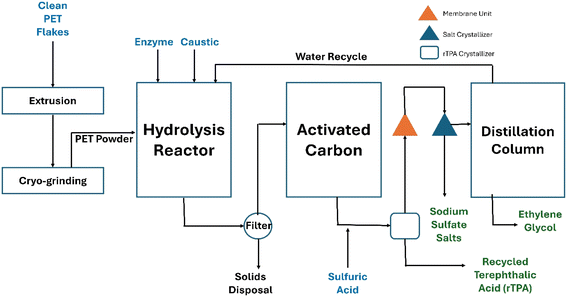 | ||
| Fig. 1 Process flow diagram of enzymatic recycling of PET to rTPA and EG [adapted from Singh et al. (2021).25 | ||
As a brief process overview, rPET flakes are pretreated and go through a series of extrusions and size reduction to yield PET powder (<1 mm diameter). The powder is fed to a series of stirred-tank reactors for depolymerization via enzymatic hydrolysis to produce rTPA and EG. The liquid fraction goes through a separation where solids are extracted from the solution, with the remaining liquid purified to remove impurities. Acidification reduces the solution pH to precipitate rTPA, followed by crystallization and drying to recover rTPA crystals. The remaining liquid is neutralized, preceding membrane separation and distillation to recover EG. The sulfate salts are crystallized and sold as coproducts.
2.1 Air emissions estimation and permitting
New manufacturing facilities or major modifications to existing facilities require an air permit under the EPA's new source review (NSR) permitting program (EPA, 2022).29 We quantify the magnitude of air emissions from the manufacturing technology (defined in terms of potential to emit [PTE]) while considering potential applicability and effects from federal regulations. New sources can fall into one of three categories based on total PTE:30• Prevention of significant deterioration permit: this permit applies to major sources in attainment areas (large geographic areas that meet air quality standards for a certain pollutant).
• Nonattainment new source review (NNSR) permit: this permit applies to major sources in nonattainment areas.
• Minor source permit: this permit applies to sources where no pollutants exceed the major source threshold for potential emissions in a typical operating year.
The steps for air emissions analysis include:
(a) Leverage process design models (e.g., Aspen Plus31) to analyze unit operations/equipment for emission points and likely air pollutants where exact values are not available.
(b) Perform a literature review of emission factors from the compilation of air pollution emission factors (EPA's AP-42) report,32 models (TANKS 4.09D),33 and air permit applications for similar unit operations.
(c) Estimate PTE for all emission sources.
(d) Conduct an in-depth review to determine permitting classification based on applicable regulations under new source performance standards (NSPS) and national emission standards for hazardous air pollutants (NESHAP).
For depolymerization of PET case study, we analyzed all unit operations (Table S1†) and air permits for similar facilities, and used three methods to calculate emissions: material balances, source-specific models, and emission factors. Emission calculations assume maximum capacity under operational design (i.e., continuous operation at design capacity with maximum throughput) and use the worst-case emission factor. Pollutants include criteria air pollutants (CAP), pollutants listed in the EPA's NSPS, and hazardous air pollutants (HAP).‡
Once the PTE for all pollutants has been calculated, any control technologies typically used (baghouses, low-NOx burners, flares, etc.) will also be considered to reduce the total PTE. If a facility exceeds an applicable major source threshold for at least one regulated pollutant under NSR, it is classified as a major source; otherwise, it is a minor source. We compared facility emissions to the corresponding major source thresholds35,36 for attainment areas. For this analysis, only a federal-level air review was completed. State regulations for air emissions may be stricter than federal limits for air toxics and would be considered once a facility location has been determined.§ These could have significant implications to the overall results in case the emission limits are stringent for the state where the facility will be sited.
We examined federal regulations in section 40 of the code of federal regulations (40 CFR) (which covers environmental protection), subchapters 60 of the NSPS and 63 of the NESHAP. We checked applicability for each regulation with a product, emission unit, or related emissions to see which requirements apply. We compared emissions and product limits to PTE emissions in case limits and additional costs would be required to meet compliance.
2.2 Applicable water and waste regulations
We began with a literature review of federal and state standards for waste streams and emissions to water bodies. We reviewed both federal regulations in 40 CFR and state requirements and selected the most restrictive measures for compliance. We examined only state-level regulations for water and waste because many federal regulations defer to states in these areas. This selection is not indicative of a prediction or preference for where a new facility would be constructed. The choice of state dictates which permitting agency would have jurisdiction: we selected South Carolina because the end product is PET, and more than 50% of all U.S. facilities and the total volume for virgin PET (vPET) is in South Carolina.37 Co-locating a site for recycled PET (rPET) with vPET is an option under consideration. 40 CFR has several subchapters that apply to water and waste. These include 40 CFR subchapters D (water programs), N (effluent guidelines and standards), and I (solid wastes). Subchapter I primarily deals with requirements for solid waste handlers with little mention of generators. Subchapter N, subpart 414 applies to organic chemicals, plastics, and synthetic fibers for this process.2.3 Cost and economic impact analysis
We used a process simulation model via Aspen to assess the costs associated with rTPA production.25 We then used material and energy balances for each unit operation from Aspen Plus to determine the equipment size and cost.38 We applied an equipment-dependent scaling exponent and installation factor to estimate the installed capital costs,38,39 as well as direct and indirect cost factors to estimate the total capital investment.38,40,41 All capital cost estimates are adjusted to 2016 U.S. dollars.42 The operating costs are based on the material and energy balance obtained from the Aspen Plus simulation. Variable operating costs—such as the costs of added chemicals, feedstock, and utilities—are based on previous modeling work and literature studies.43 The PET flakes (∼30% colored) were obtained directly from a recycler at $0.66 per kg. The fixed operating costs include the number of employees, along with maintenance, property insurance, and taxes. We adjusted all variable and fixed operating costs43 as well as labor indices44 to 2016 U.S. dollars.We used the total capital investment along with the variable and fixed operating costs in the discounted cash flow rate-of-return analysis to determine the minimum selling price (MSP) of the rTPA (in dollars per kilogram [$ per kg]) at a discount rate of 10%. The key economic assumptions used to perform the techno-economic analysis are summarized in Table S4 in the ESI.† There were several uncertainties associated with the cost of equipment based on potential process improvements and technology advancements. To address this, we performed a single-point sensitivity analysis to capture the effects of key process and non-process parameters on the MSP. In addition to estimating MSP, we evaluated the return on investment (ROI), estimated by calculating the difference between the gain (or return) and the cost of investment and dividing the number by the cost of investment. Also, we determined the internal rate of return (IRR) (used to estimate process profitability) at a percentage value when the net present value is zero for each MSP.
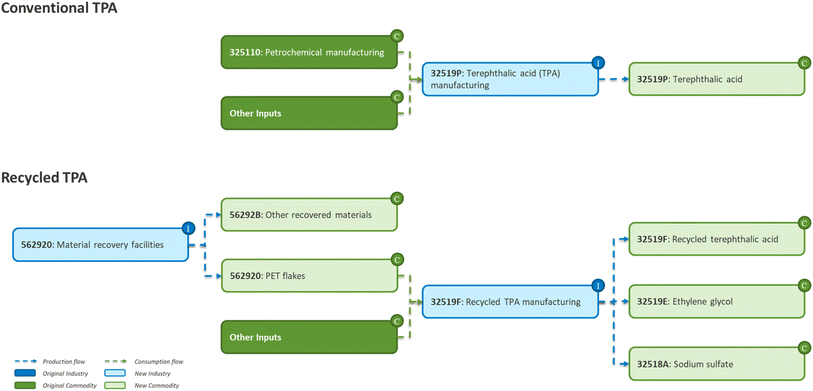
We estimated the local economic and workforce impacts of virgin terephthalic acid (vTPA) and the proposed rTPA production using input–output analysis. More specifically, we extended the EMPLOY¶ model, described in Avelino et al.,45 to include the new industries referenced in Singh et al.46 (Fig. 2). This model represents the 2012 U.S. economy using the latest available benchmark input–output table from the U.S. Bureau of Economic Analysis (2018), with total production and environmental accounts for 2017. In the EMPLOY model (originally BEIOM), vTPA production is aggregated in the more general sector other basic organic chemical manufacturing (NAICS 325190). New technologies need to be represented as new subsectors to determine the impacts from that new technology. Here, vTPA production is disaggregated from the larger sector to have a specific representation of its production technology in the model. In the case of the proposed rTPA industry, we included both the biorefinery and the material recycling facilities (MRF) as new sectors representing the pathway shown in Singh et al.|| New sectors as modeled in EMPLOY end with a letter and are added so that the model has a distinct process to reference, where the number code of the new process reflects the larger sector, and the letter indicates a new addition. In Fig. 2, new processes starting with 32![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 519 are part of the corresponding NAICS code for 32
519 are part of the corresponding NAICS code for 32![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 519, basic organic chemicals. We modeled MRFs as a single-stream plant following specifications from GBB, Inc.47 We considered a waste stream containing 3.3% of PET and a 98% recovery rate (capital intensive assumption). We obtained energy consumption from electricity and diesel fuel and employment per plant from Pressley et al.48 The recycled TPA industry uses PET recovered from MRFs as feedstock, assuming transportation costs of $8 per ton.49
519, basic organic chemicals. We modeled MRFs as a single-stream plant following specifications from GBB, Inc.47 We considered a waste stream containing 3.3% of PET and a 98% recovery rate (capital intensive assumption). We obtained energy consumption from electricity and diesel fuel and employment per plant from Pressley et al.48 The recycled TPA industry uses PET recovered from MRFs as feedstock, assuming transportation costs of $8 per ton.49
2.4 Environmental impacts
We conducted a life cycle assessment (LCA) of the rPET and vPET processes to understand the potential environmental impacts associated with the proposed process.50 Fig. 3 shows the system boundary of vPET and rPET and their associated raw materials as well as the coproducts of enzymatic depolymerization from rPET. This study primarily focuses on vPET and rPET as products and does not examine the results for monomer or coproduct life cycles.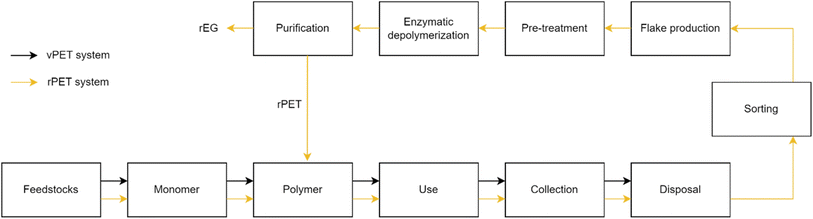 | ||
| Fig. 3 Depiction of the life cycle of raw materials virgin terephthalic acid (vTPA) and virgin ethylene glycol (vEG), products virgin polyethylene terephthalate (vPET) and enzymatically recycled polyethylene terephthalate (rPET), and co-products recycled terephthalic acid (rTPA) and recycled ethylene glycol (EG) involved in the production of vPET and enzymatically rPET (adapted from ref. 51). | ||
The rPET life cycle begins with the recovery of feedstock monomers for polymerization. Used vPET bottles from consumers (assumed to be manufactured using stretch blow molding) are collected and routed toward waste management options. The default waste management strategy assumed for vPET involves 80% of the mass being landfilled and 20% incinerated within a cradle-to-grave life cycle. For this rPET process, post-consumer vPET is routed to sorting (instead of disposal and incineration), where it is sorted into bales with an estimated 10% material loss. The enzymatic recycling reactor operates at pH 8 and 60 °C with a solids loading of 15% and an enzyme loading of 5 mg g−1 PET. The process achieves 90% depolymerization to rTPA and EG. Ninety percent of the rTPA is captured using acidic precipitation, crystallization, and drying, whereas 50% of EG is recovered through membrane concentration and distillation (with sodium sulfate as a side product). Sodium sulfate and EG are considered co-products and represent avoided primary production via negative credits. The functional unit for the LCA is 1 kg of vPET or rPET.
We used Ecoinvent version 3.3 as the data source for background processes, and modeled the system in SimaPro. Process inventory data are U.S.-specific when available and used the results from the Aspen model as data inputs.25 We used the tool for reduction and assessment of chemicals and other environmental impacts52 to quantify the system environmental impacts. Additional details are listed in the results.
2.5 Technology adoption rate
The rate of users adopting this technology is modeled using Bass diffusion curves, which have recently been used for estimating technology adoption rates in an existing market.53 This method uses variables of ‘p’ and ‘q’ from the original Bass diffusion model, which represent a coefficient of innovation (characterizing a change in adoption due to external effects) and a coefficient of imitation (characterizing a change in adoption due to internal effects), respectively. Hanes et al. (2019) developed a method to link different technology attributes to the values of ‘p’ and ‘q’ and show how technologies with different attributes would have different adoption rates; thus, we use this method and several characteristics of the rTPA process, including several results from this work, to estimate the technology adoption curves for rTPA production. We also investigate which characteristics (or attributes) of the rTPA process would have the most significant impacts on the rate of adoption. The ESI† provides additional methodological details.2.6 Supply chain risk assessment
To understand the risk factors involved with all stages of the PET recycling supply chain, first, we mapped the key raw materials, their major suppliers, and the intermediate products needed for the enzymatic depolymerization of PET for all value chain stages, as shown in Fig. S7.† Then, we compiled information on the geographic locations, annual production, annual consumption, price, and flow rates of the major suppliers of key raw materials (Table S12†). Finally, we evaluated and scored the supply chain risks associated with the raw materials in the PET recycling supply chain against a set of six criteria as defined by the U.S. Department of Energy Commercial Adoption Readiness Assessment Tool (CARAT),54 released in March 2023. Additional details on CARAT are provided in Section 3.7. The criteria span three of the four core risk areas of CARAT:• Market acceptance: market size and downstream value chain.
• Resource maturity: capital flow, manufacturing and supply chain, and materials sourcing.
• License to operate: policy environment.
We chose the six criteria based on their relevance to the supply chain of this and other novel technologies. We assigned each material a score from 0 to 5 for each of the six supply chain risk criteria, ranging from low or unknown risk to very high risk (ESI Section S7†). The findings of the supply chain risk assessment are listed in the Results and discussion section as well as the ESI.†
3. Results and discussion
3.1 Results used as modeling inputs
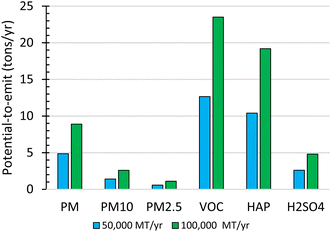
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000- and 100 000 MT year−1 plant scales (Fig. 4). As a result, either facility would be classified as a minor source, assuming they are located in an attainment area and are stand-alone facilities.
000- and 100 000 MT year−1 plant scales (Fig. 4). As a result, either facility would be classified as a minor source, assuming they are located in an attainment area and are stand-alone facilities.
We also evaluate an alternative option where we assume that the PET recycling facility would be co-located with a vPET manufacturing facility to take advantage of existing supply chains and reduce the costs associated with feedstock transportation. Note that the emissions associated with the new PET recycling facility are considered “increases in emissions” because the facility is co-located with a vPET facility (both facilities are considered as one), which requires a modification permit for the existing vPET facility. These increases in emissions are compared to EPA thresholds, which are lower than the thresholds for new facilities. Refer to Table S3† for modification thresholds.55
The type of air permit required for a PET recycling facility co-located with a vPET manufacturing facility depends on several criteria and is determined on a case-by-case basis:
• vPET located in an attainment area: adding the PET recycling facility is not expected to trigger major permitting requirements (prevention of significant deterioration) (whether the plant is a major source or not).
• vPET located in a nonattainment area (NAA) other than an extreme ozone NAA and is a not a major source: adding the PET recycling facility is not expected to trigger major (NNSR) permitting requirements.
• vPET located in an NAA other than an extreme ozone NAA and is a major source: adding the PET recycling facility is not expected to trigger major (NNSR) permitting requirements.
• vPET located in an extreme ozone NAA and is a major source: adding the PET recycling facility is expected to trigger major (NNSR) permitting requirements.
In addition, we consider federal regulations that could limit the emissions of certain pollutants from the facility. Federal regulations in 40 CFR 60 and 63 (NSPS and NESHAP, respectively) that typically apply to a facility depend on one or more of the following: (a) a specific emissions source on the facility property, (b) a specific emissions that must be controlled, or (c) the feedstock or product from the facility. For this process, there are two main federal regulations that apply based on the production of PET (from recycled or virgin feedstock):
• NSPS subpart DDD = volatile organic compound (VOC) emissions from the polymer manufacturing industry.
• NESHAP subpart JJJ = group IV polymers and resins.
NSPS subpart DDD sets limits on VOC emissions from polymer production, with different standards (listed in 40 CFR 60.562-1) for specific polymers. In 40 CFR 60.562-1(c)(2), VOC standards for PET production are listed using a terephthalic acid production process, with limits of 0.04 kg VOC per mg product in the raw material section and 0.02 kg VOC per mg product in the polymerization reaction section. There are currently zero VOC emissions from the raw material production in this process, and VOC emissions related to the clarification and crystallization sections (Fig. S1†) are assumed to be emissions from the wastewater streams that do not remain entrained in the liquid discharge, based on engineering judgement. NSPS subpart DDD has no VOC emission limits from wastewater, only raw material preparation, polymerization reaction, material recovery, product finishing, and product storage sections depending on the polymer and production method.
NESHAP subpart JJJ sets limits on the total HAP emitted from a process that produces any of several polymers, including PET. This states that there can be no continuous batch or process vents that would act as a primary source of HAP and that any applicable facilities would also need to meet the requirements of 40 CFR subpart DDD. The limits on HAP emissions are similar to those for VOC emissions, with maximum emissions of 0.04 kg Mg−1 product from the raw material preparation section and 0.02 kg Mg−1 product for the polymerization reaction section. Emissions of HAP from these sections of the process are well below the limits and require no control technologies.
Other federal regulations that would apply to the process cover the emergency backup combustion units (NSPS subpart IIII or JJJJ, and NESHAP subpart ZZZZ), cooling tower (NESHAP subpart Q), and equipment leaks (NSPS subpart VVa, NSPS subpart DD, and NESHAP subpart H, section S5). The limits required under these other federal regulations would not reduce the overall emissions from these equipment because the emissions are already below the limits in place in each regulation. In summary, the enzymatic depolymerization process would only need a minor source permit based on the potential emissions from the plant and would only be subject to non-stringent federal regulatory limits.
| Potential waste chemical | CAS # |
|---|---|
| a n/a = not available. | |
| Ethylene glycol | 107-21-1 |
| Sulfuric acid | 7664-93-9 |
| Sodium hydroxide | 1310-73-2 |
| PET particles | n/a |
| Enzyme | n/a |
| Sodium sulfate | 7757-82-6 |
| Terephthalic acid | 100-21-0 |
3.1.2.1 Federal water regulations. 40 CFR subchapter D, part 131 water quality standards58 does not list any actual standards that apply to new or existing dischargers. Instead, this section describes procedures for reviewing and approving state-level standards. Reporting requirements for all dischargers are listed in Appendix A of subpart D; this process qualifies because two categories in Appendix A are (a) plastics processing and (b) plastic and synthetic materials manufacturing. A new facility listed as a discharger shall:
“Include effluent limitations and a compliance schedule to meet the requirements of section 301(b)(2)(A), (C), (D), (E) and (F) of CWA, whether applicable effluent limitations guidelines have been promulgated.”
The applicable regulations that must be followed are in subchapter D, part 122.44 and part 122.46.59. Each regulation has more specific limitations in the subchapters for a specific process type, such as plastics processing and manufacturing. These regulations also state that all state-level standards and requirements would also apply. In summary, the discharge of wastewater from the process would likely defer to state limitations that typically have more stringent numerical limitations.
There are no NSPS regulations related to wastewater from polymer processes,** whereas NESHAP subpart G states the standards that must be followed for wastewater streams for chemical processes (including polymers). NESHAP subpart G has two classes of wastewater streams: group 1 and group 2. group 1 wastewater streams emit 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 ppm or greater of any compound from Table 8 or 9 of NESHAP subpart G, or 1000 ppm at a flow rate of greater than 10 L min−1. This process emits no compounds found in either table and it is categorized as group 2. The primary group 2 wastewater requirements are recordkeeping and reporting, with no control technologies or limits in place (true for group 1 wastewater streams). Because there are no regulations that limit operations or require large costs to ensure compliance with emissions limits, this potential barrier is not significant for the case study under consideration. Additional applicability details are included in ESI Section S5.†
000 ppm or greater of any compound from Table 8 or 9 of NESHAP subpart G, or 1000 ppm at a flow rate of greater than 10 L min−1. This process emits no compounds found in either table and it is categorized as group 2. The primary group 2 wastewater requirements are recordkeeping and reporting, with no control technologies or limits in place (true for group 1 wastewater streams). Because there are no regulations that limit operations or require large costs to ensure compliance with emissions limits, this potential barrier is not significant for the case study under consideration. Additional applicability details are included in ESI Section S5.†
3.1.2.2 State water regulations. In addition to meeting federal regulatory guidelines for water discharge, state regulations will also apply. South Carolina regulation 61-9: water control permits60 lists the necessary reporting for all facilities.†† South Carolina regulation 61-9 also includes standards that all wastewater dischargers must conform to, as opposed to standards for specific industries or equipment. Standards applicable to all dischargers include those shown in Table S7.† Wastewater must comply with limits on total suspended solids, five-day biological oxygen demand (BOD5), carbonaceous BOD5 (CBOD5), and outgoing pH level.
There are different limits on wastewater standards, depending on the averaging period, with higher limits for shorter time periods. There is also a required level of removal for each pollutant, depending on the level of treatment applied to the output stream (details in ESI†). All dischargers must follow the requirements of South Carolina regulation 61-68: water classification standards,61 depending on the type of water body discharged to. The requirements from the regulation only apply if the water body in question falls under one of several specific categories of protected waters; otherwise, the requirements do not apply. These requirements, as well as the applicable categories of water body, are shown in Table S6.† None of the potential water emissions violate state or federal standards based on this analysis; meeting all regulations related to water-based emissions will require applicable control technologies to be included during construction and following applicable discharge procedures. In summary, the process does not discharge any chemicals of concern in wastewater but would still need to meet total soluble solids and BOD5 standards, depending on state requirements.
3.1.2.3 Waste regulations. Federal waste regulations can be found in 40 CFR subchapter I, parts 261 (ref. 62) and 262 (ref. 63). These regulations deal with the identification and listing of hazardous wastes as well as standards applicable to hazardous waste generators. There are state-level equivalents in South Carolina regulation 61-79.261 and regulation 61-79.262,64 which primarily follow federal guidelines. The focus in subchapter I is on the handling, processing, storage, transportation, and eventual disposal of hazardous or solid waste. Because the PET recycling process would not be responsible for these stages of the waste process, only generation, the applicable regulations depend on which type of hazardous waste generator the facility would be classified as. Materials would also qualify as hazardous if they met certain criteria (such as reactivity or explosivity), despite not being listed in the appendix of part 261. None of the materials in Table S8† meet the criteria listed in part 261, subpart C, which would qualify the materials as hazardous. Because none of the materials in this process qualify as hazardous, there are no standards to meet.
3.1.3.1 Techno-economic analysis. We estimate the MSP of rTPA and EG from the enzymatic recycling of the PET process for two plant scales: 50
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 MT year−1 and 100
000 MT year−1 and 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 MT year−1. The MSP for the 50
000 MT year−1. The MSP for the 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 MT year−1 is based on the analysis performed by Singh et al., whereas an additional analysis is performed for an additional plant scale of 100
000 MT year−1 is based on the analysis performed by Singh et al., whereas an additional analysis is performed for an additional plant scale of 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 MT year−1. The MSP for rTPA is estimated at $1.93 per kg and $1.85 per kg (compared to ∼$1.0 per kg market price), considering sodium sulfate salts and EG sold as coproducts (plant scale of 50
000 MT year−1. The MSP for rTPA is estimated at $1.93 per kg and $1.85 per kg (compared to ∼$1.0 per kg market price), considering sodium sulfate salts and EG sold as coproducts (plant scale of 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 and 100
000 and 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 MT year−1, respectively). We assume that sodium sulfate salts can be sold at $0.15 per kg, whereas EG is sold at $0.96 per kg. Cost estimates assume a 95% recyclable PET fraction with an onstream plant factor of 90%. Without coproducts, the MSP of rTPA would increase to $2.23 per kg and $2.15 per kg for 50
000 MT year−1, respectively). We assume that sodium sulfate salts can be sold at $0.15 per kg, whereas EG is sold at $0.96 per kg. Cost estimates assume a 95% recyclable PET fraction with an onstream plant factor of 90%. Without coproducts, the MSP of rTPA would increase to $2.23 per kg and $2.15 per kg for 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000- and 100
000- and 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 MT year−1 facility scales, respectively. Considering the mass in and mass out for the feedstocks and products, the overall product yield is 0.66 tonnes rTPA per tonne PET. The plant economics for capital and operating (both variable and fixed) costs are shown in Table 2. The increase in capital costs for the 100
000 MT year−1 facility scales, respectively. Considering the mass in and mass out for the feedstocks and products, the overall product yield is 0.66 tonnes rTPA per tonne PET. The plant economics for capital and operating (both variable and fixed) costs are shown in Table 2. The increase in capital costs for the 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 MT year−1 facility is mainly attributed to larger unit operations compared to 50
000 MT year−1 facility is mainly attributed to larger unit operations compared to 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 MT year−1. For operating costs, the maintenance and overhead costs are tied to a fixed percentage of the capital costs, increasing the overall costs but with a low estimated MSP for the product.
000 MT year−1. For operating costs, the maintenance and overhead costs are tied to a fixed percentage of the capital costs, increasing the overall costs but with a low estimated MSP for the product.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 MT year−1 and 100
000 MT year−1 and 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 MT year−1 PET recycling facilities
000 MT year−1 PET recycling facilities
| Parameter | 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 MT year−1 000 MT year−1 |
100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 MT year−1 000 MT year−1 |
|---|---|---|
| Total capital cost | $124 million | $211 million |
| Total variable operating cost | $40 million | $72 million |
| Total fixed operating cost | $4 million | $6 million |
Fig. 5 shows the fraction of individual cost components and the distribution of costs across unit operations that comprise the total MSP value.
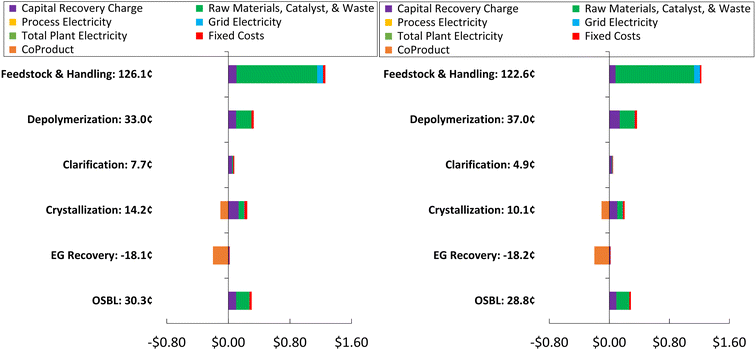 | ||
| Fig. 5 Process contributors to the overall MSP of the PET enzymatic recycling processing at 50 kt year−1 (left) and 100 kt year−1 (right) of PET, respectively. | ||
Feedstock and handling is the largest contributor to MSP, accounting for 65% and 66% of the overall production costs for 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 and 100
000 and 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 MT year−1, respectively. This is followed by depolymerization and outside battery limit (OSBL) costs. OSBL consists of the additional capital expenditures, such as piping and instrumentation, required to integrate the utilities into the plant. Overall, the raw material, catalyst, and waste comprise most of the feedstock and handling area costs. A sensitivity analysis is performed to show the key performance variables affecting the MSP of rTPA (Fig. S2†).
000 MT year−1, respectively. This is followed by depolymerization and outside battery limit (OSBL) costs. OSBL consists of the additional capital expenditures, such as piping and instrumentation, required to integrate the utilities into the plant. Overall, the raw material, catalyst, and waste comprise most of the feedstock and handling area costs. A sensitivity analysis is performed to show the key performance variables affecting the MSP of rTPA (Fig. S2†).
In addition to the cost and drivers impacting the production cost, we estimate the ROI, IRR, and payback period for the PET facility processing at 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 MT year−1. Table 3 shows the ROI, IRR, and payback period for the base case as well as other MSP costs.
000 MT year−1. Table 3 shows the ROI, IRR, and payback period for the base case as well as other MSP costs.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 and 100
000 and 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 MT year−1a
000 MT year−1a
| Selling price | @$1.93 per kg (base)b | @$2.43 per kg (+$0.5 per kg)c | @$2.93 per kg (+$1.0 per kg)d |
|---|---|---|---|
| a n/a = not applicable.b Base case price.c +$0.5 per kg based on selling price of co-products [EG and sulfate salts].d +$1.0 per kg based on selling price of co-products [EG and sulfate salts]. | |||
50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 MT year−1 000 MT year−1 |
|||
| ROI | n/a | 3.3% per year | 6.5% per year |
| IRR | 10% | 24% | 36% |
| Payback period | 15.8 years | 6.9 years | 4.1 years |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||
100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 MT year−1 000 MT year−1 |
|||
| ROI | n/a | 3.5% per year | 7.0% per year |
| IRR | 10% | 25% | 38% |
| Payback period | 15.8 years | 6.5 years | 3.9 years |
The production cost of rTPA favors economies of scale, with feedstock handling being the largest contributor to the overall cost. While for the base case, the payback period is estimated for a fixed IRR, the other two cases where rTPA is sold at high price, the IRR and payback period is calculated when the net present value is 0. If the co-products (EG and sulfate salts) are sold at higher selling price, depending on the market demand, the ROI could be increased to up to 6.5% per year, and the payback period could be reduced to 4.1 years. Moreover, there are no major impacts from federally regulated limits to the production cost of rTPA.
3.1.3.2 Jobs and local economic impact. Results from the EMPLOY model show employment numbers reported as either full-time equivalents (FTE), full-time and part-time employees, or all persons engaged in production. Operational results rely only on direct (TPA-related) and indirect (upstream and downstream supply chain of TPA) effects, whereas construction effects are not included because they were not available in the model for vPET.
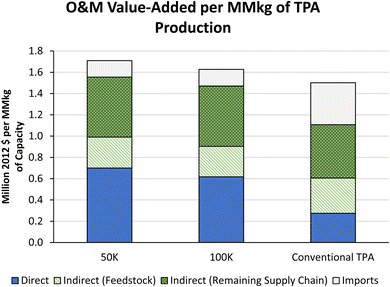
Fig. 6 shows the value added to local communities for rTPA production plants at the 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 MT year−1 and 100
000 MT year−1 and 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 MT year−1 sizes as well as a comparison to existing TPA production for operational value added. Both proposed plant sizes add more value (per MM kg capacity) than the current methods due to the direct and supply chain values. More details on these values are provided in Section S3 of the ESI.† Fig. 7 shows the jobs added (as FTE) per MM kg of TPA from the proposed and current methods, with additional details on the range of possible outcomes and other job count metrics in the ESI.†
000 MT year−1 sizes as well as a comparison to existing TPA production for operational value added. Both proposed plant sizes add more value (per MM kg capacity) than the current methods due to the direct and supply chain values. More details on these values are provided in Section S3 of the ESI.† Fig. 7 shows the jobs added (as FTE) per MM kg of TPA from the proposed and current methods, with additional details on the range of possible outcomes and other job count metrics in the ESI.†
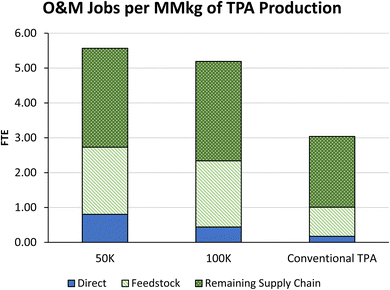 | ||
Fig. 7 O&M FTE jobs added from the 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000- and 100 000- and 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 MT year proposed rTPA plants compared to conventional methods. 000 MT year proposed rTPA plants compared to conventional methods. | ||
In summary, the jobs and economic impacts from the new recycling technology are greater than those from the current production processes when compared on a per-MM kg basis. Most operations and maintenance (O&M) jobs for rTPA production are linked to feedstock and supply chain.
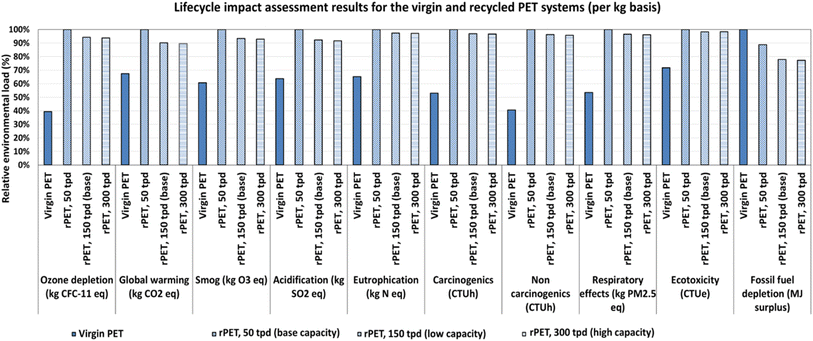
Fig. S5(a) and (b)† depict the hotspot analysis of the vPET and rPET systems, which aim to help understand the contribution of the major processes in the life cycles toward the evaluated environmental impacts. For the vPET system, the production of vPET resin and the stretch blow molding process account for most impacts in all categories. Additionally, waste PET diverted to landfills at the end of life is responsible for approximately 40% of eutrophication and 48% of the ecotoxicity impacts. In the case of the rPET systems, the enzymatic hydrolysis of the vPET powder into rTPA and rEG is the most significant process contributor across all impact categories, with the 50 tpd system having the highest relative contribution and the 300 tpd system having a lower relative contribution from the enzymatic hydrolysis compared to the 150 tpd base case. The washing and shredding of the collected PET into flakes and the stretch blow molding of rPET into bottles are additional significant contributors to the environmental impacts from the rPET process.
In summary, the PET recycling process has potentially more negative environmental impacts than the vPET process across most impact categories except fossil fuel depletion, with relative improvements when the scale of the plant is increased. Note that the scope of this study was to demonstrate the application of LCA to compare the environmental performance of both systems in their first lifetimes; however, rPET can be theoretically recycled for more than one useful life. In its subsequent life, the impacts from the preprocessing steps will be negligible except for impacts arising from any post-consumer vPET that will be needed to make up for efficiency losses; hence, a further reduction in net environmental impacts per kilogram of rPET could be possible. These impacts are also based on the scale-up of a smaller-scale process compared to an established industrial process; improvements are possible as the technology is used but not guaranteed.
3.2 New technology adoption modeling results
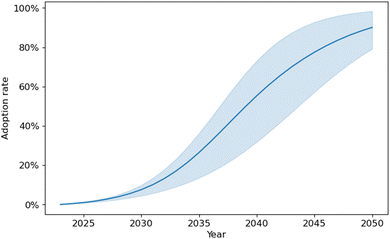
Fig. 9 represents the base case for each technology attribute, and Fig. S6† shows the effects of a sensitivity analysis that varies the available crosscutting options for TPA use, effect of economic incentives, estimated plastic lifetime, and other factors. The technology adoption rate represents adoption within a specific market—in this example, the rTPA adoption rate for the selected technology is in the specific market of rTPA, not all TPA on the market.
Compared to other technologies, the adoption rate presented in Fig. 9 is slightly below average. The adoption rate would be even smaller for the penetration of this technology in the main TPA market. In summary, the adoption of a technology depends on several factors, including the geography, market, and social and ecological context. In the case of rTPA production, product use in multiple applications, policy incentives, and payback period could increase adoption, whereas the social acceptance and permit requirements depend on where the plant is sited and could reduce its deployment rate.
Based on the results of the rankings for this technology (six categories ranked as low risk, nine ranked medium, and two ranked high), we find the adoption readiness-level score for this PET recycling process to be a 1 out of 9, which is labeled as low readiness. A high number of medium-risk rankings is what categorizes this technology as low-readiness; see the final page of the CARAT evaluation for this technology for a guide to readiness. This indicates that there are still commercialization challenges in multiple risk assessment areas that need to be addressed before implementing a quick and widespread deployment of this technology. The full results of the CARAT for the PET recycling technology can be found in Section S8 of the ESI.†
3.3 Supply chain risk assessment
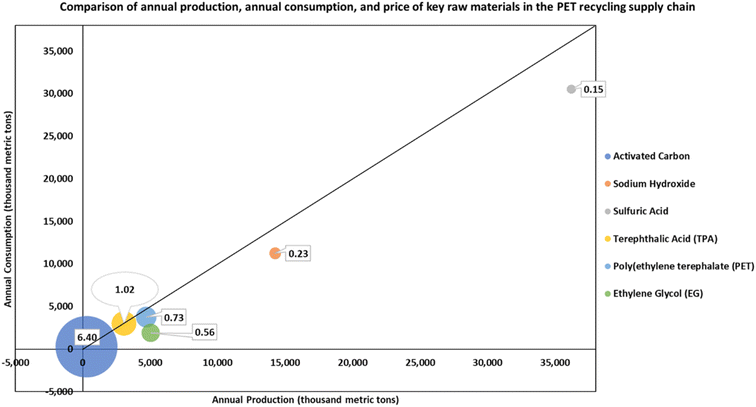
In performing the supply chain risk assessment, we modeled the adoption rate of the technology and aimed to quantify and assess the potential risks and barriers within the context of the technology's supply chain. This approach enables a holistic view of the multiple risks that lie outside of the direct geographic and operational scope of the technology, and it supports increased supply chain resilience. The bubble chart in Fig. 10 shows the comparison of the price, calculated scarcity, and flow rate (denoted by the size of the bubble) of six key raw materials. Most raw materials have a scarcity value slightly greater than 1.0 or more, indicating that current annual national production levels match or exceed the consumption, and a price less than $1.5 per kg of material. Activated carbon has a scarcity value of 0.94 and a high price of $6.4 per kg, indicating a potential source of supply chain vulnerability for technologies such as the PET depolymerization process. Even though EG is relatively abundant (scarcity value 2.66), it also has the highest flow rate (8199 kg h−1), meaning that any supply chain bottleneck that affects the continuous availability of EG can potentially disrupt normal operations.We further performed a supply chain risk assessment against six criteria that fall under three risk areas of the CARAT. The core risk areas and associated criteria are defined in Section S7 of the ESI.† For each risk criterion, we calculated an average risk score across all raw materials and components involved. A comparison of the risk scores of the six criteria indicates the relative risk embedded in the domain governed by each criterion. To demonstrate the application of the supply chain risk assessment framework, we evaluated the supply chain profile of each key material in the rPET supply chain against the selected criteria. Detailed scoring values for each material are shown in Fig. 11.
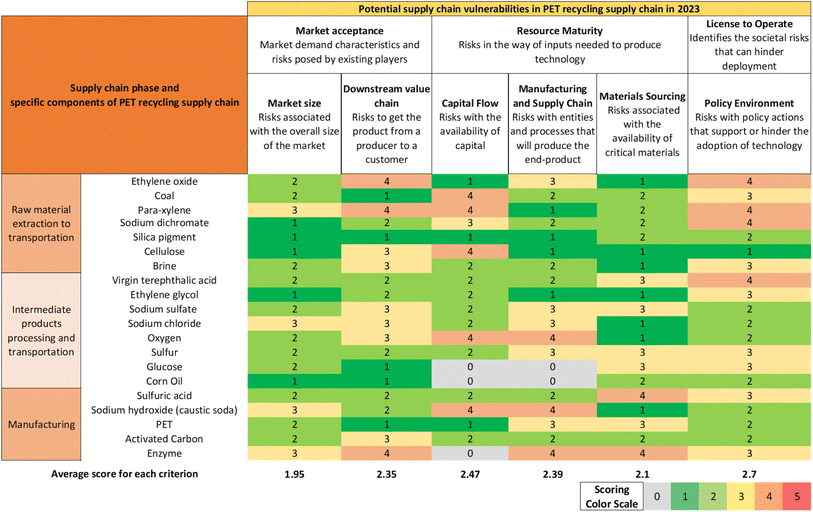 | ||
| Fig. 11 Sources of potential vulnerabilities and a semiquantitative risk assessment of key raw materials in the PET recycling supply chain. The assessment was performed by assigning a numerical score (0 to 5) and a color scale (gray to red) to each of the six risk criteria based on supply chain profiles of the listed raw materials in literature.66–80 | ||
Following is a summary of the results from the assessment of each criterion:
(a) Market size: we used factors such as existing market applications, competitors, and compound annual growth rate (CAGR) to assign risk scores in this category. Based on the supply chain profiles of the key materials, we found that the majority of the materials have a CAGR greater than the average CAGR of the chemical manufacturing industry (3.5%)81 and have a wide variety of applications, which suggests adequate market supply and continued growth.
(b) Downstream value chain: we considered factors such as transportation risks, competitiveness of technologies, and selling price stability while assigning risk scores in this category. Primary vulnerabilities are due to transportation-related dependencies for materials imported from other countries.
(c) Capital flow: no current shutdowns are observed for most of the production of the selected chemicals; however, the production of chemicals such as para-xylene was cut by Chevron in 2018, and coal and silica production also have some planned shutdowns.
(d) Manufacturing and supply chain: this factor has a higher risk score due to disruptions such as natural disasters, a pandemic, or economic downturn. Most chemicals have multiple production pathways, although limited common production routes can expose the production capacity to vulnerabilities if a technology fails to meet future regulations or requirements.
(e) Materials sourcing: the chemicals for this technology are not on the critical materials list. The high-risk scores are due to the chemical's dependency on the countries importing the raw materials. Due to the long transport distance from the key producers to the customers, risks increase as the number of transportation stages and distance increase. Moreover, some materials, such as silica and sulfuric acid, are facing shortages because of high demand or climate change affecting the locations for the common procurement of raw materials.
(f) Policy environment: most chemicals and their production processes are well established and do not require significant policy intervention for the technology to be adopted at scale. A possible exception to this could arise if some environmentally harmful chemicals involved in the PET recycling supply chain were affected by additional regulatory policies that could disrupt existing production pathways, in which case policy intervention could be needed for deploying alternative technologies to produce certain upstream materials.
In the context of the rPET supply chain, policy environment and capital flow criteria have the two highest average risk scores, whereas the market size and materials sourcing criteria have two of the lowest average risk scores, as shown in Fig. 11. This comparison brings to attention the relative risks and barriers that could impact the technology's adoption and could inform additional measures that need to be taken to ensure risk mitigation and subsequent technology adoption. Such a comparison has an important caveat—all six supply chain risk criteria are assumed to have equal importance or weight for the supply chain framework in general and for the selected rPET case study. Depending on the technology being assessed, this might or might not be applicable, and a careful evaluation of the relative importance of the supply chain risk criteria could be performed as an additional step in evaluating the overall supply chain risks that exist for a given technology.
3.4 Limitations
There are limits to fully evaluating which barriers would be significant for each technology before full commercialization or when a process is close to the first of its kind. This section examines limitations from each previous barrier applied to the technology for producing rTPA as well as possible limitations for the overall process that did not fit into an individual section.For instance, the adoption rate model we used shows only the results for a new technology as a standalone consideration, so the effects of competing technologies are not shown. Although the Bass model was initially developed to study the adoption of a product category, more complex iterations of the model could be used to consider competition.82 Other identified gaps include:
• State-specific regulatory requirements: we did not examine state air regulations. These regulations can require the use of control technologies to limit emissions based on local regulations or limit how units are operated. State air limits can be more stringent regarding the emissions of HAP and CAP, depending on the state, and new technologies might encounter resistance if they contain certain compounds.
• Water and waste regulations: we only compared water and waste emissions to state guidelines due to a lack of specific guidelines at the federal level. An in-depth water and waste analysis was not required for this process. The wastewater streams contained no hazardous compounds, and the solid waste stream qualified as a very small emitter. Additional analysis would be required for technologies with hazardous waste streams, including costs for the control or elimination of certain compounds.
• Production cost: the financial assumptions for estimating the production cost of rTPA are based on nth plant assumptions where several commercial-scale plants already exist, and there is no consideration of barriers associated with greenfield plant construction. Although the costs associated with an nth plant would be different from a pioneer plant, the technological advancements would help to overcome the barriers for faster adoption. Moreover, the emission controls required to comply with state and local regulations might increase the production costs, which are currently not considered.
• Economic impacts: we estimated the potential jobs and value added from rTPA production based on results from a single model. Although each of those results is part of the designed purpose for that model, there is no corroborating information to confirm each set of results. Also, because EMPLOY requires inputs related to the economics of the process, more specific (or state-level data) would likely affect the model results. The results for both jobs and value added are only annual from O&M rather than including temporary construction jobs and value added as well. Adoption rate modeling depends on the relative impact of a new production method compared to the current method, so if construction impacts from vPET are higher than rPET, then one of the model inputs would be different from the current values.
4. Conclusions
This study provides a framework to evaluate multiple barriers associated with the adoption of a technology and to estimate the level of market adoption based on those barriers. Although the barriers to adoption can vary based on the type of technology, the methods outlined in this research can be used and modified on a case-by-case basis. Here, we consider an enzymatic recycling of PET process as a case study for demonstrating the technology adoption framework. We provide results for several categories related to emissions, economics, and permitting that could act as barriers to entry, and we show how these factors could influence the adoption rate for a new technology. How results for other technologies scale within the categories of emissions, economics, or permitting affects how the adoption rate modeling will develop, which can subsequently predict a much lower or higher usage compared to similar technologies. Overall, barriers to enzymatic PET recycling seem to moderately hinder its adoption (with a possible near full adoption by 2050). Although the environmental and economic performance of the process need to be improved compared to vPET production, other new technologies with more stringent permitting requirements, less favorable economics, or that contain toxic chemicals could have slower adoption rates if these obstacles cannot be overcome.For the PET recycling case study, we found that air emissions for a facility processing 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 or 100
000 or 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 MT year−1 of PET would not be subject to major source permitting unless it is located in an extreme ozone NAA (for 100
000 MT year−1 of PET would not be subject to major source permitting unless it is located in an extreme ozone NAA (for 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 MT year−1) as a stand-alone facility. Also, there is no solid waste from enzymatic PET recycling that would be subject to hazardous waste regulations or contain chemicals with specific limits in wastewater streams. For the techno-economic analysis, we find that the cost of production of rTPA would be lower for a 100
000 MT year−1) as a stand-alone facility. Also, there is no solid waste from enzymatic PET recycling that would be subject to hazardous waste regulations or contain chemicals with specific limits in wastewater streams. For the techno-economic analysis, we find that the cost of production of rTPA would be lower for a 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 MT year−1 facility than for a 50
000 MT year−1 facility than for a 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 MT year−1 facility, following economies of scale, and it is comparable to the current market selling price (∼$1.0 per kg). The sensitivity analysis shows that feedstock price, solids loading, depolymerization extent, and rTPA recovery are key variables affecting production costs. In addition, both facility sizes of rPET have greater economic impacts and jobs created than the same volume of vPET. The LCA comparison indicates that the rPET process has larger environmental impacts than vPET across most impact categories except fossil fuel depletion.
000 MT year−1 facility, following economies of scale, and it is comparable to the current market selling price (∼$1.0 per kg). The sensitivity analysis shows that feedstock price, solids loading, depolymerization extent, and rTPA recovery are key variables affecting production costs. In addition, both facility sizes of rPET have greater economic impacts and jobs created than the same volume of vPET. The LCA comparison indicates that the rPET process has larger environmental impacts than vPET across most impact categories except fossil fuel depletion.
Overall, the Bass model, CARAT, and supply chain risk assessment results agree that enzymatic PET recycling face adoption challenges; however, they provide different perspectives. Although the Bass model shows potential adoption through time (i.e., a dynamic view), it accounts for fewer dimensions than CARAT (12 versus 17). The CARAT indicates relatively low adoption, which might be true in the current marketplace for rPET with no temporal changes, though the Bass model shows how the adoption rate would be expected to increase over time. It should be noted that the CARAT tool uses qualitative judgement of risks across 12 dimensions to come up with an adoption rate and the low adoption rate resulted for PET recycling technology is based on our conservative estimates, which could be different for any other user that may be aggressive in estimating the risks (low, medium, high) for dimensions included in the tool. While there are ways to improve the adoption rate (e.g., policies and incentives to provide capital incentives and reduce the overall production cost, workshops and public education to increase community perception). The supply chain risk assessment method complements both tools by adopting a material perspective and identifying potential bottlenecks within supply chains. Currently, supply chain risks are low in general, though there are certain materials with higher individual risks that will require higher monitoring if those risks materialize. Together, those methods enable a meaningful analysis of a range of technology adoption barriers.
The methodology in this framework can be used for evaluating the barriers to accelerate the deployment of all new technologies trying to enter the saturated market. The framework outlined in the study can help decision makers such as investors, regulators, and manufacturers address the barriers associated with technology adoption and deployment to make informed decisions, and it can aid in technology transitions. This framework can also serve as a complement to the CARAT when evaluating new technologies before entering the market. This method uses an estimated adoption rate that takes inputs from emissions levels, permitting requirements, economic impacts, and regulatory requirements. Although permitting and economic calculations are standard for companies looking to enter a new market, the inclusion of local economic effects, environmental impacts, technology adoption modeling, and supply chain evaluations provide a more holistic outlook of the potential barriers to the adoption of a new technology.
Abbreviation
| BOD | Biological oxygen demand |
| CAGR | Compound annual growth rate |
| CAP | Criteria air pollutants |
| CARAT | Commercial adoption readiness assessment tool |
| CFR | Code of federal regulations |
| EG | Ethylene glycol |
| EPA | Environmental Protection Agency |
| FTE | Full-time equivalent |
| HAP | Hazardous air pollutants |
| IRR | Internal rate of return |
| LCA | Life cycle assessment |
| MRF | Material recovery facilities |
| MSP | Minimum selling price |
| MT | Metric tons |
| NAA | Nonattainment area |
| NESHAP | National Emission Standards for HAP |
| NNSR | Nonattainment NSR |
| NSPS | New source performance standards |
| NSR | New source review |
| PET | Polyethylene terephthalate |
| PTE | Potential to emit |
| ROI | Return on investment |
| TPA | Terephthalic acid |
| Tpd | Tons per day |
| VOC | Volatile organic compounds |
Data availability
All supporting data for the referred article can be located in the attached ESI† file.Author contributions
A. C.: funding, supervision. A. B.: conceptualization, administration, analysis, writing (review), editing. G. A.: analysis, investigation, methods, writing (drafting), review, editing. A. F. T. A,. J. D. V., J. P.: analysis, investigation, methods. S. U., J. W.: analysis, investigation, methods, writing (drafting).Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was authored by the National Renewable Energy Laboratory, operated by Alliance for Sustainable Energy, LLC, for the U.S. Department of Energy (DOE) under Contract No. DE-AC36-08GO28308. Funding provided by the U.S. Department of Energy Industrial Efficiency and Decarbonization Office. The views expressed in the article do not necessarily represent the views of the DOE or the U.S. Government. The U.S. Government retains and the publisher, by accepting the article for publication, acknowledges that the U.S. Government retains a nonexclusive, paid-up, irrevocable, worldwide license to publish or reproduce the published form of this work, or allow others to do so, for U.S. Government purposes.References
- J. Blees, R. Kemp, J. Maas and M. Mosselman, Differences in barriers to entry for SMEs and large enterprises, in SCALES- Scientific AnaLyses of Entrepreneurship SMEs, Report No.: H200301, 2003, p. 153 Search PubMed.
- C. H. M. Lutz, R. G. M. Kemp and S. Gerhard Dijkstra, Perceptions regarding strategic and structural entry barriers, Small Bus. Econ., 2010, 35(1), 19–33 CrossRef.
- D. W. Carlton, Barriers to Entry, National Bureau of Economic Research, 2005, p. 32, Available from: https://www.nber.org/system/files/working_papers/w11645/w11645.pdf Search PubMed.
- D. Millimet, S. Roy and A. Sengupta, Environmental regulations and economic activity: influence on market structure, Ann. Rev. Resour. Econ., 2009, 1, 99–117 CrossRef.
- A. E. Ferris, R. Garbaccio, A. Marten and A. Wolverton, The impacts of environmental regulation on the U.S. Economy, in Oxford Research Encyclopedia of Environmental Science, Oxford University Press, 2017, cited 2023 December 19, Available from: https://oxfordre.com/environmentalscience/view/10.1093/acrefore/9780199389414.001.0001/acrefore-9780199389414-e-396 Search PubMed.
- M. Altman, When green isn't mean: economic theory and the heuristics of the impact of environmental regulations on competitiveness and opportunity cost, Ecol. Econ., 2001, 36(1), 31–44 CrossRef.
- M. Porter, Scientific American, America’ Green Strategy, 1991, Available from: https://www.scientificamerican.com/article/essay-1991-04 Search PubMed.
- A. Dechezleprêtre and M. Sato, The impacts of environmental regulations on competitiveness, Rev. Environ. Econ. Policy, 2017, 11(2), 183–206 CrossRef.
- X. Zhang, Y. Li, K. Shi and Y. Feng, How do environmental technology standards affect the green transformation? New evidence from China, Int. J. Environ. Res. Public Health, 2022, 19(10), 5883 CrossRef PubMed.
- F. Meng, Y. Xu and G. Zhao, Environmental regulations, green innovation and intelligent upgrading of manufacturing enterprises: evidence from China, Sci. Rep., 2020, 10(1), 14485 CrossRef CAS PubMed.
- H. Taherdoost, A review of technology acceptance and adoption models and theories, Procedia Manuf., 2018, 22, 960–967 CrossRef.
- S. Koul and A. Eydgahi, A systematic review of technology adoption frameworks and their applications, J. Technol. Manage. Innovation, 2017, 12(4), 106–113 CrossRef.
- J. C. F. Li, Roles of individual perception in technology adoption at organization level: behavioral model versus TOE framework, J. Syst. Manage. Sci., 2020, 10(3), 97–118 Search PubMed.
- S. L. Parente and E. C. Prescott, Barriers to technology adoption and development, J. Political Econ., 1994, 102(2), 298–321 CrossRef.
- L. Fang, Entry barriers, competition, and technology adoption, Econ. Inq., 2017, 55(2), 794–805 CrossRef.
- B. Herrendorf and A. Teixeira, Barriers to entry and development, Int. Econ. Rev., 2011, 52(2), 573–602 CrossRef.
- D. Xia, M. Zhang, Q. Yu and Y. Tu, Developing a framework to identify barriers of Green technology adoption for enterprises, Resour., Conserv. Recycl., 2019, 143, 99–110 CrossRef.
- S. Luthra, S. Kumar, D. Garg and A. Haleem, Barriers to renewable/sustainable energy technologies adoption: Indian perspective, Renew. Sustainable Energy Rev., 2015, 41, 762–776 CrossRef.
- R. Luken and F. Van Rompaey, Drivers for and barriers to environmentally sound technology adoption by manufacturing plants in nine developing countries, J. Clean. Product., 2008, 16(1, Supplement 1), S67–S77 CrossRef.
- A. Stornelli, S. Ozcan and C. Simms, Advanced manufacturing technology adoption and innovation: a systematic literature review on barriers, enablers, and innovation types, Res. Policy, 2021, 50(6), 104229 CrossRef.
- A. Efstathiades, S. Tassou and A. Antoniou, Strategic planning, transfer and implementation of advanced manufacturing technologies (AMT). Development of an integrated process plan, Technovation, 2002, 22(4), 201–212 CrossRef.
- A. Mallalieu, S. Isaksson Hallstedt, O. Isaksson, M. Watz and L. Almefelt, Barriers and enablers for the adoption of sustainable design practices using new design methods – accelerating the sustainability transformation in the manufacturing industry, Sustainable Product. Consumption, 2024, 51, 137–158 CrossRef.
- J. A. D. Machuca, M. S. Díaz and M. J. Á. Gil, Adopting and implementing advanced manufacturing technology: new data on key factors from the aeronautical industry, Int. J. Prod. Res., 2004, 42(16), 3183–3202 CrossRef.
- Lawrence Berkeley National Laboratory, Technology Readiness Assessment Guide, Report No.: DOE G 413.3-4A, U.S. Dept of Energy, 2011, p. 73, Available from: https://www2.lbl.gov/dir/assets/docs/TRL20guide.pdf Search PubMed.
- A. Singh, N. A. Rorrer, S. R. Nicholson, E. Erickson, J. S. DesVeaux and A. F. T. Avelino, et al., Techno-economic, life-cycle, and socioeconomic impact analysis of enzymatic recycling of poly(ethylene terephthalate), Joule, 2021, 5(9), 2479–2503 CrossRef CAS.
- SEC Edgar Filing Form 10-Q for Loop Industries, Securities and Exchange Commission, 2019, Available from: http://filings.irdirect.net/data/1504678/000165495419011528/loi_10q.pdf Search PubMed.
- Eastman. PR Newswir, Eastman to recycle discarded carpet into new materials, 2019, cited 2024 January 2, Available from: https://www.prnewswire.com/news-releases/eastman-to-recycle-discarded-carpet-into-new-materials-300951256.html Search PubMed.
- Eastman Media Center, Plastic-to-Plastic Molecular Recycling Facility in Kingsport, 2021, cited 2024 January 2, Available from: https://www.eastman.com/en/media-center/news-stories/2021/governor-lee-announce-plastic-recycling-facility Search PubMed.
- EPA, New Source Review (NSR) Permitting, 2022, Available from: https://www.epa.gov/nsr Search PubMed.
- J. S. Seitz and E. Schaeffer, Potential to Emit (PTE) Guidance for Specific Source Categories, p. 29 Search PubMed.
- ASPEN, Release 10, in AspenPlus2010, Aspen Technology Inc., Cambridge, MA, 2010, Available from: https://www.aspentech.com/en Search PubMed.
- U.S. Environmental Protection Agency Office of Air Quality Planning and Standards, Emissions Factors & AP 42, 1995, cited 2015 Mar 2, Available from: https://www.epa.gov/ttnchie1/ap42 Search PubMed.
- EPA, TANKS Emissions Estimation Software, Version 4.09D, 2006, Available from: https://www.epa.gov/ttnchie1/software/tanks Search PubMed.
- Federal Register, Environmental Protection Agency, 40 CFR Part 60: Standards of Performance for New Stationary Sources, U.S. Government Publishing Office, Available from: https://www.ecfr.gov/cgi-bin/text-idx?SID=e0866080a9e0a5afb4c4fc3fc6742a04&node=pt40.7.60&rgn=div5 Search PubMed.
- A. Bhatt, Y. Zhang, G. Heath, M. Thomas and J. Renzaglia, Federal Air Pollutant Emission Regulations and Preliminary Estimates of Potential-to-Emit from Biorefineries, Pathway #2: Conversion of Lignocellulosic Biomass to Hydrocarbon Fuels: Fast Pyrolysis and Hydrotreating Bio-Oil Pathway, National Renewable Energy Lab. (NREL), Golden, CO (United States), 2017, Report No.: NREL/TP--6A20-67333, cited 2017 February 28. Available from: https://www.osti.gov/scitech/biblio/1340174-federal-air-pollutant-emission-regulations-preliminary-estimates-potential-emit-from-biorefineries-pathway-conversion-lignocellulosic-biomass-hydrocarbon-fuels-fast-pyrolysis-hydrotreating-bio-oil-pathway Search PubMed.
- Y. Zhang, A. Bhatt, G. Heath, M. Thomas and J. Renzaglia, Federal Air Pollutant Emission Regulations and Preliminary Estimates of Potential-to-Emit from Biorefineries, Pathway #1: Dilute-Acid and Enzymatic Deconstruction of Biomass-to-Sugars and Biological Conversion of Sugars-to-Hydrocarbons, National Renewable Energy Laboratory (NREL), Golden, CO, 2016, Report No.: NREL/TP-6A20-62547, Available from: https://www.nrel.gov/docs/fy16osti/62547.pdf Search PubMed.
- S&P Global, Chemical Economics Handbook, 2023, Available from: https://connect.ihsmarkit.com/ChemicalResearch?pageId=RSRCHMRA_CEH Search PubMed.
- D. Humbird, R. Davis, L. Tao, C. Kinchin, D. Hsu, A. Aden, et al., Process Design and Economics for Biochemical Conversion of Lignocellulosic Biomass to Ethanol: Dilute-Acid Pretreatment and Enzymatic Hydrolysis of Corn Stover, National Renewable Energy Laboratory (NREL), Golden, CO, 2011, cited 2014 Dec 4, Report No.: NREL/TP-5100-47764. Available from: https://www.osti.gov/scitech/biblio/1013269 Search PubMed.
- A. Aden, M. Ruth, K. Ibsen, J. Jechura, K. Neeves, J. Sheehan, et al., Lignocellulosic Biomass to Ethanol Process Design and Economics Utilizing Co-Current Dilute Acid Prehydrolysis and Enzymatic Hydrolysis for Corn Stover, 2002, cited 2022 September 22, p. NREL/TP-510-32438, 15001119. Report No.: NREL/TP-510-32438, 15001119. Available from: https://www.osti.gov/servlets/purl/15001119-zb17aV/native Search PubMed.
- R. Davis, A. H. Bhatt, Y. Zhang, E. C. D. Tan, V. Ravi and G. Heath, Biorefinery upgrading of herbaceous biomass to renewable hydrocarbon fuels: part 1: process modeling and mass balance analysis, J. Cleaner Prod., 2022, 362, 132439 CrossRef CAS.
- R. Davis, L. Tao, E. C. D. Tan, M. J. Biddy, G. T. Beckham, C. Scarlata, et al., Process Design and Economics for the Conversion of Lignocellulosic Biomass to Hydrocarbons: Dilute-Acid and Enzymatic Deconstruction of Biomass to Sugars and Biological Conversion of Sugars to Hydrocarbons, National Renewable Energy Laboratory (NREL), Golden, CO, 2013, cited 2014 December 8, Report No.: NREL/TP-5100-60223. Available from: https://www.osti.gov/scitech/biblio/1107470 Search PubMed.
- Chemical Engineering Plant Cost Index, Chemical Engineering, cited 2021 August 6, Available from: https://www.chemengonline.com/pci Search PubMed.
- SRI Consulting, U.S. Producer Price Indexes – Chemicals and Allied Products/Industrial Inorganic Chemicals Index, Chemical Economics Handbook, Menlo Park, CA, 2008 Search PubMed.
- U.S. Dept of Labor. U.S. Bureau of Labor Statistics, Employment Cost Trends, Available from: https://www.bls.gov/eci Search PubMed.
- A. F. T. Avelino, P. Lamers, Z. Yimin and H. Chum, Creating a harmonized time series of environmentally-extended input-output tables to assess the evolution of the US bioeconomy – A retrospective analysis of corn ethanol and soybean biodiesel, J. Clean. Product., 2021, 321, 128890 CrossRef.
- A. Singh, N. A. Rorrer, S. R. Nicholson, E. Erickson, J. S. DesVeaux and A. F. T. Avelino, et al., Techno-economic, life-cycle, and socioeconomic impact analysis of enzymatic recycling of poly(ethylene terephthalate), Joule, 2021, 5(9), 2479–2503 CrossRef CAS.
- GBB, Materials Recovery Facility Feasibility Report – City of Tucson, AZ. Fairfax, VA: Gershman, Brickner & Bratton, Inc., 2008, Report No.: GBB/RRT-C08018, p. 54 Search PubMed.
- P. N. Pressley, J. W. Levis, A. Damgaard, M. A. Barlaz and J. F. DeCarolis, Analysis of material recovery facilities for use in life-cycle assessment, Waste Manage., 2015, 35, 307–317 CrossRef PubMed.
- CB&I, Material Recycling Facility Contract Development Phase I Report – City of Ann Arbor, Ann Arbor, MI: CB&I Environmental & Infrastructure, 2016 Search PubMed.
- ISO, ISO 14040:2006, Environmental management — Life cycle assessment — Principles and framework, 2006, cited 2022 September 22, Available from: https://www.iso.org/obp/ui/#iso:std:iso:14040:ed-2:v1:en Search PubMed.
- T. Uekert, J. S. DesVeaux, A. Singh, S. R. Nicholson, P. Lamers and T. Ghosh, et al., Life cycle assessment of enzymatic poly(ethylene terephthalate) recycling, Green Chem., 2022, 24(17), 6531–6543 RSC.
- U.S. EPA Office of Research & Development, Tool for the Reduction and Assessment of Chemical and Other Environmental Impacts (TRACI) TRACI version 2.1 User's Guide, 2014, cited 2022 September 27, Available from: https://cfpub.epa.gov/si/si_public_record_Report.cfm?Lab=NRMRL&dirEntryId=274755 Search PubMed.
- R. Hanes, A. Carpenter, M. Riddle, D. J. Graziano and J. Cresko, Quantifying adoption rates and energy savings over time for advanced energy-efficient manufacturing technologies, J. Clean. Product., 2019, 232, 925–939 CrossRef.
- L. Tian, J. Mees, V. Chan and W. Dean, Commercial Adoption Readiness Assessment Tool (CARAT), U.S. DOE Office of Technology Transitions, Office of Clean Energy Demonstrations, 2023, Available from: https://www.energy.gov/sites/default/files/2023-03/CARAT-R9_3-22-23.pdf Search PubMed.
- A. H. Bhatt, Y. Zhang and G. Heath, Bio-oil co-processing can substantially contribute to renewable fuel production potential and meet air quality standards, Appl. Energy, 2020, 268, 114937 CrossRef CAS.
- Code of Federal Regulations, 40 CFR Chapter I Subchapter D -- Water Programs, 2022, Available from: https://www.ecfr.gov/current/title-40/chapter-I/subchapter-D Search PubMed.
- Code of Federal Regulations, 40 CFR Part 414 -- Organic Chemicals, Plastics, and Synthetic Fibers, 2022, Available from: https://www.ecfr.gov/current/title-40/chapter-I/subchapter-N/part-414 Search PubMed.
- Code of Federal Regulations, 40 CFR Part 131 -- Water Quality Standards, 2022, Available from: https://www.ecfr.gov/current/title-40/chapter-I/subchapter-D/part-131 Search PubMed.
- Code of Federal Regulations, 40 CFR Part 122 Subpart C -- Permit Conditions, 2022, Available from: https://www.ecfr.gov/current/title-40/chapter-I/subchapter-D/part-122/subpart-C Search PubMed.
- South Carolina Dept. of Health and Environmental Control, Regulation 61-9 Water Pollution Control Permits, 2019, Available from: https://scdhec.gov/sites/default/files/Library/Regulations/R.61-9.pdf Search PubMed.
- South Carolina Dept. of Health and Environmental Control, Regulation 61-68 Water Classifications and Standards, 2019, Available from: https://scdhec.gov/sites/default/files/media/document/R.61-68_0.pdf Search PubMed.
- Code of Federal Regulations, 40 CFR Part 261 -- Identification and Listing of Hazardous Waste, 2022, Available from: https://www.ecfr.gov/current/title-40/chapter-I/subchapter-I/part-261 Search PubMed.
- Code of Federal Regulations, 40 CFR Part 262 -- Standards Applicable to Generators of Hazardous Waste, 2022, Available from: https://www.ecfr.gov/current/title-40/chapter-I/subchapter-I/part-262 Search PubMed.
- South Carolina Dept. of Health and Environmental Control, Regulation 61-79 South Carolina Hazardous Waste Management Regulations, 2019, Available from: https://scdhec.gov/sites/default/files/media/document/R.61-79_0.pdf Search PubMed.
- M. Doyle, and K. Knauer, Survey on Technology Adoption Importance, 2023 Search PubMed.
- T. Sivageerthi, S. Bathrinath, M. Uthayakumar and R. K. A. Bhalaji, A SWARA method to analyze the risks in coal supply chain management, Mater. Today Proc., 2022, 50, 935–940 CrossRef.
- Determination of the Risk of Coal Supply Chain for Thermal Power Plants in Vietnam, cited 2023 November 27, Available from: https://www.scirp.org/journal/paperinformation.aspx?paperid=110400 Search PubMed.
- ChemAnalyst, Ethylene Oxide (EO) Prices, News, Monitor, ChemAnalyst, cited 2023 November 27, Available from: https://www.chemanalyst.com/Pricing-data/ethylene-oxide-110220CEH20profile20for20ethylene20oxide Search PubMed.
- US EPA O, Water Treatment Chemical Supply Chain Profiles, 2022, cited 2023 November 27, Available from: https://www.epa.gov/waterutilityresponse/water-treatment-chemical-supply-chain-profiles Search PubMed.
- Sodium Sulphate Prices, Price, Pricing, News, Monitor, ChemAnalyst, cited 2023 November 27, Available from: https://www.chemanalyst.com/Pricing-data/sodium-sulphate-1480 Search PubMed.
- LIMITED CMRP, GlobeNewswire News Room, 2023, cited 2023 November 27, [Latest Report] Global Sodium Chloride Market Size/Share Worth USD 67.4 Billion by 2030 at a 4.4% CAGR: Custom Market Insights (Analysis, Outlook, Leaders, Report, Trends, Forecast, Segmentation, Growth, Growth Rate, Value). Available from: https://www.globenewswire.com/en/news-release/2023/05/02/2658782/0/en/Latest-Report-Global-Sodium-Chloride-Market-Size-Share-Worth-USD-67-4-Billion-by-2030-at-a-4-4-CAGR-Custom-Market-Insights-Analysis-Outlook-Leaders-Report-Trends-Forecast-Segmentat.html Search PubMed.
- Tight Supply Situation Drives Sulphur Prices Up in the United States and Europe, cited 2023 November 27, Available from: https://www.chemanalyst.com/NewsAndDeals/NewsDetails/tight-supply-situation-drives-sulphur-prices-up-in-the-united-states-and-europe-19288 Search PubMed.
- Does Sugar Production Harm the Environment? – Conserve Energy Future, 2020, cited 2023 November 27, Available from: https://www.conserve-energy-future.com/environmental-impact-sugar.php Search PubMed.
- Sulphuric Acid Prices, News, Monitor, ChemAnalyst, cited 2023 November 27, Available from: https://www.chemanalyst.com/Pricing-data/sulphuric-acid-70 Search PubMed.
- UCL, Sulfur Shortage: A Potential Resource Crisis Looming As The World Decarbonises, UCL News, 2022, cited 2023 November 27, Available from: https://www.ucl.ac.uk/news/2022/aug/sulfur-shortage-potential-resource-crisis-looming-world-decarbonises Search PubMed.
- Caustic Soda Prices, Price, Pricing, Monitor, ChemAnalyst, cited 2023 November 27, Available from: https://www.chemanalyst.com/Pricing-data/caustic-soda-3 Search PubMed.
- petnology.com, $200 Million in investment, multiple acquisitions make Evergreen among the three largest producers of Food Grade rPET in North America, 2022, cited 2023 November 27, Available from: https://www.petnology.com/online/news-detail/200-million-in-investment-multiple-acquisitions-make-evergreen-among-the-three-largest-producers-of-food-grade-rpet-in-north-america Search PubMed.
- Allied Market Research, Activated Carbon Market Size, Share, Competitive Landscape and Trend Analysis Report, by Product Type, by Application, by End-use Industry : Global Opportunity Analysis and Industry Forecast, 2020-2030, cited 2023 November 27, Available from: https://www.alliedmarketresearch.com/activated-carbon-market Search PubMed.
- ReportLinker, Activated Carbon Global Market Report 2021: COVID-19 Impact And Recovery, GlobeNewswire News Room, 2021, cited 2023 November 27, Available from: https://www.globenewswire.com/news-release/2021/07/09/2260446/0/en/Activated-Carbon-Global-Market-Report-2021-COVID-19-Impact-And-Recovery.html Search PubMed.
- Suppliers Get Creative to Battle Raw Materials and Semiconductor Shortages, cited 2023 November 27, Available from: https://www.cbtnews.com/suppliers-get-creative-to-battle-raw-materials-and-semiconductor-shortages Search PubMed.
- S&P Global, Sharing Insights Elevates Their Impact, 2021, cited 2023 November 27, Available from: https://www.spglobal.com/commodityinsights/en/ci/research-analysis/specialty-chemicals-forecast-to-grow-in-2021.html Search PubMed.
- X. Shi and P. Chumnumpan, Modelling market dynamics of multi-brand and multi-generational products, Eur. J. Operat. Res., 2019, 279(1), 199–210 CrossRef.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4su00706a |
| ‡ Criteria air pollutants—those with National Ambient Air Quality Standards—and their precursors (e.g., sulfur dioxide [SO2], carbon monoxide [CO], particulate matter [PM], including PM with diameters of less than 10 or 2.5 micrometers [PM10] and [PM2.5], nitrogen oxides [NOx], ozone regulated through its precursors such as NOx and volatile organic compounds [VOCs], and lead [Pb]). Pollutants are outlined in the NSPS (Federal register).34 Hazardous air pollutants (HAPs) are as defined by the EPA. |
| § https://scdhec.gov/sites/default/files/media/document/R.61-62.5_Std.8.pdf. |
| ¶ Environmentally-extended multi-regional projection of lifecycle and occupational energy futures model. |
| || The final model contains 425 commodities and 415 industries. |
| ** NSPS subpart QQQ for wastewater emissions from refinery streams, subpart III for air oxidation, subpart NNN for distillation processes, subpart RRR for reactor operations, and subpart VV/VVa for equipment leaks. |
| †† SC regulations 61-9.122.21(j)(3), 61-9.122.21(k)(5), and 61-9.122.34(g)(3). |
| This journal is © The Royal Society of Chemistry 2025 |