Open Access Article
Open Access ArticleAdvanced catalytic strategies for CO2 to methanol conversion: noble metal-based heterogeneous and electrochemical approaches
Soumalya
Roy
af,
Ezhava Manu
Manohar
a,
Sujoy
Bandyopadhyay
b,
Manik
Chandra Singh
c,
Yeji
Cha
f,
Soumen
Giri
 *d,
Sharad
Lande
*d,
Sharad
Lande
 *e,
Kyungsu
Na
*e,
Kyungsu
Na
 *f,
Junseong
Lee
*f,
Junseong
Lee
 *f and
Sourav
Das
*f and
Sourav
Das
 *a
*a
aDepartment of Basic Sciences, Chemistry Discipline, Institute of Infrastructure Technology Research And Management, Near Khokhra Circle, Maninagar East, Ahmedabad, 380026, Gujarat, India. E-mail: souravdas@iitram.ac.in; d.sourav245@gmail.com
bDepartment of Chemistry, Research Institute for Convergence of Basic Science and Research Institute for Natural Sciences, Hanyang University, Seoul 04763, Republic of Korea
cDept. of Physics, Balarampur P. C. H. S School, Rangadih, Purulia, West Bengal 723143, India
dSchool of Applied Sciences, Kalinga Institute of Industrial Technology, Bhubaneswar 751024, India. E-mail: giri.soumen02@gmail.com
eResearch & Development, Reliance Industries Ltd, Thane Belapur Road, Ghansoli, Navi Mumbai, 400701, India. E-mail: sharad.lande@ril.com
fDepartment of Chemistry, Chonnam National University, 77 Yongbong-ro, Buk-gu, Gwangju 61186, Republic of Korea. E-mail: leespy@chonnam.ac.kr; kyungsu_na@chonnam.ac.kr
First published on 27th January 2025
Abstract
The next generation is threatened by climate change, the significant impacts of global warming, and an energy crisis. Atmospheric CO2 levels have surpassed the critical 400 ppm threshold due to significant reliance on fossil fuels to satisfy the increasing energy demands of our fast-progressing society. An overabundance of manufactured carbon dioxide (CO2) emissions severely disrupts the ecology. The synthesis of methanol by the selective hydrogenation of CO2 is a viable approach for generating clean energy and sustainably safeguarding our biosphere. Methanol is a versatile molecule with several uses in the chemical industry as an alternative to fossil fuels. The methanol economy is recognized as a pivotal development in the pursuit of a net zero-emission fuel, representing a crucial stride toward a more sustainable planet. The developing green methanol industry, or renewable methanol initiative, primarily relies on CO2 adsorption and usage. This novel technique is essential for mitigating global warming. This review focuses on the synthesis of methanol utilizing noble metal-based catalysts and electrochemical reduction methods, examining the associated thermodynamic challenges and outlining future directions for research. It emphasizes the role of noble metals, including palladium, gold, silver, and rhodium, in enhancing catalytic activity and selectivity during the CO2 to methanol conversion process. The incorporation of these sophisticated catalytic processes improves methanol production efficiency and facilitates novel methods for carbon capture and usage, therefore advancing a more sustainable energy framework.
Sustainability spotlightA perspective on noble metal-based catalytic conversion of CO2 to methanol through heterogeneous and electrochemical approaches presents a promising solution to address the increasing concerns regarding climate change. The current situation is critical: CO2 emissions from industrial activities constitute a significant contributor to global warming, and elucidating efficient methods for converting CO2 into valuable products is crucial for achieving sustainable development. Noble metal catalysts play a pivotal role in enhancing the selectivity, efficiency, and stability of CO2 reduction reactions. The sustainable advancement of this research lies in optimizing these catalysts to facilitate CO2 conversion at reduced energy inputs, thereby rendering the process more cost-effective and scalable for industrial applications. This research aligns with SDG 7 (Affordable and Clean Energy) by enabling the production of methanol as a clean fuel, SDG 9 (Industry, Innovation, and Infrastructure) through the development of advanced catalytic technologies, and SDG 13 (Climate Action) by addressing the reduction of CO2 emissions and advancing climate solutions. |
1. Introduction
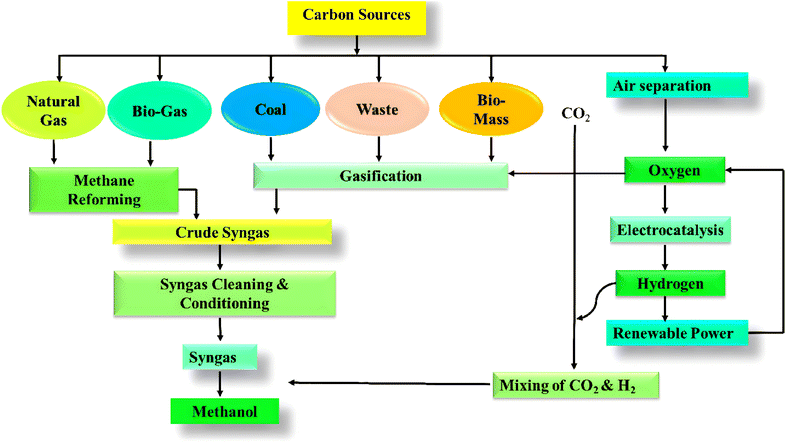
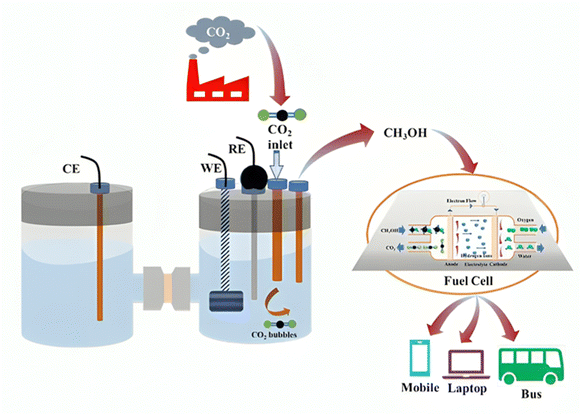
The use of fossil fuels has ushered in an era of extraordinary wealth and improved living standards for humanity.1,2 However, excessive fossil fuel burning for energy generation elevates the emission of anthropogenic greenhouse gases (GHGs), mostly in the form of pollutant CO2. According to the International Energy Agency (IEA), Global Energy & CO2 Status Report, in 2022 the worldwide CO2 emissions will have peaked at about 36.8 Gt a new all-time high, and the current concentration of atmospheric CO2 is approximately 421 ppm, which has increased by 50% since the beginning of the industrial age.3 The massive release of CO2 to the environment disrupts the Earth's natural carbon cycle and global ecology, leading to substantial and feasibly irreversible changes in the world's climate such as global warming, ocean acidification, and sea level rise, which have a direct consequence on human health.4–7 Green carbon science is the study and optimization of effective carbon resource processing, utilization, and recycling for lowering CO2 emissions, lessening the greenhouse effect, reducing reliance on fossil fuels, and lowering the global carbon footprint.8–13 Therefore, the creation of clean energy technology is the only option for the next generation.14–20 In that sense, one of the main research focuses in energy engineering is the regulation and utilization of CO2.21–29 In fact, recent years have seen the development of carbon capture and storage (CCS) technologies, which separate CO2 from combustion byproducts or chemical processes for subsequent recycling or storage.30–35 Direct capture of CO2 from the atmosphere has garnered scientific and practical attention in recent times.36–46 Despite reducing CO2 emissions to a certain degree, CCS is not a viable long-term solution due to its exorbitant expense and the possibility of leakage.47–53 Internationally, there are currently 20 active large-scale carbon capture and storage (CCS) facilities that annually separate around 40 million metric tons of carbon dioxide (CO2) and store it underground54–60 (The Global Status of CCS; (Global CCS Institute: 2020). However, this amount represents only 0.1% of the total global carbon emissions. However, the majority of these CCS projects fall under the category of enhanced oil recovery (EOR), which involves injecting CO2 underground to assist in extracting fossil fuels with the aim of creating a financially viable business model.61–63 The price for CO2 sequestration from a power plant ranges from $60 to $100 per metric ton of CO2, excluding storage and transportation expenses.64–68 Therefore, the implementation of EOR is essential to ensure the economic feasibility of CCS, which in turn leads to the introduction of more fossil fuels into the carbon cycle.69–73 For CCS to have a significant influence on climate change, it must be commercialized through the development of cost-effective and energy-efficient systems.74–82 Systems must ensure that the cost of producing CO2, regulated by authorities, exceeds the cost of capturing and storing it. This will help create an artificial carbon cycle. Additionally, we need to turn CO2 into chemicals and fuels that are in high demand (Fig. 1).83,84 This will serve to effectively utilize a significant portion of the surplus global CO2. Currently, around 230 million tons (Mt) of carbon dioxide (CO2) are utilized annually, as reported by the IEA in 2019.85,86 However, CO2 usage represents less than 1% of overall CO2 emissions.87,88 Currently, the market size limits the conversion of CO2 into chemicals. Thus, the catalytic hydrogenation of CO2 into value-added products is a key method for CO2 utilization, leading to the production of carbon-based substances such as methanol, dimethyl ether, formaldehyde, urea and various hydrocarbons.89–95 Among the products stated above, methanol (CH3OH) is a significant chemical feedstock that can be utilized as an alternative chemical for fossil fuels in internal combustion engines, fuel cells, and the production of formaldehyde, methyl-tert-butyl ether (MTBE), acetic acid, alkyl halides, etc.96–98 According to the well-accepted hypothesis of Olah in a book titled “Beyond Oil and Gas: The Methanol Economy,” methanol may be indispensable in the near future, and CO2 hydrogenation is one of the most promising and regenerative routes for methanol synthesis.99 The elevated volumetric energy density of methanol renders it a feasible substitute for gasoline in internal combustion engines (ICEs), enhancing the octane rating. Moreover, in comparison to traditional gasoline internal combustion engines, methanol internal combustion engines produce markedly lower emissions of contaminants. The Indian government has been actively encouraging the adoption of clean transportation and the use of fuel-cell vehicles.100 In 2012, the Dor Group initiated pilot testing following the decision by the Israeli government to explore alternative methods of reducing dependence on traditional fuels. One of the most promising approaches identified was the utilization of methanol as a substitute for petrol or as a component in petrol blends for internal combustion engines (Dor Group, 2019). China is also rapidly advancing the development of the methanol fuel market.It is crucial to note that the hydrogen generated via water electrolysis can function as a long-term storage solution and be directly transferred to required locations for usage as a transportation fuel or in industrial applications. Moreover, hydrogen can be converted into electrofuels following the power-to-X concept, thereby storing renewable electrical energy within the chemical bonds of gaseous or liquid fuels.101,102 Methanol can function as a carbon-neutral electrofuel when synthesized from hydrogen via electrolysis and carbon dioxide sourced from atmospheric capture, biomass, or industrial emissions103,104 (Fig. 2). Methanol is a prominent and extensively traded chemical commodity, with the global methanol market projected to expand at a compound annual growth rate (CAGR) of 4.50% from 2022 to 2028.105,106 In the chemical industry, methanol is used as both a feedstock and an energy carrier. Its applications range from organic transformations to sustainable energy solutions, highlighting its importance in both traditional and emerging chemical processes. Methanol can function as a C1 source to facilitate various C–C and C–N bond formations and dehydrogenative coupling reactions, which are significant in the realms of natural product synthesis and drug development.107,108 For instance, methanol is crucial for the production of formaldehyde, methylamine, methyl halides, methyl ethers, methyl esters, dimethyl ether, and acetic acid. It is additionally used in the manufacture of pharmaceuticals, biomolecules, agrochemicals, and polymers. Methanol may be transformed into olefins such as ethylene and propylene, which serve as essential building blocks for the petrochemical industry.109,110 Furthermore, methanol serves as a versatile compound with applications as a solvent and/or additive in diverse industries, including paint removal, windshield washing fluids, and as a fermentation substrate in microbial production processes.111
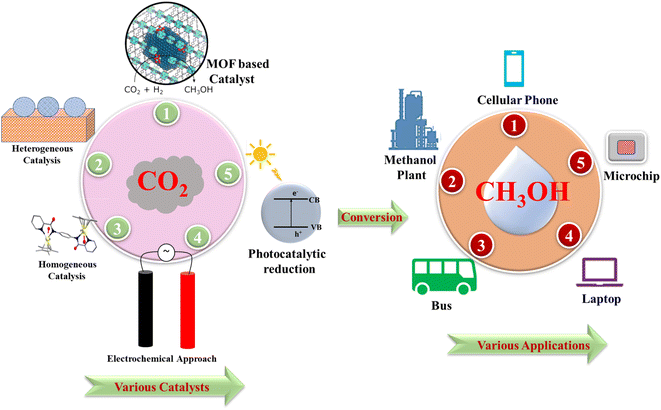
In this context, it is noteworthy to mention that homogeneous (organometallic catalysts, metal-free catalysts, and N-heterocyclic carbenes) and heterogeneous catalyst systems have recently been successfully developed, and they are excellent candidates for hydrogenation of CO2 to produce methanol.112–123 Another highly appealing strategy extensively studied by the scientific community is the direct electrocatalytic carbon dioxide reduction reaction (ECO2RR).124–126 In this instance, the term “direct ECO2RR” denotes the complete conversion of CO2 to a specific product, methanol, at a single electrode.127–129 In electrocatalytic technology, customized catalysts can improve electrode interface reaction kinetics.130,131 It lowers activation energy and helps electrolyte reactants move electrons.132,133 Electrocatalysts accelerate electron transit from noble metals to other materials by lowering voltage.134–138 To improve performance, researchers strive to augment the number of active sites and elevate the intrinsic activity of these sites. This involves fine-tuning the electronic and structural properties of the active sites to improve their catalytic efficiency.139 Recent methodologies exploit local electrostatic fields to enhance the adhesion of electrode surfaces for intermediates, facilitating proton transfer to electron-rich active species and enabling the electrochemical reduction of CO2 to produce methane.140 In another study, Zhou et al. reported the O-philic adsorption strategy to facilitate the electrosynthesis of urea.141 This study enhances our comprehension of how to control the properties of reactant adsorption–desorption dynamics for targeted conversion pathways within a comprehensive reactive multistep process pertinent to diverse electrocatalytic applications.
The first commercial methanol production unit was established in Anyang, Henan Province, China. Here methanol was synthesized using the waste carbon dioxide and hydrogen gases.142 This process used the Emissions-to-Liquids (ETL) technology which was initiated by Carbon Recycling International (CRI) and the first pilot plant was established in Iceland.143 Another emerging technology in the methanol economy is the CAMRE process, jointly developed by the Korea Institute of Science and Technology and POSCO.144 Carbon Recycling International developed a pilot plant for the commercial synthesis of methanol from CO2. In this, the source of CO2 is the Orka geothermal power plant and hydrogen is generated from the electrolysis of water from the Icelandic power grid and the methanol production capacity is 4000 tons per year. Blue Fuel Energy (BFE) is Canadian Methanol Corporation's conventional methanol production unit which produces methanol and gasoline based on the MTG process. Emerging techniques for producing methanol from CO2 include photocatalytic conversion, electrochemical reduction, photoelectrochemical reduction, chemical conversion and plasma reactor technologies.145–154
A large number of metal-based catalysts have been employed for methanol synthesis, but the most popular catalyst in CO2-derived methanol synthesis is Cu/ZnO/Al2O3.23,155–161 The major drawback of Cu based catalysts is their pyrophoric properties and low stability arising from sintering and agglomeration enhanced by the generation of water and mobility of ZnO.156,162–165 This review focuses on the synthesis of CH3OH from the hydrogenation of CO2 by employing noble metal based heterogeneous catalysts. The main advantages of noble metal-based catalysts are their excellent stability and resistance to the sintering effect, making them alternatives to Cu-based catalysts.166–170 An efficient heterogeneous catalyst possesses moderate binding strength with the reactant and intermediate.171–174 The success of catalytic reactions depends on the identification of the active site, accompanied by an understanding of structure–activity relationships that derive catalytic descriptors to explain the catalysis.175–179 Current research emphasizes that noble metal-based catalysts are superior for the conversion of CO2 to CO via the so-called reverse water gas-shift (RWGS) reaction with high reaction rates and high selectivity.180–184 Noble metal catalysts are excellent for CO2 hydrogenation due to their unique electronic and structural properties, specifically their unique tunable d-band centers.185–190 The d-band center mainly represents the energy level of the d-electrons relative to the Fermi level, strongly influencing the adsorption and activation of reactants such as CO2 and H2.191–193 The alignment of the d band center with the Fermi level enhanced the catalytic activity by optimizing the binding strength of intermediates.194,195 Pd exhibits an intermediate d band center, effectively balancing CO2 activation and methanol desorption.158,196 In contrast, Au with a lower d band center reduces the strong binding of intermediates, which enhances selectivity. Experimental evidence suggests that Pd based catalysts show high turnover frequencies (TOFs) and methanol selectivity compared to non-noble metals owing to their superior activation energies and adsorption energies.197–199 So, the manipulation of d band centers enhances the catalytic performance by tailoring the adsorption energies.200–202 It has been a widely accepted strategy to manipulate the203 d-band center of noble metals integrated with other metal compounds such as metal oxides/hydroxides and carbides by the electronic effect, which influenced the interface of atomic rearrangement with the geometric effect and strain effect.204 The catalytic reaction mainly occurs at the interface such as the metal–metal interface, metal–porous material interface or metal–metal oxides/hydroxides/carbides interface which leads to enhanced the catalytic activity of hydrogenation of CO2.205,206 The synthesis strategy of noble metal based heterogeneous catalysts with a proper understanding of structure–property relationships such as the active site of the catalyst, morphology, composition, strain, metal support interaction, crystallinity, defects etc. is one of the great achievements of the energy engineering sector.185,207,208 A promising strategy should be adopted for utilizing noble metal-based catalysts. In this respect, the strategy of incorporating bulk metals into nanostructure supported catalysts boosts the cost-effective industrial production of methanol.
Recently, several excellent reviews have been published and paved the way to catalytic CO2 conversion to value-added chemicals.209–214 However, the main aim of this comprehensive review is the hydrogenation of CO2 to CH3OH using heterogeneous noble metal-based catalysts with an electrochemical approach. This review first focuses on the physical and chemical properties of CO2 with thermodynamic considerations. Then we have briefly addressed various approaches to the catalytic conversion of CO2 to CH3OH such as the homogeneous approach, heterogeneous approach, photocatalytic approach, and electrochemical conversion approach, summarizing the catalytic journey of CO2 to CH3OH. The mechanism of the conversion of CO2 to CH3OH is one of the interesting sides of this article. Then the progress of noble metal-based heterogeneous catalysts is deeply discussed with structure–property relationships. We also provide an overview of the electrochemical reduction approach for the conversion of CO2 to CH3OH. The challenges and possibilities of CO2 reduction for industrial applications are also included. Finally, we have included the future scope and perspectives of methanol synthesized from CO2.
2. Physicochemical nature and activation of CO2
A CO2 molecule in its neutral state belongs to the D∞b point group and has a linear structure, with a bond length between carbon and oxygen of 1.17 Å. Because of the electronegativity differences, the net electronic structure of the molecule can be represented as O−δ–C+2δ–O−δ.215 In light of this, bifunctional catalytic sites are required to activate the C–O bond in an efficient manner, as the C atom is electrophilic and the O atoms are nucleophilic.216 The highest occupied molecular orbital (HOMO) of CO2 is located on the more electronegative oxygen atoms, whereas the lowest unoccupied molecular orbital (LUMO) is composed of the hybrid orbitals. The reduction of carbon dioxide (CO2) introduces an electron into its LUMO. This results in the molecule undergoing a conformational shift to accommodate the energy differences between each orbital, thereby enhancing its ability to interact with nucleophiles. Simultaneously, the localized electron density of oxygen lone pairs allows the HOMO to interact with electrophiles. As a result, a meta-stable radical adduct (CO2˙−) is generated in a bent shape that is kinetically stable (an activation energy barrier of ∼0.4 eV) with an average lifetime of 60–90 μs. The common metal center (metal oxide), which is usually used for CO2 reduction, where CO2 is physisorbed, exhibits several coordination modes, such as monodentate, bidentate, or even tridentate, depending on the surface atomic arrangement.2173. Conversion of CO2 to methanol: thermodynamic aspects
In the present era, syngas (CO and H2 mixture) is one of the main resources for the commercial production of methanol. In methanol synthesis, the H/C ratio is crucial, so to balance this ratio, a small amount of CO2, i.e. 2–8% is added to the CO/H2 mixture to enhance the reaction rate.218| CO + 2H2 = CH3OH ΔH298K = −90.6 kJ mol−1 | (1) |
| CO2 + 3H2 = CH3OH + H2O ΔH298K = −49.5 kJ mol−1 | (2) |
Reverse water gas shift (RWGS) reaction:
| CO2 + H2 = CO + H2O ΔH298K = 41.2 kJ mol−1 | (3) |
The processes of making methanol from CO2 hydrogenation and the RWGS reaction are limited by thermodynamics. As the reaction temperature increases, the CO2 conversion rate drops. It is important to note that the synthesis of methanol from CO2 is an exothermic process that competes with the RWGS reaction, an endothermic reaction. According to Le Chatelier's principle, high pressure and low temperature thermodynamically favor CO2 conversion to methanol. But considering the chemical inertness of CO2, a higher temperature makes CO2 activation easier for methanol production, but it can also lead to increased selectivity towards the undesirable RWGS reaction thermodynamically to produce CO and H2O. In this way, the RWGS process not only consumes hydrogen but also lowers the CO2 equilibrium conversion rate, hence reducing the generation of methanol.70 It was observed that less than 40% methanol was obtained from CO2 at 200 °C but at the aforesaid temperature, more than 80% yield was obtained from the syngas process. Therefore, high pressure and lower temperature are required to control the RWGS process219 (Fig. 3). According to the reaction (eqn (1)), as compared to the syngas strategy, methanol synthesis from CO2 hydrogenation requires an additional quantity of hydrogen for the removal of one oxygen atom from CO2, yielding water as a by-product (eqn (2)).220 It is important to note that water, a byproduct of both processes, enhances catalyst sintering. This phenomenon deactivates the catalyst and reduces the efficiency of subsequent steps in methanol production.221 Therefore, the foregoing explanation implies that thermodynamic equilibrium limits the maximum methanol yield; nevertheless, the equilibrium constraint on methanol yield may be bypassed by optimizing reaction conditions, activating the catalyst, using promoters, developing new reactor designs, etc.222,223
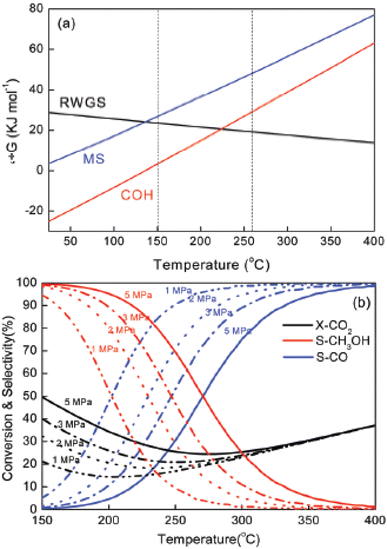 | ||
| Fig. 3 (a) Free energy of methanol synthesis (MS) from CO2, RWGS and syngas hydrogenation (COH). (b) Equilibrium conversion-selectivity values of the CO2-hydrogenation reaction at various pressures Reprinted with permission from ref. 156. Copyright 2020 Royal Society of Chemistry. | ||
However, if we take into account the CO2 thermodynamic potential, the following reduction reactions occur:
| CO2 + e− → CO2˙−; E0 = −1.9 V | (4) |
| CO2 + 2H+ + 2e− → CO + H2O; E0 = −0.52 V | (5) |
| CO2 + 2H+ + 2e− → HCOOH; E0 = −0.61 V | (6) |
| CO2 + 4H+ + 4e− → HCHO + H2O; E0 = −0.51 V | (7) |
| CO2 + 8H+ + 8e− → CH4 + 2H2O; E0 = −0.24 V | (8) |
| CO2 + 12H+ + 12e− → C2H4 + 4H2O; E0 = −0.34 V | (9) |
| CO2 + 6H+ +6e− → CH3OH + H2O; E0 = −0.38 V | (10) |
| 2H+ + 2e− → H2E0 = −0.42 V | (11) |
From the above reaction sequences, it can be observed that the overpotential of CO2 is high (eqn (4)) and it's a multi-electron process. The CO2 reduction is challenging as it generates the hydrogen evolution reaction (HER) if we compare it with the last two reactions. Again, for the CO2 reduction reaction, the following processes are involved in the cathode (reduction) and anode (oxidation):
| Cathode: CO2 + 6H+ + 6e− ⇌ CO2+H2O, E0 = 0.02 vs. SHE |
| Anode: 3H2O ⇌ 1.5O2 + 6H+ + 6e−, E0 = 1.23 vs. SHE |
| Overall: CO2+2H2O⇌CH3OH+1.5O2, E0 = 1.21 vs. SHE |
If we look at water splitting at 1.23 V vs. SHE and the overall CO2 reduction reaction, then a positive difference of only 20 mV exists. Therefore, a catalyst with a high hydrogen overpotential is preferred as it allows the carbon dioxide reduction reaction to achieve high selectivity and excellent rates before water reduction occurs, which is a process bottleneck (Fig. 4).
 | ||
| Fig. 4 Schematic representation of ECO2R to CH3OH.138 | ||
In order to be effective in CO2 reduction, noble-metal-based catalysts must be able to reduce the overpotential by which their activity is enhanced and able to show remarkable selectivity and stability. The below figure depicts the groupwise metal activity on CO2 reduction and the overall idea (Fig. 5).
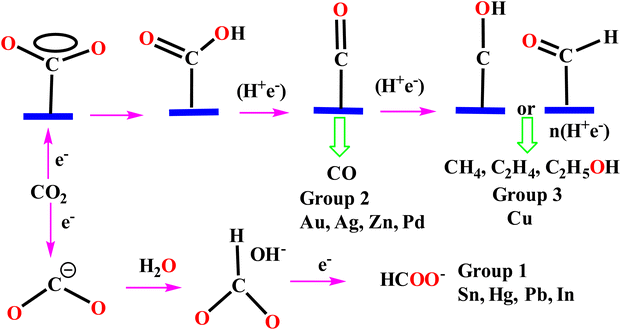 | ||
| Fig. 5 Reaction mechanism of the electrochemical CO2RR on metal electrodes in aqueous solutions.224 | ||
4. Mechanism of CO2 conversion to CH3OH
The CO2 hydrogenation mechanism depends on various factors such as stability, activity and selectivity of the catalyst.157,225 According to the prevailing theory, the first step deals with the CO2 conversion to CO, which acts as a scavenger to eliminate the H2O and the function of derived oxygen species is to inhibit active metal sites.226–228 The use of isotope tracer tests has further supported the notion that CO2 serves as the elementary carbon source for the production of methanol from syngas or CO2 (ref. 229) (Fig. 6).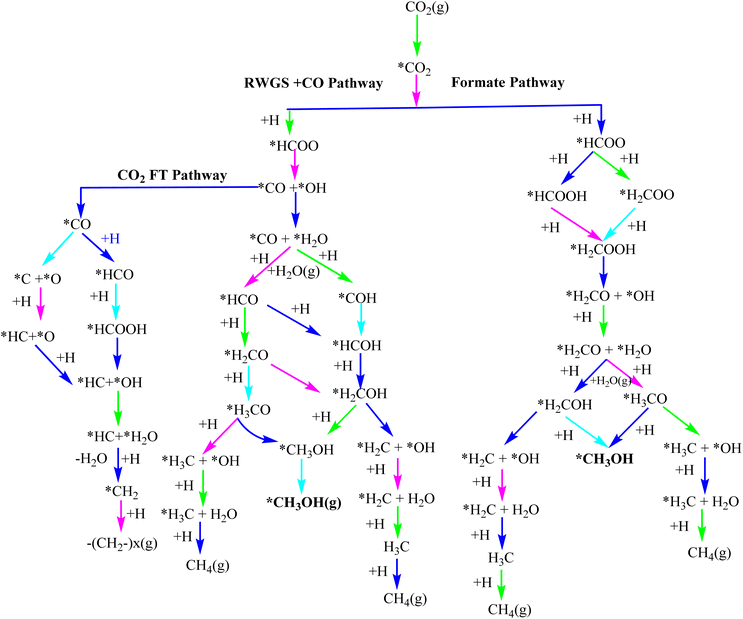 | ||
| Fig. 6 Reaction mechanism of CH3OH synthesis from CO2.157 | ||
The theoretical and computational tools provide the validation of this reaction mechanism with experimental results. In this respect, DFT analysis, microkinetic modeling, and Monte Carlo simulation offer novel methods for developing CO2 hydrogenation catalysts.230–235
4.1 The HCOO mechanism
Researchers have followed the formate mechanistic path where CO2 interacts with formate (HCOO) via the Eley–Rideal (ER) or Langmuir–Hinshelwood (LH) mechanism, and the reaction proceeds through H2COO*, H2CO*, and CH3OH.236–242 The RWGS approach generates unstable HCO* species which further dissociate into CO and H. Investigations using XPS, TPD, and IRAS techniques demonstrate that bidentate formate compounds play a crucial role in modifying the surface under reaction conditions and serve as stable intermediates during the production of CH3OH.243,244 The methanol synthesis proceeds through the formate mechanistic pathway over Pd based catalysts Pd/β-Ga2O3, Pd@Zn core shell catalyst, and Pd4/In2O3.231,245,246In this mechanism, the C–O bond gets dissociated by the hydrogenation of HCOO*/H2COO* and is the rate determining step of the reaction.247–249 According to the microkinetic model, the rate determining step is the hydrogenation of HCOO to H2COO on Pd–Ga2O3/silica.250,251
4.2 The revised HCOO mechanism
According to studies on a revised formate pathway, HCOO* is hydrogenated to form HCOOH*, which is then hydrogenated to form H2COOH* and dissociates to form H2CO* and OH. H2CO* is then hydrogenated via intermediate CH3O* formation to form methanol.252,253 A similar r-HCOO mechanism was proposed by Behrens et al. The DFT calculation studies revealed that compared to PsCu3(111), the surface of PdCu (111) with an unsaturated Pd atom is more active for the adsorption and activation of CO2 and H2.2534.3 The RWGS + CO-hydro mechanism
In this mechanism, CO2 is transformed to CO via a COOH* intermediate, then CO is hydrogenated to produce methanol using intermediates HC, H2CO, and H3CO and CO is the byproduct.254–256 While HCO, H2CO, and H3CO intermediates directly contribute to the hydrogenation of methanol, CO can still be generated by dissociating CO2 without an intermediate. This mechanism has been suggested for Au-based systems such as Au/CeOx/TiO2.2574.4 The trans-COOH mechanism
Hydrocarboxyl COOH* is the first hydrogenated species in this molecular route. COOH is generated when CO2 and the H atom of H2O react.226,258–260 Methanol is formed when COOH* is further hydrogenated to COHOH*, which is then dissociated to COH* via intermediates such as HCOH and H2COH. This mechanism was proposed over the surface of Au/Cu–ZnO–Al2O3 and Ga3Ni5.261,262One of the major challenges in methanol synthesis from CO2 over heterogeneous catalysts is severe operating conditions including high pressure and temperature. Additionally, the thermodynamics and the continuous separation of methanol from CO2 and byproducts in the recirculating process constrain CO2 thermocatalytic hydrogenation. Various methods for CO2 reduction to methanol have been developed in recent years, including homogeneous catalysis, photocatalysis, and electrocatalysis. The benefits of the new technologies encompass lower temperatures compared to heterogeneous catalysis, the utilization of alternate energy sources (light or electricity), and the potential for enhanced methanol selectivity.
4.5 Electrocatalytic mechanism
Electrocatalysis is a process in which the catalyst plays a pivotal role by decreasing the activation energy of the reaction i.e. accelerating the reaction with faster electron transfer and it remains unchanged at the end. Typically, these electrocatalysts are heterogeneous catalysts as the reactions take place on the surface of the electrocatalyst. The process involves three major steps: (a) reactants adsorb onto the catalyst surface, (b) the chemical reaction takes place on the surface, and (c) the products desorb from the catalyst surface. To evaluate the catalytic activity of different electrode materials, researchers commonly compare the current density at a fixed overpotential or determine the overpotential required to achieve a specific current density. An efficient electrocatalyst demonstrates high current density at low overpotential, indicating both high activity and energy efficiency.263Electrocatalytic processes generally occur in cathodes and anodes of fuel cells and also in electrolysis devices. In the anode, fuel gets oxidized and at the cathode, the oxygen reduction reaction occurs, producing electrical energy. In the anode, various fuels can be used for oxidation purposes, such as methane, ammonia, alcohols, hydrogen and sugars. For the cathode, another alternative can be H2O2 as an oxidant. A single atom catalyst (SAC) represents the ultimate small size limit of metal particles, which is generally used as a catalyst with the help of a support such as metal oxides, metal surfaces or graphene. The SAC contains isolated atoms singly dispersed on a support. With well-defined dispersion on the support surface, it exhibits maximum efficiency and selectivity. Furthermore, the most efficient way to maximize the utilization of every metal atom in supported metal catalysts is to reduce the metal nanostructures to well-defined, atomically dispersed active centers, known as single-atom catalysts (SACs) (Fig. 7). This represents the ultimate achievement in fine dispersion. For example, Zhang et al. developed a practical platinum single-atom catalyst supported on iron oxide designated as Pt1/FeOx.264 Moreover, the preparation of SACs is very challenging as single atoms tend to get agglomerated. The SACs can be applied in different electrochemical applications, such as the hydrogen evolution reaction (HER), oxygen evolution reaction (OER), oxygen reduction reaction (ORR), nitrogen reduction reaction, and carbon dioxide reaction. The mechanisms of these reactions are discussed in detail in ref. 265.
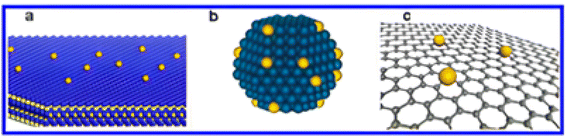 | ||
| Fig. 7 Schematic diagrams illustrating different types of SACs. Reprinted with permission from ref. 264. Copyright permission from 2013 American Chemical Society. | ||
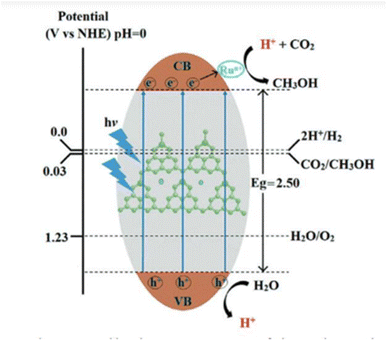
P. Sharma et al. reported a straightforward synthesis strategy for a single-atom ruthenium (Ru) catalyst integrated into a photoactive mesoporous C3N4 (mC3N4) support. Using the RuSA-mC3N4 catalyst under ambient conditions and visible light, efficient photocatalytic conversion of CO2 into methanol was achieved. The process yielded an impressive 1500 μmol g−1 of methanol within 6 hours, utilizing a low ruthenium loading of just 0.4 wt%.266 The image depicts the possible photocatalytic mechanism of the RuSA-mC3N4 catalyst under visible light irradiation during the conversion of CO2 to methanol. They have narrowed the band gap compared to other prepared materials which helps to generate more electrons upon visible light irradiation. The incorporated Ru single atom on the C3N4 network traps electrons from C3N4 which helps to sustain the photogenerated electron–hole pair for a longer time. As a result, easy electron transfer occurs to reduce CO2 which adsorbs on its surface and transforms into methanol (Fig. 8).
 | ||
| Fig. 8 A plausible schematic illustration of the photocatalysis pathway under visible light irradiation. Reprinted with permission from ref. 266. Copyright permission from 2021 Wiley-VCH GmbH. | ||
Here's an explanation of the process:
1. Photoexcitation (light absorption):
(a) When visible light is irradiated on the catalyst, the electrons present in the valence band of the mC3N4 photoactive support get excited.
(b) These excited electrons are promoted to the conduction band (CB), leaving behind holes (h+) in the VB of the same metal.
(c) In this particular case i.e. the RuSA-mC3N4 catalyst has a band gap (energy difference between the CB and VB) of approx. 2.50 eV, enabling the absorption of photons from visible light.
(2) Electron transfer and catalysis:
(a) After excitation of the electrons to the CB, the electrons are transferred to the Ru single atoms (Ru+) embedded in the mC3N4 matrix, also reducing the photocarrier transfer barrier. These Ru atoms act as active sites for catalytic reactions.
(b) The electrons reduce CO2, leading to the formation of methanol (CH3OH) and side products identified as CO and CH4 gases.
3. Hole utilization:
(a) The holes (h+) in the VB participate in the oxidation of water (H2O), producing oxygen (O2) and releasing additional protons (H+).
(b) This step regenerates protons necessary for the reduction process.
4. Reduction and oxidation reactions:
(a) On the CB side, the reduction reactions include: first, CO2 gets adsorbed on the surface of the catalyst, reduced to a COOH* intermediate, and then further reduced to CO. Later CO transformed into methanol after 6 steps, as given below:265
| 1e− + 1H+ + CO2 → *COOH |
| 1e− + 1H+ + *COOH → H2O + *CO |
| *CO + 1e− + H+ → *C–OH |
| *C–OH + 1e− + H+ → *CH–OH |
| *CH–OH + 1e− + H+ → *CH2–OH |
| *CH2–OH + 1e− + H+ → *OH–CH3 |
| *O–CH3 + 1e− + H+ → *OH–CH3 |
| *OH–CH3 → CH3–OH + * |
(b) On the VB side, oxidation reactions occur:
| ˙H2O → O2 + H+ + e− |
5. The catalytic journey of the CO2 hydrogenation reaction
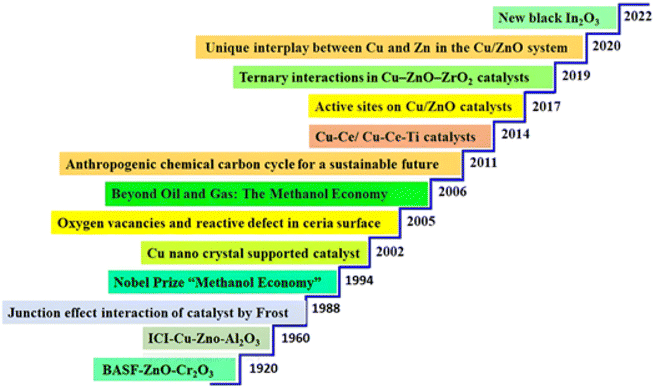
The review provides a strategic action plan to address the negative impacts of CO2 emissions on Earth's ecosystems. The strategy places an emphasis on reducing the use of fossil fuels and implementing catalytic technologies for CO2 conversion. Specifically, it focuses on the mixed and electrocatalytic transformation of carbon dioxide to methanol using noble metals. It goes into great detail about thermodynamics, problems, and techniques used in CO2 hydrogenation. We present a study of the historical development of catalysts and their industrial applications, emphasizing the novel approach towards achieving carbon neutrality. The transformation of carbon dioxide (CO2) into methanol is a subject of ongoing research and development in the field of catalytic science (Fig. 9). Over time, the discovery of numerous catalysts and the increasing understanding of their behavior and effectiveness have led to significant progress towards a sustainable future. The recognition of the BASF-ZnO-Cr2O3 compound as a catalyst for the reduction of carbon dioxide to methanol in 1920 marked a significant achievement in this area.267 The discovery of ICI–Cu–ZnO–Al2O3 as an effective catalyst for the same process in 1960 significantly expanded the potential of this conversion.267 The discovery that Frost made in 1988 on the “Junction Effect Interaction” of catalysts opened up new doors for the creation of catalysts that were more effective.268 The Nobel Prize for the “Methanol Economy” in 1994 highlighted the importance of converting carbon dioxide into methanol as a means of promoting sustainability. The year 1994 further solidified this concept. Significant progress in the conversion of carbon dioxide to methanol was made possible by the development of catalysts that were supported on nanocrystals of copper in 2002. Subsequently, researchers conducted an investigation into the correlation between oxygen vacancies and reactive flaws in ceria surfaces, leading to the development of even more effective catalysts.269 The publication of the book “Beyond Oil and Gas: The Methanol Economy” in 2006 heightened awareness of methanol's potential as an environmentally friendly alternative to fossil fuels.270In 2011, the idea of an “Anthropogenic Chemical Carbon Cycle for a Sustainable Future” was presented, with the primary focus being placed on the necessity of converting carbon dioxide into methanol in order to reduce greenhouse gas emissions.83 The creation of copper-ceria/copper-ceria-titania catalysts in 2014 was a significant step forward, as these catalysts demonstrated increased efficiency in the process of converting carbon dioxide to methanol.274 In 2017, active sites on Cu/ZnO catalysts were investigated, yielding significant knowledge on the mechanism of CO2 reduction to methanol.275 The catalyst support primarily scatters the active phase to prevent metal particles from sintering, while also ensuring thermal stability. Numerous experiments have demonstrated that the catalyst support influences the activity and selectivity of the methanol reaction. There are several types of carbon nanofibers (CNFs), including zirconia, silica, alumina, and carbon nanotubes (CNTs).276–285 In certain instances, a promoter may not yield the desired effect. Furthermore, it has the potential to influence the catalyst's efficiency by either promoting the intended reaction or minimizing the incidence of undesirable reactions. As a result, incorporating promoters has the potential to improve both the product's selectivity and the catalyst's activity. There are several researchers who have documented the utilization of promoters for CH3OH synthesis catalysts. Some examples of these promoters are gallium oxide (Ga2O3), zinc oxide (ZnO), niobium oxide (Nb2O5), and others.286–290 The year 2019 saw the discovery of ternary interactions in Cu–ZnO–ZrO2 catalysts, which extended the possibilities for catalyst development that are even more effective.291 The year 2020 saw the discovery of the one-of-a-kind interaction that occurs between copper and zinc in the Cu/ZnO system. This discovery opened up new possibilities for the creation of catalysts that are even more efficient.292 The most recent breakthrough in this area is the creation of a new black indium oxide catalyst in 2022. This catalyst facilitates the effective conversion of carbon dioxide into methanol, which serves as a source of energy and a chemical feedstock, and represents a significant step towards a more sustainable future.293 The constant process of transforming carbon dioxide into methanol through the application of catalytic technology, defined by countless discoveries and breakthroughs, has been a journey. These achievements are critical milestones in developing a sustainable future and highlight the potential of catalytic science in making the world a better place (Fig. 10).
 | ||
| Fig. 10 Some remarkable journeys of hydrogenation of CO2 to CH3OH using heterogeneous catalysts.83,225,268–273 | ||
5.1 Noble metal-based heterogeneous catalysts
In the mid-19th century, heterogeneous catalysts were utilized for the production of CH3OH from syngas, employing a Cu–ZnO based heterogeneous catalyst in this exothermic process.294,295 The Cu–ZnO catalyst exhibited significant deactivation and diminished activity with standard syngas, which improved in the late 1990s, followed by the introduction of noble metal-based heterogeneous catalysts that enhanced the hydrogenation conversion of CO2 to CH3OH. Compared with transition metal-based catalysts, noble metal-based catalysts generally have higher activity in the CO2 hydrogenation process. For successful CO2 hydrogenation, both CO2 and H2 should be activated simultaneously, and the noble metal nanoparticles are usually more active for H2 activation with the enhanced hydrogen spillover phenomenon than transition metal nanoparticles, hence noble metal-based catalysts usually outperform transition metal-based catalysts in CO2 hydrogenation. Furthermore, noble metal nanoparticles are more endurable than transition metal nanoparticles in terms of coke formation, which allows noble metal-based catalysts to have a longer catalytic lifetime. The details about the noble metal-based heterogeneous catalysts for CO2 to methanol conversion are discussed as follows.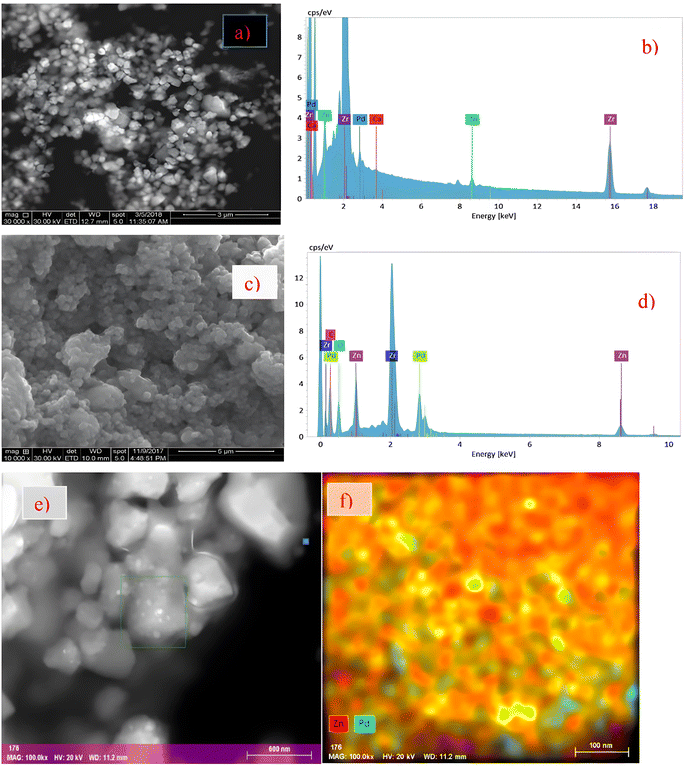 | ||
| Fig. 11 Electron microscopy and spectroscopy of spent catalyst 0.5Ca5P5ZZr-IMP. SEM image (a) and corresponding energy dispersive spectrum (EDS) (b) of 0.5Ca5P5ZZr-IMP, SEM image (c) and EDS (d) of 5P5ZZr-IMP. Surface mapping (e) and PdZn distribution (f) in 5P5ZZr-IMP.323 Copyright 2019 Elsevier. | ||
This group reported another study that elaborated on the connection between the size effect, surface alloy composition and catalytic efficiency. This work mainly focused on the manufacture of a Pd–Cu bimetallic catalyst; the ratio of Pd/(Pd + Cu) lies within 0.25–0.34 and is used for the hydrogenation of CO2 to produce CH3OH. The main observation is that Pd–Cu(2.4)/SiO2, which has a lower metal loading than a commercial catalyst, produces 2 times as much CH3OH at 523 K and 4.1 MPa concerning metal-based space-time yield (STY).328 Lin et al. conducted an investigation into the use of various supports, including TiO2, ZrO3, CeO3, Al2O3, and SiO2, for the Pd–Cu catalyst. The results indicated that TiO2 and ZrO2 showed the highest activity for methanol synthesis. The adsorption capacity for methanol synthesis is attributed to the influence of metal support interaction on the process, with the Pd–Cu catalysts facilitating the bonding between H2 and CO2 on the surface.329 The changes in metal–support interactions were primarily attributed to enhancing moderate interactions between the metal and support, which aimed to boost adsorption capacity and promote methanol synthesis. This study supported the idea that Pd–Cu catalysts play a significant role in shifting adsorption toward bonding between H2 and CO2 on the alloy surface.
Morgan and coworkers336 synthesized a nanodispersed intermetallic GaPd2/SiO2 catalyst; industrially relevant high-surface-area SiO2 is impregnated with Pd and Ga nitrates, then dried, calcined, and reduced in hydrogen (Fig. 12). The GaPd2/SiO2 catalyst outperforms the traditional Cu/ZnO/Al2O3 catalyst for CO2 hydrogenation to methanol at ambient pressure. Additionally, it produces less unwanted CO. The GaPd2/SiO2 catalyst is investigated using both in situ and ex situ approaches (Fig. 13).
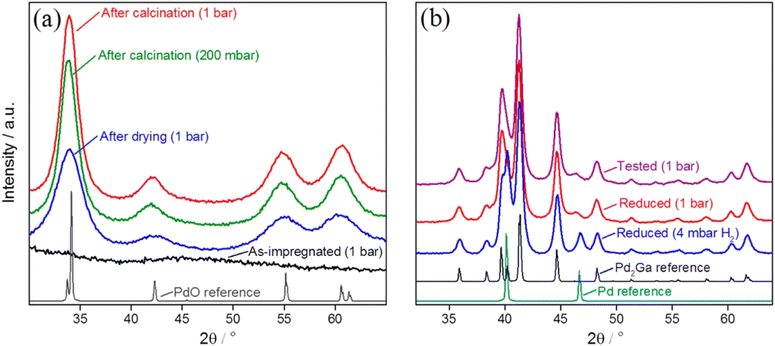 | ||
| Fig. 12 (a) XRD patterns obtained from the impregnated, dried, and calcined catalyst precursor at room temperature and atmospheric pressure, and (b) from the reduced and tested GaPd2/SiO2 catalyst for CO2 hydrogenation. Reprinted with permission from ref. 333. Copyright 2015 American Chemical Society. | ||
 | ||
| Fig. 13 IL-TEM images of the GaPd2/SiO2 catalyst acquired after drying (a), calcination (b), reduction (c), and methanol synthesis (d).333 Copyright 2015 American Chemical Society. | ||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0, 2
0, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, and 0
2, and 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and enhanced the methanol synthesis process from CO2/H2 at 40 bar and 300 °C. The highest methanol selectivity of 61% was achieved using the In
1) and enhanced the methanol synthesis process from CO2/H2 at 40 bar and 300 °C. The highest methanol selectivity of 61% was achieved using the In![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Pd (2
Pd (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)/SiO2 catalyst. In situ XAS characterization technique revealed that the synergistic effect of the intermetallic compound indium–palladium enhances the selectivity and activity of methanol synthesis.338 Liu et al. synthesized Pd/In2O3 catalysts by thermal treatment of In2O3 powder with a Pd/peptide composite. The Pd/In2O3 catalyst showed higher activity of methanol synthesis from CO2 conversion i.e. >20% and methanol selectivity of 70%.339 Jeschke and coworkers developed the catalyst Pd–In2O3–ZrO2 by flame spray pyrolysis methods for the conversion of CO2 to methanol. Its high and persistent methanol productivity can be explained by the improved formation of oxygen vacancies revealed by this particular catalyst architecture, according to a ground-breaking procedure designed to quantify them using in situ electron paramagnetic resonance spectroscopy.340 Recently, Cai et al. have reported that hollow In2O3 supported Pd based catalysts are suitable for CO2 hydrogenation to methanol. The main finding of this work is that the hollow morphology of In2O3 supported Pd based catalysts led to higher catalytic performance (10.5% CO2 conversion, a methanol selectivity of 72.4% and a methanol space time of 0.53 gMeOH h−1 gcat−1 over 100 h on stream at 3 MPa and 295 °C) compared to In2O3 nanotube or nanorod morphology.341 Jiang and coworkers reported the use of an In2O3/SBA-15-supported Pd-based catalyst and observed a CO2 conversion rate of 12.6% with 83.9% methanol selectivity.342 Hua and coworkers developed the Pd/In2O3 nanocatalyst by employing innovative electrostatic self-assembly methods. This catalyst exhibited an increase in the concentration of oxygen vacancies which enhanced the methanol production. This catalyst led to a space time yield (STY) of 0.54 gMeOH h−1 gcat−1 (300 °C, 3.5 MPa, 21
1)/SiO2 catalyst. In situ XAS characterization technique revealed that the synergistic effect of the intermetallic compound indium–palladium enhances the selectivity and activity of methanol synthesis.338 Liu et al. synthesized Pd/In2O3 catalysts by thermal treatment of In2O3 powder with a Pd/peptide composite. The Pd/In2O3 catalyst showed higher activity of methanol synthesis from CO2 conversion i.e. >20% and methanol selectivity of 70%.339 Jeschke and coworkers developed the catalyst Pd–In2O3–ZrO2 by flame spray pyrolysis methods for the conversion of CO2 to methanol. Its high and persistent methanol productivity can be explained by the improved formation of oxygen vacancies revealed by this particular catalyst architecture, according to a ground-breaking procedure designed to quantify them using in situ electron paramagnetic resonance spectroscopy.340 Recently, Cai et al. have reported that hollow In2O3 supported Pd based catalysts are suitable for CO2 hydrogenation to methanol. The main finding of this work is that the hollow morphology of In2O3 supported Pd based catalysts led to higher catalytic performance (10.5% CO2 conversion, a methanol selectivity of 72.4% and a methanol space time of 0.53 gMeOH h−1 gcat−1 over 100 h on stream at 3 MPa and 295 °C) compared to In2O3 nanotube or nanorod morphology.341 Jiang and coworkers reported the use of an In2O3/SBA-15-supported Pd-based catalyst and observed a CO2 conversion rate of 12.6% with 83.9% methanol selectivity.342 Hua and coworkers developed the Pd/In2O3 nanocatalyst by employing innovative electrostatic self-assembly methods. This catalyst exhibited an increase in the concentration of oxygen vacancies which enhanced the methanol production. This catalyst led to a space time yield (STY) of 0.54 gMeOH h−1 gcat−1 (300 °C, 3.5 MPa, 21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 mL gcat−1 h−1).343
000 mL gcat−1 h−1).343
The CNT-supported Pd-based catalyst shows high catalytic activity for methanol synthesis. Zhang and coworkers synthesized Pd–ZnO supported multi-walled carbon nanotube-based catalysts showing a high rate of catalytic conversion of methanol from CO2. A large amount of hydrogen is reversibly adsorbed using CNT-supported Pd–ZnO catalysts, which boosts the concentration of active H-species at the surface of the working catalyst and accelerates the kinetics of the hydrogenation reaction.347 Kong and co-workers developed a MWCNT supported Pd–Ga catalyst for the synthesis of methanol by hydrogenation. This catalyst improved the molar percentage of catalytic activity of Pd0 species and also increased the catalytic activity of adsorbing/activating H2.348 Li et al. investigated the Pd nanoparticle supported inside and outside CNT-based catalysts. Higher catalytic activity and methanol selectivity are displayed inside the Pd/CNTs than outside. The turnover frequency of Pd/CNTs (inside) is 3.7 times higher than that of outside-supported CNTs. The selectivity difference between inside and outside CNT-based catalysts is due to the relative concentration of Pd+ species.349
Pd metal actively influences the chemical and electronic properties of the catalyst surface. Furthermore, it is impossible to overstate the importance of Pd's role in converting H2 into H-add species and permitting them to overflow onto support surfaces because this is still an essential step in the synthesis of methanol, without which hydrogenation cannot proceed properly. The Pd precursor employed for the synthesis of the catalyst will also affect its overall performance.
The promoters on a Pd surface enhance the CO2 adsorption capacity by generating an electrostatic field. Cabrera et al. observed that Pd itself acts as a promoter which increases the catalytic activity of Cu + ZnO (Al2O3) and improves the rate of hydrogenation of CO2 due to the hydrogen dissociated on the surface of Pd particles and spillover on the surface of the Cu + ZnO catalyst. In another remarkable study established by Lee and co-workers, the catalytic activity of Cu/CeO2 was increased by the addition of a small amount of Pd. A small amount of Pd is added to the Cu/CeO2 catalyst to facilitate the reactivity of Cu sites, which in turn increases methanol productivity. The interaction with highly dispersed Pd increases the surface and dispersion concentration of Cu. The transfer of electrons from palladium to copper leads to a reduction in copper sites, while the addition of palladium as a promoter diminishes the surface area of cerium dioxide. So, it can be concluded that the rate of hydrogenation of CO2 and methanol productivity can be increased with the addition of a small amount of Pd to the Ce/CeO2 catalyst.350 Hu et al. reported Pd promoted Cu–ZnO catalysts for the synthesis of a series of Pd doped Cu–ZnO catalysts with a constant Cu/Zn ratio (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and varied Pd molar ratios from 0.01 to 0.04 mol%. It was observed that the 1% Pd doped Cu–ZnO catalyst promotes the methanol space-time yield by 2.5 times and turnover frequency (TOF) by 3.5 times at 543 K as compared to the undoped Cu–ZnO catalyst. Tsang et al. investigated the effect of the promoter Pd on the Pd/ZnO/Al2O3 catalyst. The result indicated that methanol selectivity depends on the ratio of Pd0/PdZn and the EXAFS study showed that the Pd-modified ZnOx possesses an active site for methanol synthesis.305 Koizumi et al. investigated the incorporation of Pd nanoparticles into mesoporous supports such as MCM-41, and SBA-15 with promotion using alkali/alkaline earth metals synthesized by incipient wetness impregnation. It was observed that without impregnation of any promoters, Pd-supported mesoporous silica showed weak activities towards methanol synthesis, but the addition of alkali or alkaline earth metals such as K, Mg, and Ca increased the catalytic activities of K(Ca) promoted Pd-supported mesoporous SBA-15 producing two times higher methanol yield than that of Pd-supported mesoporous silica. Song and co-workers synthesized Pd supported catalysts by the impregnation method with Pd(NO3)2. CaO promoted the CO2 hydrogenation activity with the highest selectivity for methanol over Pd/MCM-41.344 Yin and co-workers reported a metal–organic framework-based PdZnO catalyst from the Pd@zeolitic imidazolate framework (ZIF-8) for hydrogenation of CO2. The formation of small-sized PdZn alloy particles after H2 reduction and the abundance of surface oxygen defects on ZnO are crucial for such a high methanol production. The SMSI between PdZn and ZnO also ensures the long-term stability of the PdZn catalysts. Lastly, it can be proposed that the active site strongly associated with methanol formation is the PdZn alloy rather than metallic Pd.351
1) and varied Pd molar ratios from 0.01 to 0.04 mol%. It was observed that the 1% Pd doped Cu–ZnO catalyst promotes the methanol space-time yield by 2.5 times and turnover frequency (TOF) by 3.5 times at 543 K as compared to the undoped Cu–ZnO catalyst. Tsang et al. investigated the effect of the promoter Pd on the Pd/ZnO/Al2O3 catalyst. The result indicated that methanol selectivity depends on the ratio of Pd0/PdZn and the EXAFS study showed that the Pd-modified ZnOx possesses an active site for methanol synthesis.305 Koizumi et al. investigated the incorporation of Pd nanoparticles into mesoporous supports such as MCM-41, and SBA-15 with promotion using alkali/alkaline earth metals synthesized by incipient wetness impregnation. It was observed that without impregnation of any promoters, Pd-supported mesoporous silica showed weak activities towards methanol synthesis, but the addition of alkali or alkaline earth metals such as K, Mg, and Ca increased the catalytic activities of K(Ca) promoted Pd-supported mesoporous SBA-15 producing two times higher methanol yield than that of Pd-supported mesoporous silica. Song and co-workers synthesized Pd supported catalysts by the impregnation method with Pd(NO3)2. CaO promoted the CO2 hydrogenation activity with the highest selectivity for methanol over Pd/MCM-41.344 Yin and co-workers reported a metal–organic framework-based PdZnO catalyst from the Pd@zeolitic imidazolate framework (ZIF-8) for hydrogenation of CO2. The formation of small-sized PdZn alloy particles after H2 reduction and the abundance of surface oxygen defects on ZnO are crucial for such a high methanol production. The SMSI between PdZn and ZnO also ensures the long-term stability of the PdZn catalysts. Lastly, it can be proposed that the active site strongly associated with methanol formation is the PdZn alloy rather than metallic Pd.351
(i) Au based catalysts
Researchers have discovered that gold nanoparticles act as highly active catalysts for CO2 hydrogenation. Vourros et al. successfully investigated the activity of Au nanoparticles supported on various oxides (MxOY – Al2O3, TiO2, Fe2O3, CeO2, and ZnO) to catalyze the synthesis of methanol from the hydrogenation of CO2. Au nanoparticles based on Au/CeO2 (82%) and Au/ZnO (90%) show high selectivity towards CH3OH synthesis at temperature below 250 °C.352 Behm and co-workers synthesized metal oxide-supported Au catalysts (Au/Al2O3, Au/TiO2/Au/ZnO, and Au/ZrO2) for the synthesis of ‘green methanol’ from CO2. The Au/ZnO catalyst is one of the potential candidates for green methanol technology. With an increase in the diameter of Au nanoparticles in the Au/ZnO catalyst, the rate of methanol formation reduces and the selectivity of methanol increases from 56% to approximately 82%.353 This group also reported better catalytic behaviour of the Au/ZnO-based catalyst for the synthesis of methanol than the conventional Cu–Zn–Al catalyst. From the kinetic and IR spectrophotometer studies, it is revealed that the conversion of CO2 to methanol proceeds through the formation of formate and methoxy intermediates initiated using the proposed Au/ZnO catalyst.354 Han et al. reported an Au-supported ZrO2 catalyst for methanol synthesis with a selectivity rate of 73% at 180 °C.355 The electrical polarization at the metal oxide CeOx/TiO2 anchored with Au nanoparticle-based substrates, as proposed by Chen et al., actively promoted CO2 adsorption under low pressure conditions and led to greater selectivity of methanol production. According to the AP-XPS study, Au possesses a partial negative charge and CeOx shows a Ce3+ state. The DFT calculation studies supported the substantial charge redistribution occurring at Au3/CeOx/TiO2(110), with Au atoms becoming negatively charged, and negatively charged Au and the positively charged Ce3+ site bind to the positively charged C atom and the negatively charged O atom of CO2, respectively.356 Rodriguez and coworkers investigated the catalytic properties of In/Au(111) alloys and InOx/Au(111) inverse systems for CO2 hydrogenation by employing AP-XPS studies and batch reactors. The experimental evidence shows that InOx/Au(111) demonstrates significant potential for CO2 hydrogenation combining high activity and selectivity for methanol production.357
(ii) Ag-based catalysts
Ag-based catalysts received less attention than Pd and Au-supported catalysts for the hydrogenation of CO2.358 Grabowski et al. investigated the catalytic activity of Ag/ZrO2 and Ag/ZrO2/ZnO catalysts. The XPS and Auger spectroscopic techniques confirmed the electronic state of silver and the contents of the zirconia polymorphic phases (tetragonal and monoclinic). The presence of oxygen vacancies in ZrO2 directly influenced the thermodynamically unstable t-ZrO2 and Ag+ species connected with oxygen vacancies thereby accelerating the active phase for methanol synthesis.359
(iii) Pt/Rh based catalysts
In heterogeneous reactions, dispersed catalysts have a remarkable place due to the low coordination environment of the metal center, quantum size, and SMSI effect.360 Zheng et al. prepared atomically dispersed Pt/MoS2 catalysts with Pt amounts up to 7.5% and reported that the synergetic interaction between neighboring Pt monomers reduces the activation energy and enhances the catalytic activity relative to isolated Pt monomers in CO2 hydrogenation. Unlike isolated Pt monomers, neighboring Pt monomers hydrogenate CO2 sequentially into formic acid and methanol.361 Song et al. investigated a PtCo alloy-based catalyst for methanol synthesis where zigzag Pt–Co nanowires with a Pt-rich surface and abundant step/edges (defects) were utilized to obtain high catalytic activity. Pt4Co NWs/C showed the best performance among other PtCo alloys.303 Analysis using Fourier transform infrared spectroscopy demonstrated that when Pt4Co NWs/C was used as a catalyst, the process involved CO2 adsorption and activation via a carboxylate intermediate, resulting in enhanced CH3OH yield. Shimizu et al. investigated a Pt nanoparticle loaded MoOx/TiO2 catalyst for the hydrogenation of CO2 to produce CH3OH in 73% yield under mild conditions. The X-ray absorption fine structure study suggested that reducing the MoOx species promoted the hydrogenation reaction.362 Han et al. investigated that the methanol selectivity of In2O3 increased (72.2% to 91.1%) by introducing a small amount of Pt.363 Zeng and co-workers reported that the RhW alloy with a nanosheet structure acts as a catalyst for the synthesis of methanol by hydrogenation of CO2. The 1D quantum confinement effect leads to a larger d band level in the RhW alloy-based nanosheet, while an electronic effect associated with the alloy mechanism results in a negatively charged Rh surface (Table 1).364
| Catalyst | CO2/H2 ratio | Temp./K | MPa | Space velocity | Conv. | Selectivity | STY | Ref. |
|---|---|---|---|---|---|---|---|---|
| Pd/ZnO | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
523 K | 2 | 3600(W) | 10.7 | 60 | 77.4 | 320 |
| PdZn-400 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
543 K | 4.5 | 21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600(W) 600(W) |
15.1 | 56.2 | 650 | 365 |
| Pd/Ga2O3 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
523 K | 5 | — | 19.6 | 51.5 | c.a 20.8 | 334 |
| Pd–In2O3 (Co-precipitation) | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
553 K | 5 | 24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
75 | 0.061 | 366 | |
| Pd(0.25–Cu/SiO2 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
250 °C | 41 | 3600 | 6.7 | 30 | 0.03 | 367 |
| Pd/SiO2 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
523 K | 2 | — | 0.33 | 31.6 | 0.013 | 349 |
| Pd/Cu–P25 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
523 K | 4.1 | — | 16.4 | 25.7 | 1.80 | 368 |
| Pd/In2O3 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
300 °C | 50 | 21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
20 | 72 | 0.89 | 369 |
| Pd–Pt/In2O3 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
300 °C | 5 | 21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
17.7 | 68.3 | 0.725 | 370 |
| PdZn/ZnO-3.93Al | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
523 K | 3.0 | 14.2 | 51.6 | c.a 4.51 | 371 | |
| Pd/Zn SiO2 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
260 °C | 3 | 2.6 | 11.2 | 0.03 | 372 | |
| 2Pd/CeO2−R | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
240 | 3 | 2000 | 6.2 | 29 | 0.012 | 373 |
| 5% Pd/ZnO, IM | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
523 K | 2.0 | — | 8.7 | 1 | 0.055 | 320 |
| 0.5Ca5Pd5ZnZr | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
523 K | 3.0 | 2400 | 14.2 | 51.6 | c.a 4.51 | 374 |
| Pd–Cu/SBA-15 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
250 °C | 4.1 | 3600(W) | 6.5 | 23 | 23 | 375 |
| Pd/Zn/CNTs | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
250 °C | 3 | 1800(W) | 6.3 | 99.6 | 37.1 | 376 |
| Pd Cu/CeO2 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
523 K | 4.1 | — | 9.9 | 28.4 | 1.37 | 368 |
| Pd/TiO2 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
523 K | 5 | — | 15.5 | 3.9 | c.a. 1.21 | 377 |
| Pd–P/In2O3 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
498 K | 5 | Ca 3 | Ca 95 | 6.01 | 339 | |
| Pd/In2O3/SBA-15 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
533 K | 5 | 12.6 | 83.9 | 11 | 314 | |
| Pd–ZnZrOx | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
320 °C | 5 | 24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
16 | 58 | 0.63 | 378 |
| Pt/In2O3 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
300 °C | 5 | 21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
17.6 | 54 | 0.542 | 379 |
| Pt/In2O3 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
300 °C | 4 | 24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
8.8 | 71.5 | 0.480 | 380 |
| Rh/In2O3–ZrO2 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
300 | 5 | 21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
18.1 | 66.5 | 0.684 | 381 |
| UiO-67-Pt | 170 | 0.8 | 6000 | 1.5 | 19.0 | 0.0024 | 382 | |
| Au/ZnO | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
240 °C | 0.5 | 4800(G) | 0.3 | 82 | N/A | 383 |
| Ag/ZrO2 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
240 | 80 | 3600 | 55 | 359 | ||
| Ag/In2O3 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
300 °C | 5 | 21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
13.6 | 58.2 | 0.450 | 384 |
| Au/In2O3–ZrO2 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
250 | 50 | 21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000(G) 000(G) |
5.0 | 90 | 0.59 | 385 |
| Au/Al2O3 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
220 | 5 | 24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000(W) 000(W) |
2.0 | 3.8 | 383 | |
| Au/CeO2 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
240 | 5 | 9000 | 1–2 | 40 | 0.412 | 386 |
| Au/In2O3 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
300 | 5 | 21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
11.7 | 67.8 | 0.47 | 387 |
Electrochemical catalytic conversion of CO2 to methanol using precious metal catalysts is another interesting approach to CO2 reduction that can be made through the electrochemical pathway. In this regard, several electrocatalysts such as CuAu, CuPd, and AgZn were found to be excellent candidates for enhancing the CO, CH4, and CH3OH productions, respectively. In addition, palladium-based electrocatalysts have not received much attention despite Pd's exceptional ability to show low overpotentials for the electrochemical reduction of CO2. In this case, when Pd is supported on carbon (Pd/C), the C added to metals improves their different properties by making them more active because carbon materials are porous. Additionally, we can use C materials with a high surface area and good electrical conductivity to stabilize high metal loadings and enhance the electron transfer rate in both directions (from and to) of metal catalyst sites.
Similarly, Pd/C started producing formate at 0.00 V vs. RHE, with hydrogen being the only by-product (0.00 to −0.20 V vs. RHE).388 So, the unwanted hydrogen evolution happens at the same time at Pd (supported on C) electrodes, along with the electrocatalytic reduction and hydrogenation of CO2 to formate. However, the combination of Pd with Zn led to the formation of formate species that remain adsorbed on the PdZn alloy surface for long enough to be converted into CH3OH. Another interesting study on Pd-based materials was reported by Han et al. Herein, the Pd combined with SnO2, forming plentiful Pd–O–Sn interfaces, undergoes four-electron transfer mechanisms for selective CO2 reduction to methanol.389 This excellent design helped to achieve a faradaic efficiency (FE) of 54.8% ± 2% for CH3OH at −0.24 V vs. RHE, and was able to deliver higher energy density as compared to sole Pd nanosheets (NSs) and SnO2 nanoparticles. They also reported that the current densities of Pd/SnO2 NSs didn't change much, and a high FE of 54% retains over 24 hours, indicating that it is more stable. This structure not only facilitated CO2 adsorption on the SnO2 surface, but also made it difficult for CO to remain on Pd due to the Pd–O–Sn interface weakening the binding strength of CO to Pd. In turn, enhancing Pd catalysts' CO2RR performance is crucial in such a circumstance. Different types of electrochemical studies performed are given in Fig. 14.
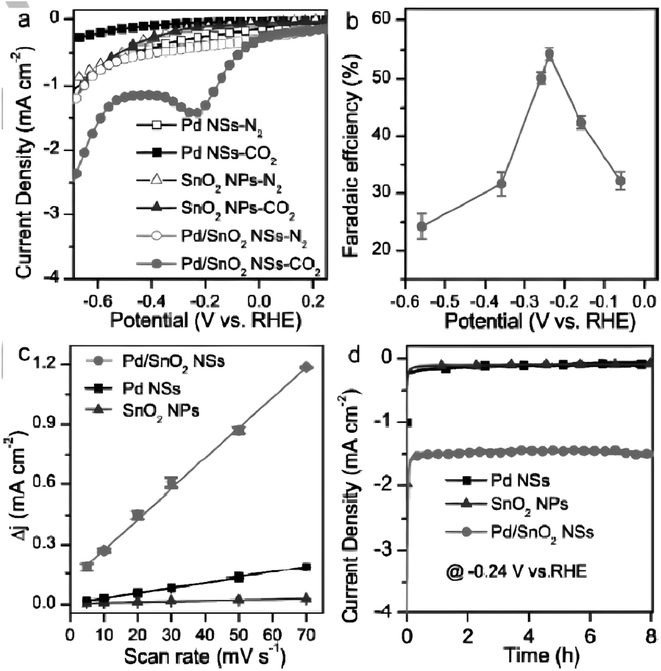 | ||
Fig. 14 Electrochemical characterization studies of Pd/SnO2 NSs (Pd![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Sn = 1 Sn = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) in comparison with Pd NSs and SnO2 NPs. (a) LSV curves obtained in 0.1 M NaHCO3 solution. (b) FE for CH3OH at various applied potentials. (c) Charging current density differences plotted against scan rates. (d) Chronoamperometry studies of different catalysts. Reprinted with permission from ref. 390. Copyright permission from 2018 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim. 1) in comparison with Pd NSs and SnO2 NPs. (a) LSV curves obtained in 0.1 M NaHCO3 solution. (b) FE for CH3OH at various applied potentials. (c) Charging current density differences plotted against scan rates. (d) Chronoamperometry studies of different catalysts. Reprinted with permission from ref. 390. Copyright permission from 2018 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim. | ||
Another excellent study was carried out by Buxing Han et al.;389 they developed a PdxCuy aerogel electrocatalyst and investigated it in a CO2-saturated aqueous ionic liquid, 1-butyl-3-methylimidazolium tetrafluoroborate ([BmimBF4) electrolyte containing 25 mol% [Bmim]BF4 and 75 mol% water via a H-type cell. The characterization studies of that sample were carried out by SEM, TEM, HRTEM, SAED and XRD and the results are given in Fig. 15.
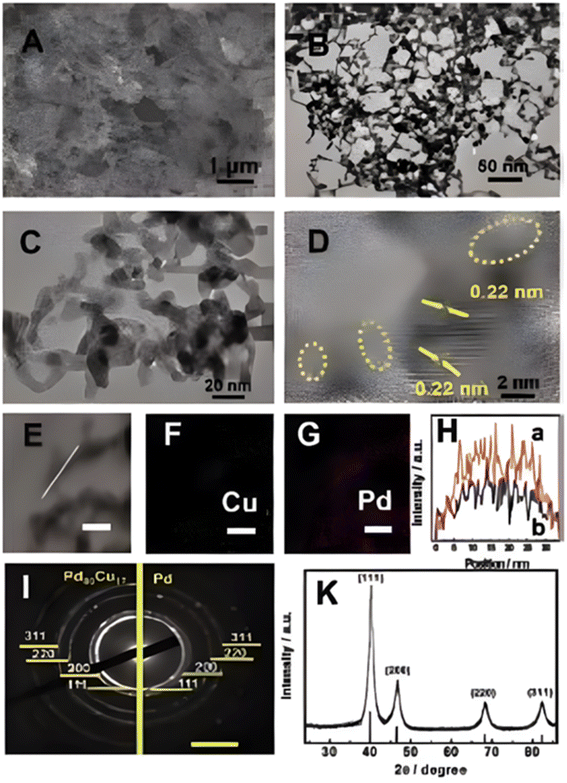 | ||
| Fig. 15 (A) SEM, (B) and (C) TEM, (D) HR-TEM, and (E)–(G) EDX mapping images (scale bar: 20 nm). (H) Distribution of Pd (a) and Cu (b) in Pd83Cu17 by line-scan analysis. (I) SAED pattern of the Pd83Cu17 and pure Pd (scale bar: 10 1 nm@1). (K) XRD pattern of the Pd83Cu17 aerogel. Reprinted with permission from ref. 389 Copyright permission from 2018 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim. | ||
They achieved the highest Brunauer–Emmett–Teller (BET) surface area of 76.9 m2 g−1 for Pd67Cu33 aerogels. This aerogel was capable of delivering FE efficiency for CH3OH within the range of −1.9 V to −2.4 V (vs. Ag/Ag+). It has been found that the proportion of Cu present in the aerogel also played a crucial role in the FE of CH3OH. In a [Bmim]BF4 aqueous solution with 25 mol% [Bmim]BF4 and 75 mol% water, the FE for CH3OH production for Pd83Cu17 is highest (80.0%) among others at −2.1 V (vs. Ag/Ag+) after 5 hours of electrolysis. For this material, the results of the electrochemical characterization studies are given in Fig. 16.
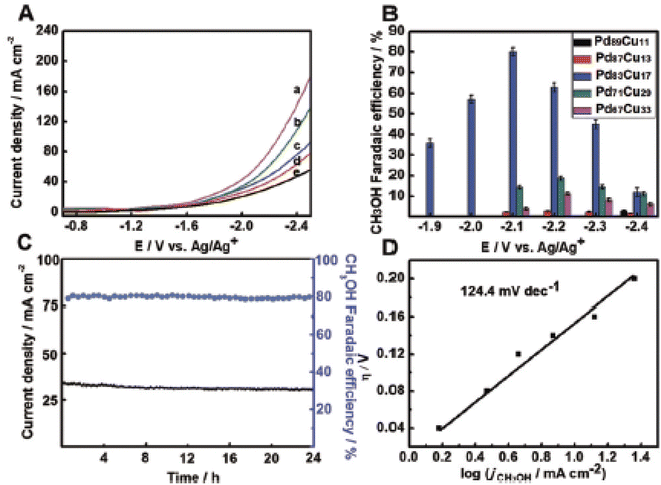 | ||
| Fig. 16 The as performed CO2 electroreduction reactions in ionic liquid [Bmim]BF4 aqueous solution combined with 25 mol% [Bmim]BF4 and 75 mol% water. (A) Different LSV curves for PdxCuy aerogels are given such as: (a) Pd67Cu33, (b) Pd71Cu29, (c) Pd83Cu17, (d) Pd87Cu13, and (e) Pd89Cu11. (B) The FE for CH3OH over PdxCuy aerogels. (C) Current density and FE over Pd83Cu17 aerogel electrodes at @2.1 V vs. Ag/Ag+. (D) Tafel plots for CH3OH production over Pd83Cu17 aerogel electrodes. Reprinted with permission from ref. 389. Copyright permission from 2018 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim. | ||
Unlike Pd, the intermediate cannot be stabilized long enough for the reverse water-gas shift (RWGS) reaction to proceed without the intermediate first being decomposed to give CO and H2O.320
| CO2 + H2 ⇌ CO + H2O |
| CO2 + 3H2 → CH3OH + H2O, ΔH298 = −49.5 kJ mol−1; ΔG298=3.30 kJ mol−1 |
| CO2+H2 → CO + H2O, ΔH298 = 41.2 kJ mol−1; ΔG298 = 28.6 kJ mol−1 |
| CO2+2H2 → CH3H3, ΔH298 = −91 kJ mol−1; ΔG298 = 25.34 kJ mol−1 |
The water formation at the 2nd step inhibits the methanol synthesis through a catalytic way. Hence, the design of materials is important in which the metal's tolerance towards the water is essential. Brisard et al., using differential electrochemical mass spectrometry, studied the electroreduction of carbon dioxide at a Pt porous electrode.391 With their findings, it was demonstrated that a Pt electrodeposited electrode could be used to produce CH3OH in the presence of acidic media. These findings further indicated that CO was formed as an intermediate leading to the production of CH3OH. As part of efforts to develop a reversible fuel cell based on CH3OH, Shironita et al. recently studied the electroreduction of CO2 on the carbon-supported Pt (Pt/C) and Pt–Ru (Pt–Ru/C) electrodes through a single cell. Faradaic efficiency estimates for the electroreduction of CO2 range from 30–50%. They concluded that the reduction current decreases over time because of the accumulation of CH3OH on the reaction active sites of the electrocatalyst (Pt/C). This is the reason the efficiency of yielding CH3OH went from 0.03% at Pt/C compared to 7.5% in the case of Pt–Ru/C. Also, cyclic voltammetry measurements showed that the faradaic efficiencies of Pt/C and Pt–Ru/C were 35% and 75%, respectively. In addition to having a significant impact on the selectivity, reduction mechanisms, and efficiency of the CO2 electroreduction process, the metal surface was also affected by a variety of other factors. In fact, it has been discovered that changing supporting electrolytes and operating circumstances has a significant impact on current density, product selectivity, and energy efficiency in CO2 reduction even for the same metal electrode with the same purity.392
The binding energy of the reaction intermediates on the catalyst surface affects both the catalyst's reaction activity and selectivity. During the electrochemical reduction of CO2, a complex reaction occurs that involves multi-electron transfers and a diverse array of intermediates and products, hence it is necessary to regulate the adsorption energy of the various intermediate species that are produced. In order to modify the binding energy of intermediates for desired end products, it is rational to create specialized surface structures.
6. Challenges and possibilities of CO2 conversion
A diverse range of problems, along with intriguing prospects, exists for the conversion of CO2 into frequently utilized formal products. Despite CO2 being a plentiful and inexpensive source, its chemical inertness frequently makes conversion procedures energy-intensive and economically impractical at large scales. Recent advancements in catalysis, electrochemistry, and process engineering are generating new opportunities for effective CO2 usage for methanol production (Fig. 17). | ||
| Fig. 17 Different Challenges and possibilities of CO2 to methanol conversion (with various catalysts used). | ||
(i) CO2 is a linear, kinetically inert, nonpolar molecule with two opposite reactive sites, i.e., carbonyl carbon has an electron-donating nature, and oxygen is an electron withdrawing species. Therefore, breaking the two double bonds present in the inert CO2 molecule requires a higher energy, which poses the main challenge for the reduction of CO2 to methanol.257 The conversion of CO2 to CO or other value-added products is facilitated using the metal surface catalysts.258
The methanol synthesis from CO2 generates water as a byproduct, which directly influences the rapid sintering and deactivation of the Cu/ZnO catalyst. As a result, if the RWGS reaction occurs at a high temperature, it continues in an endothermic mode, and if it occurs at a lower temperature, it reduces the catalytic activity, which can be considered the main drawback of CO2.69,259,260
(ii) Intermediate stabilization
In methanol synthesis from the hydrogenation of CO2, controlling the intermediate stabilization is another critical challenge. CO is a crucial intermediate in the catalytic hydrogenation of methanol synthesis. From a thermodynamic perspective, the bonding between CO and the catalyst surface develops a stronger binding energy that increases the reaction rate of methanol or any other hydrocarbon synthesis process, while the bonding between the catalyst surface and CO initiates the RWGS reaction with weaker binding energy.261 For methanol synthesis, controlling the stabilization of the intermediate, i.e., CO, is crucial for increasing the yields.123 DFT calculations and studies reveal that multiple factors influence catalytic properties. These include the key intermediate, oxygen adsorption energy, the relationship between catalyst structure and properties, and CO2 adsorption phenomena. This understanding provides a novel approach for catalyst development.393
(iii) Inhibition of hydrogenation by water
The production of large amounts of water during the reduction of CO2 by H2 reduces the catalytic activity of the catalyst through the formation of hydroxyl. The main challenge of researchers is to develop a hydrothermal catalyst that resists catalyst poisoning. However, some research groups improved the catalytic activity of methanol production from CO2 by using promising noble metals, primarily Pd, Pt-based microporous SiO2-supported catalysts, and carbon shell-supported catalysts.394,395
(iv) Development of economically favorable catalysts
One of the challenges researchers face is developing a low-cost, economically advantageous catalyst with good recycling and reducible qualities that would produce large amounts of methanol from the CO2 hydrogenation process.396 Recently, some research groups developed a Mo2C catalyst; however, this catalyst's main disadvantage is coke formation because, during the reaction, it binds firmly to intermediate hydrocarbons.397
(v) CO2 reduction by alkanes
Researchers are trying to develop an alternative path for the reduction of CO2. An alternate substance that can be used to reduce CO2 might be light alkanes.398 Although methane dry reforming can be employed as an alternative to hydrogen gas, researchers have observed that the catalyst rapidly loses effectiveness when exposed to elevated temperatures. But in the case of ethane, thermodynamically favorable conditions are available for CO2 hydrogenation. In the near future, one of the hotspot research topics for energy engineering would be CO2 reduction via light alkanes.389
7. Conclusion & future perspectives
This review emphasizes the use of noble metal-based catalysts for the hydrogenation of CO2 to produce methanol. It is evident that several aspects of catalysts are important including promoters, support types, synthetic procedures, reaction circumstances, large surface area, and particle size, all of which must be taken into consideration to design catalysts to achieve higher performance.The “Methanol Economy” offers a promising strategy for addressing global warming by utilizing CO2 as a renewable energy resource. Methanol can serve as an energy storage medium, hydrocarbon feedstock, and alternative fuel. Various catalysts, including those based on noble metals, have been developed for the hydrogenation of methanol from CO2. The conversion of CO2 to methanol using heterogeneous and electrochemical catalysts holds industrial promise but faces significant challenges. Heterogeneous catalysts, such as noble metal-based systems, are advantageous due to ease of separation and recyclability but are hindered by mass transport and diffusion limitations. Electrochemical catalysts offer high selectivity and efficiency but require precise control of operating conditions and are sensitive to electrode materials and electrolytes. Nanostructured catalysts can enhance surface area and tunability, improving reaction rates and stability. However, scaling these technologies from lab to industry involves challenges in reactor design, mass transport, and heat management, essential for commercial viability. Addressing these issues is crucial for the success of CO2 to methanol conversion technologies. The procedure's efficacy depends on understanding fundamental chemistry, catalyst characterization, and reactor characteristics. Researchers aim to develop dual-function promoters to improve CO2 to CH3OH conversion and reduce CO via the RWGS reaction. While noble metal-based catalysts show exceptional efficacy, their limited activity and high cost make them economically impractical. To commercialize CO2-derived methanol synthesis, researchers need to integrate multiple elements into a single material or process. This may involve creating innovative materials that combine the catalytic abilities of noble metals with more abundant and cost-effective components, enhancing efficiency and reducing costs. Additionally, improving reactor designs to increase CO2–catalyst interaction could further boost conversion rates and overall feasibility.
The hydrogenation of CO2 to methanol, despite its limitations, offers promising pathways for sustainable fuel production. Although progress has been made in understanding reaction mechanisms and catalyst design, critical areas still require further research. This study establishes a foundation for sustainable fuel production methods, potentially reducing fossil fuel dependence. By identifying key areas for future research, it paves the way for advances in CO2 utilization and catalyst design, which could significantly impact climate change mitigation and the shift to sustainable energy. Priority areas include developing low-temperature reactive catalysts, optimizing reaction conditions and reactor designs, and employing guided rational catalyst design and synthesis. Future efforts may utilize advanced analytical techniques and computational methods to further elucidate reaction mechanisms. Bridging the gap between model catalysts and industrial-scale processing remains crucial for commercialization. The field shows promise through the development of novel materials, particularly noble metal-based catalysts, and the exploration of energy-efficient technologies. Ultimately, achieving sustainable methanol production from CO2 hydrogenation will necessitate interdisciplinary collaboration and innovative strategies to overcome existing challenges, contributing significantly to global initiatives aimed at reducing atmospheric CO2.
Data availability
No primary research results, software or code have been included and no new data were generated or analysed as part of this review.Author contributions
Soumalya Roy: conceptualization, investigation, data generation, and writing–original draft. Ezhava Manu Manohar: administration and data validation. Dr Sujoy Bandyopadhyay: administration and data validation. Dr Manik Chandra Singh: administration and data validation. Yeji Cha: administration and data validation. Dr Soumen Giri: writing and editing. Dr Sharad Lande: writing and editing. Dr Kyungsu Na: conceptualization, result validation, supervision, writing, editing, and methodology. Dr Junseong Lee: conceptualization, result validation, supervision, writing, editing, and methodology. Dr Sourav Das: conceptualization, result validation, supervision, writing, editing, and methodology.Conflicts of interest
The authors declare no competing financial interest.Acknowledgements
The authors express their gratitude to the Institute of Infrastructure Technology Research & Management (IITRAM, Ahmedabad), Reliance Industries Limited, and C. V. Raman Global University. J. L. acknowledges the financial support from the National Research Foundation of Korea (NRF) grant funded by the Korean government (RS-2023-00218219 and 2022R1A2C100611312) and the ‘Regional Innovation Strategy (RIS)’ through the National Research Foundation of Korea (NRF), funded by the Ministry of Education (MOE) (2021RIS-002). S. B. is thankful for the financial support provided by the National Research Foundation of Korea (NRF) grant funded by the Korea government Ministry of Science and NRF BP (Project Number RS-2023-00263849).References
- M. E. Boot-Handford, J. C. Abanades, E. J. Anthony, M. J. Blunt, S. Brandani, N. Mac Dowell, J. R. Fernández, M.-C. Ferrari, R. Gross and J. P. Hallett, Energy Environ. Sci., 2014, 7, 130–189 RSC.
- J. Chen, M. J. Dahlin, L. Luuppala, D. Bickford, L. Boljka, V. Burns and M. S. Johnson, Nature, 2021, 279–325 Search PubMed.
- IEA, Defying Expectations, CO2 Emissions from Global Fossil Fuel Combustion are Set to Grow in 2022 by Only a Fraction of Last Year’s Big Increase, 2022, https://www.iea.org/news/defying-expectations-co2-emissions-from-global-fossil-fuel-combustion-are-set-to-grow-in-2022-by-only-a-fraction-of-last-year-s-big-increase Search PubMed.
- S. Seneviratne, N. Nicholls, D. Easterling, C. Goodess, S. Kanae, J. Kossin, Y. Luo, J. Marengo, K. McInnes, M. Rahimi, M. Reichstein, A. Sorteberg, C. Vera and X. Zhang, Changes in climate extremes and their impacts on the natural physical environment, in Managing the Risks of Extreme Events and Disasters to Advance Climate Change Adaptation: A Special Report of Working Groups I and II of the Intergovernmental Panel on Climate Change (IPCC), ed. C. B. Field, V. Barros, T. F. Stocker, D. Qin, D. J. Dokken, K. L. Ebi, M. D. Mastrandrea, K. J. Mach, G.-K. Plattner, S. K. Allen, M. Tignor and P. M. Midgley, Cambridge University Press, Cambridge, UK, New York, NY, USA, 2012, pp. 109–230 Search PubMed.
- O. Hoegh-Guldberg, D. Jacob, M. Bindi, S. Brown, I. Camilloni, A. Diedhiou, R. Djalante, K. Ebi, F. Engelbrecht and J. Guiot, Global Warming of 1.5 °C, 2018 Search PubMed.
- J. Hansen, M. Sato, R. Ruedy, K. Lo, D. W. Lea and M. Medina-Elizade, Proc. Natl. Acad. Sci. U. S. A., 2006, 103, 14288–14293 CrossRef CAS PubMed.
- B. Soergel, E. Kriegler, I. Weindl, S. Rauner, A. Dirnaichner, C. Ruhe, M. Hofmann, N. Bauer, C. Bertram and B. L. Bodirsky, Nat. Clim. Change, 2021, 11, 656–664 CrossRef.
- G. A. Olah, G. S. Prakash and A. Goeppert, J. Am. Chem. Soc., 2011, 133, 12881–12898 CrossRef CAS PubMed.
- G. Flato, J. Marotzke, B. Abiodun, P. Braconnot, S. C. Chou, W. Collins, P. Cox, F. Driouech, S. Emori and V. Eyring, in Climate Change 2013: the Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change, Cambridge University Press, 2014, pp. 741–866 Search PubMed.
- M. He, Y. Sun and B. Han, Angew. Chem., Int. Ed., 2013, 52, 9620–9633 CrossRef CAS PubMed.
- P. Nema, S. Nema and P. Roy, Renewable Sustainable Energy Rev., 2012, 16, 2329–2336 CrossRef.
- J. A. Patz, M. L. Grabow and V. S. Limaye, Ann. Glob. Health, 2014, 80, 332–344 CrossRef PubMed.
- A. Mikhaylov, N. Moiseev, K. Aleshin and T. Burkhardt, Entrep., Sustain. Issues, 2020, 7, 2897 Search PubMed.
- N. Linares, A. M. Silvestre-Albero, E. Serrano, J. Silvestre-Albero and J. García-Martínez, Chem. Soc. Rev., 2014, 43, 7681–7717 RSC.
- J. J. Spivey, Catal, 2005, 100, 171–180 CAS.
- L. Grandell, A. Lehtilä, M. Kivinen, T. Koljonen, S. Kihlman and L. S. Lauri, Renewable Energy, 2016, 95, 53–62 CrossRef CAS.
- S. Perathoner and G. Centi, ChemSusChem, 2014, 7, 1274–1282 CrossRef CAS PubMed.
- E. A. Quadrelli, G. Centi, J. L. Duplan and S. Perathoner, ChemSusChem, 2011, 4, 1194–1215 CrossRef CAS PubMed.
- S. Perathoner, K. M. Van Geem, G. B. Marin and G. Centi, ChemComm., 2021, 57, 10967–10982 RSC.
- S. Carley and D. M. Konisky, Nat. Energy, 2020, 5, 569–577 CrossRef CAS.
- W. Wang, S. Wang, X. Ma and J. Gong, Chem. Soc. Rev., 2011, 40, 3703–3727 RSC.
- M. Ding, R. W. Flaig, H.-L. Jiang and O. M. Yaghi, Chem. Soc. Rev., 2019, 48, 2783–2828 RSC.
- X. Jiang, X. Nie, X. Guo, C. Song and J. G. Chen, Chem. Rev., 2020, 120, 7984–8034 CrossRef CAS PubMed.
- W. Gao, S. Liang, R. Wang, Q. Jiang, Y. Zhang, Q. Zheng, B. Xie, C. Y. Toe, X. Zhu and J. Wang, Chem. Soc. Rev., 2020, 49, 8584–8686 RSC.
- O. Hoegh-Guldberg and J. F. Bruno, Science, 2010, 328, 1523–1528 CrossRef CAS PubMed.
- K. Lee, H. Yan, Q. Sun, Z. Zhang and N. Yan, Acc. Mater. Res., 2023, 4, 746–757 CrossRef CAS.
- S. Valluri, V. Claremboux and S. Kawatra, J. Environ. Sci., 2022, 113, 322–344 CrossRef CAS PubMed.
- Z. Zhang, S.-Y. Pan, H. Li, J. Cai, A. G. Olabi, E. J. Anthony and V. Manovic, Renew. Sustain. Energy Rev., 2020, 125, 109799 CrossRef CAS.
- Z. Zhang, S.-Y. Pan, H. Li, J. Cai, A. G. Olabi, E. J. Anthony and V. Manovic, Renew. Sustain. Energy Rev., 2020, 125, 109799 CrossRef CAS.
- T. Wilberforce, A. Olabi, E. T. Sayed, K. Elsaid and M. A. Abdelkareem, Sci. Total Environ., 2021, 761, 143203 CrossRef CAS PubMed.
- N. Zhu, Y. Liu, L. Yang, C. Jiang and N. Wei, Renew. Sustain. Energy Rev., 2025, 207, 114899 CrossRef CAS.
- J. Ma, L. Li, H. Wang, Y. Du, J. Ma, X. Zhang and Z. Wang, Engineering, 2022, 14, 33–43 CrossRef CAS.
- S. Paltsev, J. Morris, H. Kheshgi and H. Herzog, Appl. Energy, 2021, 300, 117322 CrossRef CAS.
- T. Kazlou, A. Cherp and J. Jewell, Nat. Clim. Change, 2024, 1–9 Search PubMed.
- L. Rosa, D. L. Sanchez, G. Realmonte, D. Baldocchi and P. D'Odorico, Renew. Sustain. Energy Rev., 2021, 138, 110511 CrossRef CAS.
- E. S. Sanz-Pérez, C. R. Murdock, S. A. Didas and C. W. Jones, Chem. Rev., 2016, 116, 11840–11876 CrossRef PubMed.
- A. Sodiq, Y. Abdullatif, B. Aissa, A. Ostovar, N. Nassar, M. El-Naas and A. Amhamed, Environ. Technol. Innov., 2023, 29, 102991 CrossRef CAS.
- X. Shi, H. Xiao, H. Azarabadi, J. Song, X. Wu, X. Chen and K. S. Lackner, Angew. Chem. Int. Ed., 2020, 59, 6984–7006 CrossRef CAS PubMed.
- M. Erans, E. S. Sanz-Pérez, D. P. Hanak, Z. Clulow, D. M. Reiner and G. A. Mutch, Energy Environ. Sci., 2022, 15, 1360–1405 RSC.
- X. Zhu, W. Xie, J. Wu, Y. Miao, C. Xiang, C. Chen, B. Ge, Z. Gan, F. Yang and M. Zhang, Chem. Soc. Rev., 2022, 51, 6574–6651 RSC.
- D. Fu and M. E. Davis, Chem. Soc. Rev., 2022, 51, 9340–9370 RSC.
- H. Li, A. Dilipkumar, S. Abubakar and D. Zhao, Chem. Soc. Rev., 2023, 52, 6294–6329 RSC.
- R. Castro-Munoz, M. Z. Ahmad, M. Malankowska and J. Coronas, J. Chem. Eng., 2022, 446, 137047 CrossRef CAS.
- J. Wang, R. Fu, S. Wen, P. Ning, M. H. Helal, M. A. Salem, B. B. Xu, Z. M. El-Bahy, M. Huang and Z. Guo, Adv. Compos. Hybrid Mater., 2022, 5, 2721–2759 CrossRef CAS.
- M. Ding, X. Liu, P. Ma and J. Yao, Coord. Chem. Rev., 2022, 465, 214576 CrossRef CAS.
- C. Xiao, J. Tian, Q. Chen and M. Hong, Chem. Sci., 2024, 15, 1570–1610 RSC.
- L. James, From Capture to Storage: Understanding the Viability and Challenges of Carbon Capture and Sequestration Initiatives, Massachusetts Institute of Technology, 2024 Search PubMed.
- H. Herzog, Environ. Sci. Technol., 2001, 35, 148A CrossRef CAS PubMed.
- E. Martin-Roberts, V. Scott, S. Flude, G. Johnson, R. S. Haszeldine and S. Gilfillan, One Earth, 2021, 4, 1569–1584 CrossRef.
- T. M. Gür, Prog. Energy Combust. Sci., 2022, 89, 100965 CrossRef.
- M. Bui, C. S. Adjiman, A. Bardow, E. J. Anthony, A. Boston, S. Brown, P. S. Fennell, S. Fuss, A. Galindo and L. A. Hackett, Energy Environ. Sci., 2018, 11, 1062–1176 Search PubMed.
- R. L. Siegelman, E. J. Kim and J. R. Long, Nat. Mater., 2021, 20, 1060–1072 CrossRef CAS PubMed.
- S. Deutz and A. Bardow, Nat. Energy, 2021, 6, 203–213 CrossRef CAS.
- B. Dziejarski, R. Krzyżyńska and K. Andersson, Fuel, 2023, 342, 127776 Search PubMed.
- H. Liu, C. Consoli and A. Zapantis, Overview of Carbon Capture and Storage (CCS) facilities globally, in 14th Greenhouse Gas Control Technologies Conference Melbourne, 2018, pp. 1–10 Search PubMed.
- A. Olabi, T. Wilberforce, K. Elsaid, E. T. Sayed, H. M. Maghrabie and M. A. Abdelkareem, J. Clean. Prod., 2022, 362, 132300 CrossRef CAS.
- N. Darraj, M. M. Abdelrahman and M. Y. Alklih, Regional Review for Large-Scale Deployment of Carbon Capture and Storage (CCS) in the Middle East, 2024 Search PubMed.
- D. Cha, Australian Energy Producers Journal, 2024, 64, S119–S124 CrossRef.
- A. E. Ikpe, I. Ekanem and K. R. Ekanem, Int. J. Energy Environ. Eng., 2024, 1, 1–15 Search PubMed.
- J. Li, C. Zhang, M. R. Davidson and X. Lu, Appl. Energy, 2025, 377, 124459 CrossRef CAS.
- O. Massarweh and A. S. Abushaikha, Petroleum, 2022, 8, 291–317 CrossRef.
- L. W. Lake, R. Johns, B. Rossen and G. A. Pope, Fundamentals of Enhanced Oil Recovery, Society of Petroleum Engineers, Richardson, TX, 2014 Search PubMed.
- S. Davoodi, M. Al-Shargabi, D. A. Wood, M. Mehrad and V. S. Rukavishnikov, Fuel, 2024, 366, 131313 CrossRef CAS.
- E. S. Rubin, J. E. Davison and H. J. Herzog, Int. J. Greenhouse Gas Control, 2015, 40, 378–400 CrossRef CAS.
- J. Rissman, C. Bataille, E. Masanet, N. Aden, W. R. Morrow III, N. Zhou, N. Elliott, R. Dell, N. Heeren and B. Huckestein, Appl. Energy, 2020, 266, 114848 CrossRef CAS.
- G. Brändle, M. Schönfisch and S. Schulte, Appl. Energy, 2021, 302, 117481 CrossRef.
- J. A. Garcia, M. Villen-Guzman, J. M. Rodriguez-Maroto and J. M. Paz-Garcia, J. Environ. Chem. Eng., 2022, 10, 108470 CrossRef CAS.
- S. Griffiths, B. K. Sovacool, J. Kim, M. Bazilian and J. M. Uratani, Energy Res. Soc. Sci., 2021, 80, 102208 CrossRef.
- A. Mirzaei-Paiaman, R. Okuno, T. Lawal, K. Sheng, C. Chen, I. Lai, S. Chen and L. Hu, Techno-Economic-Environmental Analysis of CO2 Storage and EOR in an Underdeveloped Field, 2024 Search PubMed.
- P. Roefs, M. Moretti, K. Welkenhuysen, K. Piessens and T. Compernolle, J. Environ. Manag., 2019, 239, 167–177 CrossRef CAS PubMed.
- X. Yao, P. Zhong, X. Zhang and L. Zhu, Energy Pol., 2018, 121, 519–533 CrossRef.
- X. Wu, Z. Tian and J. Guo, Sustain. Oper. Comput., 2022, 3, 54–66 CrossRef.
- B. Lin and Z. Tan, J. Clean. Prod., 2021, 298, 126768 CrossRef CAS.
- A. Al-Mamoori, A. Krishnamurthy, A. A. Rownaghi and F. Rezaei, Energy Technol., 2017, 5, 834–849 CrossRef.
- T. Wilberforce, A. Baroutaji, B. Soudan, A. H. Al-Alami and A. G. Olabi, Sci. Total Environ., 2019, 657, 56–72 CrossRef CAS PubMed.
- J. C. Pires, F. G. Martins, M. C. Alvim-Ferraz and M. Simões, Chem. Eng. Res. Des., 2011, 89, 1446–1460 CrossRef CAS.
- A. I. Osman, M. Hefny, M. Abdel Maksoud, A. M. Elgarahy and D. W. Rooney, Environ. Chem. Lett., 2021, 19, 797–849 CrossRef CAS.
- M. L. Szulczewski, C. W. MacMinn, H. J. Herzog and R. Juanes, Proc. Natl. Acad. Sci. U. S. A., 2012, 109, 5185–5189 CrossRef CAS PubMed.
- R. Wennersten, Q. Sun and H. Li, J. Clean. Prod., 2015, 103, 724–736 CrossRef CAS.
- Y.-M. Wei, J.-N. Kang, L.-C. Liu, Q. Li, P.-T. Wang, J.-J. Hou, Q.-M. Liang, H. Liao, S.-F. Huang and B. Yu, Nat. Clim. Change, 2021, 11, 112–118 CrossRef.
- J. Ladenburg, J. Kim, M. Zuch and U. Soytas, Renewable Energy, 2024, 220, 119582 CrossRef CAS.
- J. Rowland, S. López-Asensio, A. Bagci, A. Delicado and A. Prades, J. Assoc. Inf. Sci. Technol., 2024, 75, 625–639 CrossRef.
- G. A. Olah, G. S. Prakash and A. Goeppert, J. Am. Chem. Soc., 2011, 133, 12881–12898 CrossRef CAS PubMed.
- Z. Caineng, B. Xiong, X. Huaqing, D. Zheng, G. Zhixin, W. Ying, L. Jiang, P. Songqi and W. Songtao, Petrol. Explor. Dev., 2021, 48, 480–491 CrossRef.
- R. M. Andrew, Earth Syst. Sci. Data, 2020, 12, 1437–1465 CrossRef.
- H. Ritchie and M. Roser, Our World in Data, 2024 Search PubMed.
- S. J. Davis and K. Caldeira, Proc. Natl. Acad. Sci. U. S. A., 2010, 107, 5687–5692 CrossRef CAS PubMed.
- Z. Wang and X. Jia, Energy Rep., 2022, 8, 1667–1679 CrossRef.
- S. Saeidi, N. A. S. Amin and M. R. Rahimpour, J. CO2 Util., 2014, 5, 66–81 CrossRef CAS.
- S. Saeidi, S. Najari, V. Hessel, K. Wilson, F. J. Keil, P. Concepción, S. L. Suib and A. E. Rodrigues, Prog. Energy Combust. Sci., 2021, 85, 100905 CrossRef.
- Z. Hua, Y. Yang and J. Liu, Coord. Chem. Rev., 2023, 478, 214982 CrossRef CAS.
- M. Aktary, H. S. Alghamdi, A. M. Ajeebi, A. S. AlZahrani, M. A. Sanhoob, M. A. Aziz and M. Nasiruzzaman Shaikh, Chem.–Asian J., 2024, e202301007 CrossRef CAS PubMed.
- N. Yusuf, F. Almomani and H. Qiblawey, Fuel, 2023, 345, 128178 CrossRef CAS.
- S. Saeidi, S. Najari, F. Fazlollahi, M. K. Nikoo, F. Sefidkon, J. J. Klemeš and L. L. Baxter, Renew. Sustain. Energy Rev., 2017, 80, 1292–1311 CrossRef CAS.
- G. Wang, J. Chen, Y. Ding, P. Cai, L. Yi, Y. Li, C. Tu, Y. Hou, Z. Wen and L. Dai, Chem. Soc. Rev., 2021, 50, 4993–5061 RSC.
- M. Alias, S. Kamarudin, A. Zainoodin and M. Masdar, Int. J. Hydrogen Energy, 2020, 45, 19620–19641 CrossRef CAS.
- Y. Liu, F. M. Kirchberger, S. Müller, M. Eder, M. Tonigold, M. Sanchez-Sanchez and J. A. Lercher, Nat. Commun., 2019, 10, 1462 CrossRef PubMed.
- M. Bertau, H. Offermanns, L. Plass, F. Schmidt and H.-J. Wernicke, Methanol: the Basic Chemical and Energy Feedstock of the Future, Springer, 2014 Search PubMed.
- G. A. Olah, Angew. Chem., 2005, 44, 2636–2639 CrossRef CAS PubMed.
- M. Nouni, P. Jha, R. Sarkhel, C. Banerjee, A. K. Tripathi and J. Manna, Fuel, 2021, 305, 121583 CrossRef CAS.
- A. Goldmann, W. Sauter, M. Oettinger, T. Kluge, U. Schröder, J. R. Seume, J. Friedrichs and F. Dinkelacker, Energies, 2018, 11, 392 CrossRef.
- P. Su-Ungkavatin, L. Tiruta-Barna and L. Hamelin, Prog. Energy Combust. Sci., 2023, 96, 101073 CrossRef.
- A. Goeppert, M. Czaun, G. S. Prakash and G. A. Olah, Energy Environ. Sci., 2012, 5, 7833–7853 RSC.
- International Energy Agency (IEA), Technology Roadmap: Delivering Sustainable Bioenergy, IEA, Paris, France, 2017 Search PubMed.
- M. Fasihi and C. Breyer, Energy Environ. Sci., 2024, 17, 3503–3522 RSC.
- S. S. Tabibian and M. Sharifzadeh, Renew. Sustain. Energy Rev., 2023, 179, 113281 CrossRef CAS.
- H.-V. Tran, T. T. Dang, N. H. Nguyen, H. T. Tran, D. T. Nguyen, D. V. Do, T. S. Le, T. H. Ngo, Y. K. Late and P. N. Amaniampong, ChemSusChem, 2024, e202401974 CrossRef PubMed.
- J. Bao, G. Yang, Y. Yoneyama and N. Tsubaki, ACS Catal., 2019, 9, 3026–3053 CrossRef CAS.
- W. Zhang, M. Song, Q. Yang, Z. Dai, S. Zhang, F. Xin, W. Dong, J. Ma and M. Jiang, Biotechnol. Biofuels, 2018, 11, 1–11 Search PubMed.
- G. Garcia, E. Arriola, W.-H. Chen and M. D. De Luna, Energy, 2021, 217, 119384 CrossRef CAS.
- T. Klein: Methanol: A Future-Proof Fuel A Primer Prepared for the Methanol Institute, Methanol Institute, Alexandria, VA, USA, 2020 Search PubMed.
- N. Onishi and Y. Himeda, Coord. Chem. Rev., 2022, 472, 214767 CrossRef CAS.
- M. Saito, M. Takeuchi, T. Fujitani, J. Toyir, S. Luo, J. Wu, H. Mabuse, K. Ushikoshi, K. Mori and T. Watanabe, Appl. Organomet. Chem., 2000, 14, 763–772 CrossRef CAS.
- N. Onishi and Y. Himeda, Chem Catal., 2021, 2, 242–251 CrossRef.
- K. Klier, in Advances in Catalysis, Elsevier, 1982, vol. 31, pp. 243–313 Search PubMed.
- S. Barman, A. Singh, F. A. Rahimi and T. K. Maji, J. Am. Chem. Soc., 2021, 143, 16284–16292 CrossRef CAS PubMed.
- S. Dang, H. Yang, P. Gao, H. Wang, X. Li, W. Wei and Y. Sun, Catal., 2019, 330, 61–75 CAS.
- J. Strunk, K. Kähler, X. Xia and M. Muhler, Surf. Sci., 2009, 603, 1776–1783 CrossRef CAS.
- X. Chang, X. Han, Y. Pan, Z. Hao, J. Chen, M. Li, J. Lv and X. Ma, Ind. Eng. Chem. Res., 2022 Search PubMed.
- J.-L. Dubois, K. Sayama and H. Arakawa, Chem. Lett., 1992, 21, 5–8 CrossRef.
- Y. Kim, S. Kwon, Y. Song and K. Na, J. CO2 Util., 2020, 36, 145–152 CrossRef CAS.
- V. Bressi, T. Len, S. Abate, C. Espro and R. Luque, Chem. Eng. J., 2024, 485, 149989 CrossRef CAS.
- F. Zhang, B. Li, X. Quan, K. Wang, J. Xu, T. Wu, Z. Li, M. Yan, S. Liu and Y. He, ACS Catal., 2024, 14, 7136–7148 CrossRef CAS.
- Q. Lu, J. Rosen, Y. Zhou, G. S. Hutchings, Y. C. Kimmel, J. G. Chen and F. Jiao, Nat. Commun., 2014, 5, 3242 CrossRef PubMed.
- B. P. Sullivan, K. Krist and H. Guard, Electrochemical and Electrocatalytic Reactions of Carbon Dioxide, Elsevier, 2012 Search PubMed.
- W.-H. Wang, Y. Himeda, J. T. Muckerman, G. F. Manbeck and E. Fujita, Chem. Rev., 2015, 115, 12936–12973 CrossRef CAS PubMed.
- D. Johnson, Z. Qiao and A. Djire, ACS Appl. Energy Mater., 2021, 4, 8661–8684 CrossRef CAS.
- L. Li, X. Li, Y. Sun and Y. Xie, Chem. Soc. Rev., 2022, 51, 1234–1252 RSC.
- H. Lee, N. Park, T.-H. Kong, S. Kwon, S. Shin, S. G. Cha, E. Lee, J. Cha, S. Sultan and Y. Kwon, Nano Energy, 2024, 130, 110099 CrossRef CAS.
- X. Zhang, S.-X. Guo, K. A. Gandionco, A. M. Bond and J. Zhang, Mater. Today Adv., 2020, 7, 100074 CrossRef.
- P. Saha, S. Amanullah and A. Dey, Acc. Chem. Res., 2022, 55, 134–144 CrossRef CAS PubMed.
- P. Sun, S. Liu, X. Zheng, G. Hu, Q. Zhang, X. Liu, G. Zheng and Y. Chen, Nano Today, 2024, 55, 102152 CrossRef CAS.
- R. Aziz, S. Abad and S. A. Onaizi, Chemosphere, 2024, 143312 CrossRef CAS PubMed.
- W. Cheng, L. Sun, X. He and L. Tian, Dalton Trans., 2022, 51, 7763–7774 RSC.
- A. Al Harthi, M. Al Abri, H. A. Younus and R. Al Hajri, J. CO2 Util., 2024, 83, 102819 CrossRef CAS.
- A. Sheppard, V. Del Angel Hernandez, C. F. Faul and D. J. Fermin, Chemelectrochem, 2023, 10, e202300068 CrossRef CAS.
- K. Wiranarongkorn, K. Eamsiri, Y.-S. Chen and A. Arpornwichanop, J. CO2 Util., 2023, 71, 102477 CrossRef CAS.
- A. Roy, H. S. Jadhav, S. J. Park and J. G. Seo, J. Alloys Compd., 2021, 887, 161449 CrossRef CAS.
- Z. W. Seh, J. Kibsgaard, C. F. Dickens, I. Chorkendorff, J. K. Nørskov and T. F. Jaramillo, Science, 2017, 355, eaad4998 CrossRef PubMed.
- Z. Guo, P. Zhou, L. Jiang, S. Liu, Y. Yang, Z. Li, P. Wu, Z. Zhang and H. Li, Adv. Mater., 2024, 36, 2311149 CrossRef CAS PubMed.
- M. Zhou, Y. Zhang, H. Li, Z. Li, S. Wang, X. Lu and S. Yang, Angew. Chem. Int. Ed., 2024, e202414392 Search PubMed.
- G. C. Chinchen, P. Denny, J. Jennings, M. Spencer and K. Waugh, Appl. Catal., 1988, 36, 1–65 CrossRef CAS.
- Q. Zhu, Clean Energy, 2019, 3, 85–100 CrossRef.
- C. Feliciano-Bruzual, J. Mater. Res. Technol., 2014, 3, 233–243 CrossRef CAS.
- L. Spadaro, F. Arena and A. Palella, in Methanol, Elsevier, 2018, pp. 429–472 Search PubMed.
- S. Chakrabortty, J. Nayak, B. Ruj, P. Pal, R. Kumar, S. Banerjee, M. Sardar and P. Chakraborty, Environ. Chem. Eng., 2020, 8, 103935 CrossRef CAS.
- O. Martin, A. J. Martín, C. Mondelli, S. Mitchell, T. F. Segawa, R. Hauert, C. Drouilly, D. Curulla-Ferré and J. Pérez-Ramírez, Angew. Chem., 2016, 55, 6261–6265 CrossRef CAS PubMed.
- T. Kobayashi and H. Takahashi, Energy Fuels, 2004, 18, 285–286 CrossRef CAS.
- W. Sheng, S. Kattel, S. Yao, B. Yan, Z. Liang, C. J. Hawxhurst, Q. Wu and J. G. Chen, Energy Environ. Sci., 2017, 10, 1180–1185 RSC.
- J. M. Spurgeon, N. Theaker, C. A. Phipps, S. S. Uttarwar and C. A. Grapperhaus, ACS Sustain. Chem. Eng., 2022, 10, 12882–12894 CrossRef CAS.
- A. A. Kiss, J. Pragt, H. Vos, G. Bargeman and M. De Groot, Chem. Eng. J., 2016, 284, 260–269 CrossRef CAS.
- Z. Cui, S. Meng, Y. Yi, A. Jafarzadeh, S. Li, E. C. Neyts, Y. Hao, L. Li, X. Zhang and X. Wang, ACS Catal., 2022, 12, 1326–1337 CrossRef CAS.
- D. Mignard, M. Sahibzada, J. Duthie and H. Whittington, Int. J. Hydrogen Energy, 2003, 28, 455–464 CrossRef CAS.
- S. Kwon, H. Yang, Y. Yu, Y. Choi, N. Kim, G. H. Kim, K. C. Ko and K. Na, Appl. Catal. B Environ., 2023, 339, 123120 CrossRef CAS.
- J. Niu, H. Liu, Y. Jin, B. Fan, W. Qi and J. Ran, Int. J. Hydrogen Energy, 2022, 47, 9183–9200 CrossRef CAS.
- J. Zhong, X. Yang, Z. Wu, B. Liang, Y. Huang and T. Zhang, Chem. Soc. Rev., 2020, 49, 1385–1413 RSC.
- M. D. Porosoff, B. Yan and J. G. Chen, Energy Environ. Sci., 2016, 9, 62–73 RSC.
- H. Sugiyama, M. Miyazaki, M. Sasase, M. Kitano and H. Hosono, J. Am. Chem. Soc., 2023, 145, 9410–9416 CrossRef CAS PubMed.
- F. Cannizzaro, E. J. Hensen and I. A. Filot, ACS Catal., 2023, 13, 1875–1892 CrossRef CAS PubMed.
- H. Zhang, D. Mao, J. Zhang and D. Wu, Chem. Eng. J., 2023, 452, 139144 CrossRef CAS.
- J. Wu, M. Saito, M. Takeuchi and T. Watanabe, Appl. Catal., A, 2001, 218, 235–240 CrossRef CAS.
- M. B. Fichtl, D. Schlereth, N. Jacobsen, I. Kasatkin, J. Schumann, M. Behrens, R. Schlögl and O. Hinrichsen, Appl. Catal., A, 2015, 502, 262–270 CrossRef CAS.
- O. Martin and J. Pérez-Ramírez, Catal. Sci. Technol., 2013, 3, 3343–3352 RSC.
- T. P. Araújo, J. Morales-Vidal, G. Giannakakis, C. Mondelli, H. Eliasson, R. Erni, J. A. Stewart, S. Mitchell, N. López and J. Pérez-Ramírez, Angew. Chem. Int. Ed., 2023, 62, e202306563 CrossRef PubMed.
- Y. Wang, B. Yang, B. Gao, L. Li, Y. Zhou, Y. Zhang, T. Ishihara and L. Guo, Appl. Catal., A, 2023, 665, 119374 CrossRef CAS.
- J. Díez-Ramírez, J. L. Valverde, P. Sánchez and F. Dorado, Catal. Lett., 2016, 146, 373–382 CrossRef.
- A. S. Malik, S. F. Zaman, A. A. Al-Zahrani, M. A. Daous, H. Driss and L. A. Petrov, Appl. Catal., A, 2018, 560, 42–53 CrossRef CAS.
- O. A. Ojelade, S. F. Zaman, M. A. Daous, A. A. Al-Zahrani, A. S. Malik, H. Driss, G. Shterk and J. Gascon, Appl. Catal., A, 2019, 584, 117185 CrossRef CAS.
- L. Oar-Arteta, A. Remiro, F. Epron, N. Bion, A. T. Aguayo, J. Bilbao and A. G. Gayubo, Ind. Eng. Chem. Res., 2016, 55, 3546–3555 CrossRef CAS.
- S. Ghoshal and P. Sarkar, J. Phys. Chem. C, 2024, 128, 2392–2405 CrossRef CAS.
- F. Lyu, M. Cao, A. Mahsud and Q. Zhang, J. Mater. Chem. A, 2020, 8, 15445–15457 RSC.
- Z. Seh, J. Kibsgaard, C. Dickens, I. Chorkendorff, J. Nørskov and T. Jaramillo, Science, 2017, 355, 6321 CrossRef PubMed.
- X. Liu, Q. Gu, Y. Zhang, X. Xu, H. Wang, Z. Sun, L. Cao, Q. Sun, L. Xu and L. Wang, J. Am. Chem. Soc., 2023, 145, 6702–6709 CrossRef CAS PubMed.
- X. Li, M. Song, Y. Zhou, P. Zhou, D. Xu, T. Liu and X. Hong, ChemCatChem, 2024, e202301577 CrossRef CAS.
- S. Natesakhawat, J. W. Lekse, J. P. Baltrus, P. R. Ohodnicki Jr, B. H. Howard, X. Deng and C. Matranga, ACS Catal., 2012, 2, 1667–1676 CrossRef CAS.
- M. Abdelgaid and G. Mpourmpakis, ACS Catal., 2022, 12, 4268–4289 CrossRef CAS.
- F. Hu, R. Ye, Z.-H. Lu, R. Zhang and G. Feng, Energy Fuels, 2021, 36, 156–169 CrossRef.
- R. Singh, K. Kundu and K. K. Pant, Chem. Eng. J., 2024, 479, 147783 CrossRef CAS.
- K. Liu, J. Niu, Y. Bai, J. Qi, L. Han, N. Zhu and L. Yan, J. Colloid Interface Sci., 2025, 683, 1030–1040 CrossRef CAS PubMed.
- A. M. Bahmanpour, M. Signorile and O. Kröcher, Appl. Catal., B, 2021, 295, 120319 CrossRef CAS.
- N. M. Martin, P. Velin, M. Skoglundh, M. Bauer and P.-A. Carlsson, Catal. Sci. Technol., 2017, 7, 1086–1094 RSC.
- M. González-Castaño, P. Tarifa, A. Monzon and H. Arellano-Garcia, in Circular Economy Processes for CO2 Capture and Utilization, Elsevier, 2024, pp. 307–323 Search PubMed.
- Y. He, F. H. Müller, R. Palkovits, F. Zeng and C. Mebrahtu, Appl. Catal. B Environ., 2024, 123663 CrossRef CAS.
- J. Guo, L. Wang, Z. Jin, Z. Liu, H. Hao, J. Gong and Z. Wang, Chem. Eng. J., 2024, 155160 CrossRef CAS.
- L. Guo, J. Zhou, F. Liu, X. Meng, Y. Ma, F. Hao, Y. Xiong and Z. Fan, ACS Nano, 2024, 18, 9823–9851 CrossRef CAS PubMed.
- X. Dong, X. Li, H. Tan, J. Zhu, G. Wang, S. Wang, W. Xie, T. Zhan and V. Polshettiwar, Mol. Catal., 2023, 547, 113369 CrossRef CAS.
- J. Fan, H. Du, Y. Zhao, Q. Wang, Y. Liu, D. Li and J. Feng, ACS Catal., 2020, 10, 13560–13583 CrossRef CAS.
- S. Jiao, X. Fu and H. Huang, Adv. Funct. Mater., 2022, 32, 2107651 CrossRef CAS.
- H. Chen, Q. Wu, Y. Wang, Q. Zhao, X. Ai, Y. Shen and X. Zou, ChemComm, 2022, 58, 7730–7740 RSC.
- M. Wang, Y. Xiang, Y. Lin, Y. Sun, Z.-z. Zhu, S. Wu and X. Cao, J. Mater. Chem. A, 2024, 12, 31902–31913 RSC.
- W. Li, H. Wang, X. Zheng, L. Ricardez-Sandoval, Q. Wu and G. Bai, Chem. Eng. J., 2023, 453, 139779 CrossRef CAS.
- K.-Q. Lu, Y.-H. Li, F. Zhang, M.-Y. Qi, X. Chen, Z.-R. Tang, Y. M. Yamada, M. Anpo, M. Conte and Y.-J. Xu, Nat. Commun., 2020, 11, 5181 CrossRef CAS PubMed.
- Z. Chen, A. Huang, K. Yu, T. Cui, Z. Zhuang, S. Liu, J. Li, R. Tu, K. Sun and X. Tan, Energy Environ. Sci., 2021, 14, 3430–3437 RSC.
- L. Fu, R. Wang, C. Zhao, J. Huo, C. He, K.-H. Kim and W. Zhang, Chem. Eng. J., 2021, 414, 128857 CrossRef CAS.
- J. Klankermayer, S. Wesselbaum, K. Beydoun and W. Leitner, Angew. Chem. Int. Ed., 2016, 55, 7296–7343 CrossRef CAS PubMed.
- U. B. Kim, D. J. Jung, H. J. Jeon, K. Rathwell and S.-g. Lee, Chem. Rev., 2020, 120, 13382–13433 CrossRef CAS PubMed.
- J. Xu, X. Su, X. Liu, X. Pan, G. Pei, Y. Huang, X. Wang, T. Zhang and H. Geng, Appl. Catal., A, 2016, 514, 51–59 CrossRef CAS.
- L. E. Briand, A. M. Hirt and I. E. Wachs, J. Catal., 2001, 202, 268–278 CrossRef CAS.
- Z. Liu, F. Huang, M. Peng, Y. Chen, X. Cai, L. Wang, Z. Hu, X. Wen, N. Wang and D. Xiao, Nat. Commun., 2021, 12, 6194 CrossRef PubMed.
- J. Liang, F. Ma, S. Hwang, X. Wang, J. Sokolowski, Q. Li, G. Wu and D. Su, Joule, 2019, 3, 956–991 CrossRef CAS.
- M. A. Z. G. Sial, M. A. U. Din and X. Wang, Chem. Soc. Rev., 2018, 47, 6175–6200 RSC.
- W. Xiao, W. Lei, M. Gong, H. L. Xin and D. Wang, ACS Catal., 2018, 8, 3237–3256 CrossRef CAS.
- X. Wang, J. Pan, H. Wei, W. Li, J. Zhao and Z. Hu, J. Phys. Chem. C, 2022, 126, 1761–1769 CrossRef CAS.
- Q. Shao, P. Wang, T. Zhu and X. Huang, Acc. Chem. Res., 2019, 52, 3384–3396 CrossRef CAS PubMed.
- P. Yang, J. Zheng, Y. Xu, Q. Zhang and L. Jiang, Adv. Mater., 2016, 28, 10508–10517 CrossRef CAS PubMed.
- S. W. Lee, M. L. Luna, N. Berdunov, W. Wan, S. Kunze, S. Shaikhutdinov and B. R. Cuenya, Nat. Commun., 2023, 14, 4649 CrossRef CAS PubMed.
- H.-H. Wang, J.-F. Zhang, Z.-L. Chen, M.-M. Zhang, X.-P. Han, C. Zhong, Y.-D. Deng and W.-B. Hu, Rare Met., 2019, 38, 115–121 CrossRef CAS.
- J. Zhu, P. Wang, X. Zhang, G. Zhang, R. Li, W. Li, T. P. Senftle, W. Liu, J. Wang and Y. Wang, Sci. Adv., 2022, 8, eabm3629 CrossRef CAS PubMed.
- B. Gao, Z. Wen, Y. Wang, D. Chen, B. Yang, T. Ishihara and L. Guo, ChemCatChem, 2024, 16, e202400814 CrossRef CAS.
- J. Zhu, S. Shaikhutdinov and B. R. Cuenya, Chem. Sci., 2025, 16, 1071–1092 RSC.
- P. Schwiderowski, H. Ruland and M. Muhler, Curr. Opin. Green Sustainable Chem., 2022, 38, 100688 CrossRef CAS.
- N. Onishi and Y. Himeda, Acc. Chem. Res., 2024, 57, 2816–2825 CrossRef CAS PubMed.
- S. Navarro-Jaén, M. Virginie, J. Bonin, M. Robert, R. Wojcieszak and A. Y. Khodakov, Nat. Rev. Chem, 2021, 5, 564–579 CrossRef PubMed.
- M. A. Adnan and M. G. Kibria, Appl. Energy, 2020, 278, 115614 CrossRef CAS.
- A. Álvarez, M. Borges, J. J. Corral-Pérez, J. G. Olcina, L. Hu, D. Cornu, R. Huang, D. Stoian and A. Urakawa, ChemPhysChem, 2017, 18, 3135–3141 CrossRef PubMed.
- J. O. Edwards and R. G. Pearson, J. Am. Chem. Soc., 1962, 84, 16–24 CrossRef CAS.
- D. Qin, Y. Zhou, W. Wang, C. Zhang, G. Zeng, D. Huang, L. Wang, H. Wang, Y. Yang and L. Lei, J. Mater. Chem. A, 2020, 8, 19156–19195 RSC.
- H. Ahouari, A. Soualah, A. Le Valant, L. Pinard, P. Magnoux and Y. Pouilloux, catalysis, React. Kinet. Mech. Catal., 2013, 110, 131–145 CrossRef CAS.
- S. L. Suib, New and Future Developments in Catalysis: Activation of Carbon Dioxide, Newnes, 2013 Search PubMed.
- M. Aresta, A. Dibenedetto and E. Quaranta, J. Catal., 2016, 343, 2–45 CrossRef CAS.
- K. Stangeland, H. Li and Z. Yu, Ind. Eng. Chem. Res., 2018, 57, 4081–4094 CrossRef CAS.
- R. Schlögl, Chemical Energy Storage, de Gruyter, Berlin, 2013 Search PubMed.
- J. Ma, N. Sun, X. Zhang, N. Zhao, F. Xiao, W. Wei and Y. Sun, Catal. Today, 2009, 148, 221–231 CrossRef CAS.
- H.-J. Yin, J.-H. Zhou and Y.-W. Zhang, Inorg. Chem. Front., 2019, 6, 2582–2618 RSC.
- S. Kattel, P. J. Ramírez, J. G. Chen, J. A. Rodriguez and P. Liu, Science, 2017, 355, 1296–1299 CrossRef CAS PubMed.
- S. Kattel, P. Liu and J. G. Chen, J. Am. Chem. Soc., 2017, 139, 9739–9754 CrossRef CAS PubMed.
- J. Wang, G. Li, Z. Li, C. Tang, Z. Feng, H. An, H. Liu, T. Liu and C. Li, Sci. Adv., 2017, 3, e1701290 CrossRef PubMed.
- X. Su, X.-F. Yang, Y. Huang, B. Liu and T. Zhang, Acc. Chem. Res., 2018, 52, 656–664 CrossRef PubMed.
- D. Xu, P. Wu and B. y. Yang, Catal. Sci. Technol., 2020, 10, 3346–3352 RSC.
- G. Yan, Z. Gao, M. Zhao, W. Yang and X. Ding, Appl. Surf. Sci., 2020, 517, 146200 CrossRef CAS.
- J. Ye, C.-j. Liu, D. Mei and Q. Ge, J. Catal., 2014, 317, 44–53 CrossRef CAS.
- C. Panzone, R. Philippe, C. Nikitine, A. Bengaouer, A. Chappaz and P. Fongarland, Ind. Eng. Chem. Res., 2022, 61, 4514–4533 CrossRef CAS.
- Q.-J. Hong and Z.-P. Liu, Surf. Sci., 2010, 604, 1869–1876 CrossRef CAS.
- D. Kopac, M. Hus, M. Ogrizek and B. Likozar, J. Phys. Chem. C, 2017, 121, 17941–17949 CrossRef CAS.
- Q.-L. Tang, Q.-J. Hong and Z.-P. Liu, J. Catal., 2009, 263, 114–122 CrossRef CAS.
- Y. Yang, J. Evans, J. A. Rodriguez, M. G. White and P. Liu, Phys. Chem. Chem. Phys., 2010, 12, 9909–9917 RSC.
- C. Gilbert, L. Gilbey, A. Caragheorgheopol, F Savonea, D. B. Jackson, B. Onida, E. Garrone and R. Luque, J. Mater. Chem., 2005, 15, 3946–3951 RSC.
- Q. Tang, Z. Shen, C. K. Russell and M. Fan, J. Phys. Chem. C, 2018, 122, 315–330 CrossRef CAS.
- W. Liu, D. Wang and J. Ren, Appl. Surf. Sci., 2020, 505, 144528 CrossRef CAS.
- Y. Kim, T. S. B. Trung, S. Yang, S. Kim and H. Lee, ACS Catal., 2016, 6, 1037–1044 CrossRef CAS.
- Y. Zhuang, R. Currie, K. B. McAuley and D. S. Simakov, Appl. Catal., A, 2019, 575, 74–86 CrossRef CAS.
- F. Sha, Z. Han, S. Tang, J. Wang and C. Li, ChemSusChem, 2020, 13, 6160–6181 CrossRef CAS PubMed.
- T. Fujitani and J. Nakamura, Catal. Lett., 1998, 56, 119–124 CrossRef CAS.
- Y. Choi, K. Futagami, T. Fujitani and J. Nakamura, Appl. Catal., A, 2001, 208, 163–167 CrossRef CAS.
- S. E. Collins, M. A. Baltanas and A. L. Bonivardi, J. Catal., 2004, 226, 410–421 CrossRef CAS.
- F. Liao, X.-P. Wu, J. Zheng, M.-J. Li, Z. Zeng, X. Hong, A. Kroner, Y. Yuan, X.-Q. Gong and S. C. E. Tsang, Catal. Sci. Technol., 2016, 6, 7698–7702 RSC.
- M. Huš, D. Kopač, N. S. Štefančič, D. L. Jurković, V. D. Dasireddy and B. Likozar, Catal. Sci. Technol., 2017, 7, 5900–5913 RSC.
- S. G. Neophytides, A. J. Marchi and G. F. Froment, Appl. Catal., A, 1992, 86, 45–64 CrossRef CAS.
- E. L. Kunkes, F. Studt, F. Abild-Pedersen, R. Schlögl and M. Behrens, J. Catal., 2015, 328, 43–48 CrossRef CAS.
- Z. Zhang, Y. Zheng, L. Qian, D. Luo, H. Dou, G. Wen, A. Yu and Z. Chen, Adv. Mater., 2022, 2201547 CrossRef CAS PubMed.
- D. L. Chiavassa, S. E. Collins, A. L. Bonivardi and M. A. Baltanás, Chem. Eng. J., 2009, 150, 204–212 CrossRef CAS.
- L. Grabow and M. Mavrikakis, ACS Catal., 2011, 1, 365–384 CrossRef CAS.
- M. Behrens, F. Studt, I. Kasatkin, S. Kühl, M. Hävecker, F. Abild-Pedersen, S. Zander, F. Girgsdies, P. Kurr and B.-L. Kniep, Science, 2012, 336, 893–897 CrossRef CAS.
- J. A. Rodriguez, J. Evans, L. Feria, A. B. Vidal, P. Liu, K. Nakamura and F. Illas, J. Catal., 2013, 307, 162–169 CrossRef CAS.
- J. Artz, T. E. Müller, K. Thenert, J. Kleinekorte, R. Meys, A. Sternberg, A. Bardow and W. Leitner, Chem. Rev., 2018, 118, 434–504 CrossRef CAS PubMed.
- S. Roy, A. Cherevotan and S. C. Peter, ACS Energy Lett., 2018, 3, 1938–1966 CrossRef CAS.
- X. Yang, S. Kattel, S. D. Senanayake, J. A. Boscoboinik, X. Nie, J. Graciani, J. A. Rodriguez, P. Liu, D. J. Stacchiola and J. G. Chen, J. Am. Chem. Soc., 2015, 137, 10104–10107 CrossRef CAS PubMed.
- Y.-F. Zhao, Y. Yang, C. Mims, C. H. Peden, J. Li and D. Mei, J. Catal., 2011, 281, 199–211 CrossRef CAS.
- C. Liu and P. Liu, ACS Catal., 2015, 5, 1004–1012 CrossRef CAS.
- S. Kattel, B. Yan, Y. Yang, J. G. Chen and P. Liu, J. Am. Chem. Soc., 2016, 138, 12440–12450 CrossRef CAS PubMed.
- Q. Tang, Z. Shen, L. Huang, T. He, H. Adidharma, A. G. Russell and M. Fan, Phys. Chem. Chem. Phys., 2017, 19, 18539–18555 RSC.
- N. Pasupulety, H. Driss, Y. A. Alhamed, A. A. Alzahrani, M. A. Daous and L. Petrov, Appl. Catal., A, 2015, 504, 308–318 CrossRef CAS.
- P. Banoth, C. Kandula and P. Kollu, in Noble Metal-free Electrocatalysts: New Trends in Electrocatalysts for Energy Applications, vol. 2, ACS Publications, 2022, pp. 1–37 Search PubMed.
- X.-F. Yang, A. Wang, B. Qiao, J. Li, J. Liu and T. Zhang, Acc. Chem. Res., 2013, 46, 1740–1748 CrossRef CAS PubMed.
- M. Humayun, M. Israr, A. Khan and M. Bououdina, Nano Energy, 2023, 113, 108570 CrossRef CAS.
- P. Sharma, S. Kumar, O. Tomanec, M. Petr, J. Zhu Chen, J. T. Miller, R. S. Varma, M. B. Gawande and R. Zbořil, Small, 2021, 17, 2006478 CrossRef CAS PubMed.
- J. Zhong, X. Yang, Z. Wu, B. Liang, Y. Huang and T. Zhang, Chem. Soc. Rev., 2020, 49, 1385–1413 RSC.
- J. Frost, Nature, 1988, 334, 577–580 CrossRef CAS.
- P. L. Hansen, J. B. Wagner, S. Helveg, J. R. Rostrup-Nielsen, B. S. Clausen and H. Topsøe, Science, 2002, 295, 2053–2055 CrossRef CAS PubMed.
- G. A. Olah, A. Goeppert and G. S. Prakash, Beyond Oil and Gas: the Methanol Economy, John Wiley & Sons, 2011 Search PubMed.
- C. T. Campbell and C. H. Peden, Science, 2005, 309, 713–714 CrossRef CAS PubMed.
- M. Zabilskiy, V. L. Sushkevich, D. Palagin, M. A. Newton, F. Krumeich and J. A. van Bokhoven, Nat. Commun., 2020, 2409 CrossRef CAS PubMed.
- Z. Zhang, C. Mao, D. M. Meira, P. N. Duchesne, A. A. Tountas, Z. Li, C. Qiu, S. Tang, R. Song and X. Ding, Nat. Commun., 2022, 13, 1512 CrossRef CAS PubMed.
- J. Graciani, K. Mudiyanselage, F. Xu, A. E. Baber, J. Evans, S. D. Senanayake, D. J. Stacchiola, P. Liu, J. Hrbek and J. F. Sanz, Science, 2014, 345, 546–550 CrossRef CAS PubMed.
- P. Gao, S. Li, X. Bu, S. Dang, Z. Liu, H. Wang, L. Zhong, M. Qiu, C. Yang and J. Cai, Nat. Chem., 2017, 9, 1019–1024 CrossRef CAS PubMed.
- B. Liu, C. Li, G. Zhang, X. Yao, S. S. Chuang and Z. Li, ACS Catal., 2018, 8, 10446–10456 CrossRef CAS.
- K. Li and J. G. Chen, ACS Catal., 2019, 9, 7840–7861 CrossRef CAS.
- G. Wang, D. Mao, X. Guo and J. Yu, Int. J. Hydrogen Energy, 2019, 44, 4197–4207 CrossRef CAS.
- B. Min, A. Santra and D. Goodman, Catal. Today, 2003, 85, 113–124 CrossRef CAS.
- F. Chen, C. Kreyenschulte, J. r. Radnik, H. Lund, A.-E. Surkus, K. Junge and M. Beller, ACS Catal., 2017, 7, 1526–1532 CrossRef CAS.
- R. P. Mogorosi, N. Fischer, M. Claeys and E. van Steen, J. Catal., 2012, 289, 140–150 CrossRef CAS.
- R. Takahashi, S. Sato, T. Sodesawa, M. Yoshida and S. Tomiyama, Appl. Catal., A, 2004, 273, 211–215 CrossRef CAS.
- A. T. Aguayo, J. Ereña, D. Mier, J. M. Arandes, M. Olazar and J. Bilbao, Ind. Eng. Chem. Res., 2007, 46, 5522–5530 CrossRef CAS.
- J. Díez-Ramírez, P. Sanchez, A. Rodriguez-Gomez, J. L. Valverde and F. Dorado, Ind. Eng. Chem. Res., 2016, 55, 3556–3567 CrossRef.
- V. I. Bogdan, Y. A. Pokusaeva, A. E. Koklin, S. V. Savilov, S. A. Chernyak, V. V. Lunin and L. M. Kustov, Energy Technol., 2019, 7, 1900174 CrossRef.
- J. A. Rodriguez, P. Liu, D. J. Stacchiola, S. D. Senanayake, M. G. White and J. G. Chen, ACS Catal., 2015, 5, 6696–6706 CrossRef CAS.
- H. Sakurai, S. Tsubota and M. Haruta, Appl. Catal., A, 1993, 102, 125–136 CrossRef CAS.
- F. Liao, Z. Zeng, C. Eley, Q. Lu, X. Hong and S. C. E. Tsang, Angew. Chem., Int. Ed., 2012, 24, 5832–5836 CrossRef PubMed.
- I. A. Da Silva and C. J. Mota, Front. Energy Res., 2019, 7, 49 CrossRef.
- I. U. Din, M. S. Shaharun, D. Subbarao, A. Naeem and F. Hussain, Catal. Today, 2016, 259, 303–311 CrossRef CAS.
- Y. Wang, S. Kattel, W. Gao, K. Li, P. Liu, J. G. Chen and H. Wang, Nat. Commun., 2019, 10, 1166 CrossRef PubMed.
- M. Zabilskiy, V. L. Sushkevich, D. Palagin, M. A. Newton, F. Krumeich and J. A. van Bokhoven, Nat. Commun., 2020, 11, 2409 CrossRef CAS PubMed.
- L. Wang, Y. Dong, T. Yan, Z. Hu, F. M. Ali, D. M. Meira, P. N. Duchesne, J. Y. Y. Loh, C. Qiu and E. E. Storey, Nat. Commun., 2020, 11, 2432 CrossRef CAS PubMed.
- A. e. Prasnikar, A. Pavlisic, F. Ruiz-Zepeda, J. Kovac and B. Likozar, Ind. Eng. Chem. Res., 2019, 58, 13021–13029 CrossRef CAS.
- A. H. Leung, A. García-Trenco, A. Phanopoulos, A. Regoutz, M. E. Schuster, S. D. Pike, M. S. Shaffer and C. K. Williams, J. Mater. Chem. A, 2020, 8, 11282–11291 RSC.
- F. Studt, M. Behrens, E. L. Kunkes, N. Thomas, S. Zander, A. Tarasov, J. Schumann, E. Frei, J. B. Varley and F. Abild-Pedersen, ChemCatChem, 2015, 7, 1105–1111 CrossRef CAS.
- S. G. Jadhav, P. D. Vaidya, B. M. Bhanage and J. B. Joshi, Chem. Eng. Res. Des., 2014, 92, 2557–2567 CrossRef CAS.
- O. A. Ojelade and S. F. Zaman, Catal. Surv. Asia, 2020, 24, 11–37 CrossRef CAS.
- S. G. Jadhav, P. D. Vaidya, B. M. Bhanage and J. B. Joshi, Chem. Eng. Res. Des., 2014, 92, 2557–2567 CrossRef CAS.
- J. Schittkowski, H. Ruland, D. Laudenschleger, K. Girod, K. Kähler, S. Kaluza, M. Muhler and R. Schlögl, Chem. Ing. Tech., 2018, 90, 1419–1429 CrossRef CAS.
- X.-M. Liu, G. Lu, Z.-F. Yan and J. Beltramini, Ind. Eng. Chem. Res., 2003, 42, 6518–6530 CrossRef CAS.
- T. T. N. Vu, A. Desgagnés and M. C. Iliuta, Appl. Catal., A, 2021, 617, 118119 CrossRef CAS.
- X. Nie, X. Jiang, H. Wang, W. Luo, M. J. Janik, Y. Chen, X. Guo and C. Song, ACS Catal., 2018, 8, 4873–4892 CrossRef CAS.
- A. S. Malik, S. F. Zaman, A. A. Al-Zahrani, M. A. Daous, H. Driss and L. A. Petrov, Catal. Today, 2020, 357, 573–582 CrossRef CAS.
- B. Hu, Y. Yin, G. Liu, S. Chen, X. Hong and S. C. E. Tsang, J. Catal., 2018, 359, 17–26 CrossRef CAS.
- A. Ota, E. L. Kunkes, I. Kasatkin, E. Groppo, D. Ferri, B. Poceiro, R. M. N. Yerga and M. Behrens, J. Catal., 2012, 293, 27–38 CrossRef CAS.
- M. Bowker, N. Lawes, I. Gow, J. Hayward, J. R. Esquius, N. Richards, L. R. Smith, T. J. Slater, T. E. Davies and N. F. Dummer, ACS Catal., 2022, 12, 5371–5379 CrossRef CAS PubMed.
- S. Kanuri, S. Roy, C. Chakraborty, S. P. Datta, S. A. Singh and S. Dinda, Int. J. Energy Res., 2022, 46, 5503–5522 CrossRef CAS.
- Y. Zeng, Y. Chen, Y. Wu, D. Wang, X. Liu and L. Li, Organometallics, 2022, 41, 2001–2010 CrossRef CAS.
- S. Bai, Q. Shao, P. Wang, Q. Dai, X. Wang and X. Huang, J. Am. Chem. Soc., 2017, 139, 6827–6830 CrossRef CAS PubMed.
- R. Manrique, J. Rodríguez-Pereira, S. A. Rincón-Ortiz, J. J. Bravo-Suárez, V. G. Baldovino-Medrano, R. Jiménez and A. Karelovic, Catal. Sci. Technol., 2020, 10, 6644–6658 RSC.
- A. Erdöhelyi, M. Pásztor and F. Solymosi, J. Catal., 1986, 98, 166–177 CrossRef.
- J. Wang, G. Zhang, J. Zhu, X. Zhang, F. Ding, A. Zhang, X. Guo and C. Song, ACS Catal., 2021, 11, 1406–1423 CrossRef CAS.
- H. Jiang, J. Lin, X. Wu, W. Wang, Y. Chen and M. Zhang, J. CO2 Util., 2020, 36, 33–39 CrossRef CAS.
- X.-L. Liang, X. Dong, G.-D. Lin and H.-B. Zhang, Appl. Catal., B, 2009, 88, 315–322 CrossRef CAS.
- I. U. Din, M. S. Shaharun, D. Subbarao, A. Naeem and F. Hussain, Catal. Today, 2016, 259, 303–311 CrossRef CAS.
- M. Chatterjee, T. Ishizaka and H. Kawanami, in Advances in CO2 Capture, Sequestration, and Conversion, ACS Publications, 2015, pp. 191–250 Search PubMed.
- N. Iwasa, H. Suzuki, M. Terashita, M. Arai and N. Takezawa, Catal. Lett., 2004, 96, 75–78 CrossRef CAS.
- J. Xu, X. Su, X. Liu, X. Pan, G. Pei, Y. Huang, X. Wang, T. Zhang and H. Geng, Appl. Catal., 2016, 514, 51–59 CrossRef CAS.
- H. Bahruji, M. Bowker, G. Hutchings, N. Dimitratos, P. Wells, E. Gibson, W. Jones, C. Brookes, D. Morgan and G. Lalev, J. Catal., 2016, 343, 133–146 CrossRef CAS.
- H. Bahruji, M. Bowker, W. Jones, J. Hayward, J. R. Esquius, D. Morgan and G. Hutchings, Faraday Discuss., 2017, 197, 309–324 RSC.
- F. Liao, X.-P. Wu, J. Zheng, M. M.-J. Li, A. Kroner, Z. Zeng, X. Hong, Y. Yuan, X.-Q. Gong and S. C. E. Tsang, Green Chem., 2017, 19, 270–280 RSC.
- A. S. Malik, S. F. Zaman, A. A. Al-Zahrani, M. A. Daous, H. Driss and L. A. Petrov, Catal, 2020, 357, 573–582 CAS.
- J. Diez-Ramirez, P. Sanchez, A. Rodriguez-Gomez, J. L. Valverde and F. Dorado, Ind. Eng. Chem. Res., 2016, 55, 3556–3567 CrossRef CAS.
- C. Quilis, N. Mota, B. Pawelec, E. Millan and R. M. N. Yerga, Appl. Catal., B, 2023, 321, 122064 CrossRef CAS.
- X. Yao, S. Yuan, C. Li, L. Wang, X. Yu, P. Tian and S.-t. Tu, Fuel, 2024, 358, 130133 CrossRef CAS.
- X. Jiang, X. Wang, X. Nie, N. Koizumi, X. Guo and C. Song, Catal, 2018, 316, 62–70 CAS.
- X. Jiang, X. Nie, X. Wang, H. Wang, N. Koizumi, Y. Chen, X. Guo and C. Song, J. Catal., 2019, 369, 21–32 CrossRef CAS.
- F. Lin, X. Jiang, N. Boreriboon, Z. Wang, C. Song and K. Cen, Appl. Catal., 2019, 585, 117210 CrossRef.
- T. Fujitani, M. Saito, Y. Kanai, T. Watanabe, J. Nakamura and T. Uchijima, Appl. Catal., 1995, 125, L199–L202 CrossRef CAS.
- S. E. Collins, M. A. Baltanás, J. L. G. Fierro and A. L. Bonivardi, J. Catal., 2002, 211, 252–264 CrossRef CAS.
- L. Li, B. Zhang, E. Kunkes, K. Föttinger, M. Armbrüster, D. S. Su, W. Wei, R. Schlögl and M. Behrens, ChemCatChem, 2012, 4, 1764–1775 CrossRef CAS.
- E. M. Fiordaliso, I. Sharafutdinov, H. W. Carvalho, J.-D. Grunwaldt, T. W. Hansen, I. Chorkendorff, J. B. Wagner and C. D. Damsgaard, ACS Catal., 2015, 5, 5827–5836 CrossRef CAS.
- X. Zhou, J. Qu, F. Xu, J. Hu, J. S. Foord, Z. Zeng, X. Hong and S. C. E. Tsang, Chem. Commun., 2013, 49, 1747–1749 RSC.
- A. s. García-Trenco, E. R. White, A. Regoutz, D. J. Payne, M. S. Shaffer and C. K. Williams, ACS Catal., 2017, 7, 1186–1196 CrossRef.
- N. Lawes, N. F. Dummer, S. Fagan, O. Wielgosz, I. E. Gow, L. R. Smith, T. J. Slater, T. E. Davies, K. J. Aggett and D. J. Morgan, Appl. Catal., A, 2024, 679, 119735 CrossRef CAS.
- A. García-Trenco, A. Regoutz, E. R. White, D. J. Payne, M. S. Shaffer and C. K. Williams, Appl. Catal., B, 2018, 220, 9–18 CrossRef.
- J. L. Snider, V. Streibel, M. A. Hubert, T. S. Choksi, E. Valle, D. C. Upham, J. Schumann, M. S. Duyar, A. Gallo and F. Abild-Pedersen, ACS Catal., 2019, 9, 3399–3412 CrossRef CAS.
- N. Rui, Z. Wang, K. Sun, J. Ye, Q. Ge and C.-J. Liu, Appl. Catal., B, 2017, 218, 488–497 CrossRef CAS.
- T. Pinheiro Araújo, C. Mondelli, M. Agrachev, T. Zou, P. O. Willi, K. M. Engel, R. N. Grass, W. J. Stark, O. V. Safonova and G. Jeschke, Nat. Commun., 2022, 13, 1–12 Search PubMed.
- Z. Cai, J. Dai, W. Li, K. B. Tan, Z. Huang, G. Zhan, J. Huang and Q. Li, ACS Catal., 2020, 10, 13275–13289 CrossRef CAS.
- H. Jiang, J. Lin, X. Wu, W. Wang, Y. Chen and M. Zhang, J. CO2 Util., 2020, 36, 33–39 CrossRef CAS.
- J. Wu, B. Lu, S. Yang, J. Huang, W. Wang, R. Dun and Z. Hua, ChemSusChem, 2024, e202400543 CrossRef CAS PubMed.
- N. Koizumi, X. Jiang, J. Kugai and C. Song, Catal, 2012, 194, 16–24 CAS.
- A. Velty and A. Corma, Chem. Soc. Rev., 2023, 52, 1773–1946 RSC.
- P. Romero-Marimon, J. J. Gutiérrez-Sevillano and S. Calero, Chem. Mater., 2023, 35, 5222–5231 CrossRef CAS.
- X.-L. Liang, X. Dong, G.-D. Lin and H.-B. Zhang, Appl. Catal., B, 2009, 88, 315–322 CrossRef CAS.
- H. Kong, H.-Y. Li, G.-D. Lin and H.-B. Zhang, Catal. Lett., 2011, 141, 886–894 CrossRef CAS.
- J. Wang, S.-m. Lu, J. Li and C. Li, Chem. Commun., 2015, 51, 17615–17618 RSC.
- E. J. Choi, Y. H. Lee, D.-W. Lee, D.-J. Moon and K.-Y. Lee, Mol. Catal., 2017, 434, 146–153 CrossRef CAS.
- Y. Yin, B. Hu, X. Li, X. Zhou, X. Hong and G. Liu, Appl. Catal., B, 2018, 234, 143–152 CrossRef CAS.
- A. Vourros, I. Garagounis, V. Kyriakou, S. Carabineiro, F. Maldonado-Hódar, G. Marnellos and M. Konsolakis, J. CO2 Util., 2017, 19, 247–256 CrossRef CAS.
- Y. Hartadi, D. Widmann and R. J. Behm, Phys. Chem. Chem. Phys., 2016, 18, 10781–10791 RSC.
- Y. Hartadi, D. Widmann and R. J. Behm, J. Catal., 2016, 333, 238–250 CrossRef CAS.
- C. Wu, P. Zhang, Z. Zhang, L. Zhang, G. Yang and B. Han, ChemCatChem, 2017, 9, 3691–3696 CrossRef CAS.
- X. Yang, S. Kattel, S. D. Senanayake, J. A. Boscoboinik, X. Nie, J. Graciani, J. A. Rodriguez, P. Liu, D. J. Stacchiola and J. G. Chen, J. Am. Chem. Soc., 2015, 137, 10104–10107 CrossRef CAS PubMed.
- K. Prabhakar Reddy, Y. Tian, P. J. Ramírez, A. Islam, H. Lim, N. Rui, Y. Xie, A. Hunt, I. Waluyo and J. A. Rodriguez, ACS Catal., 2024, 14, 17148–17158 CrossRef CAS.
- J. Słoczyński, R. Grabowski, A. Kozłowska, P. Olszewski, J. Stoch, J. Skrzypek and M. Lachowska, Appl. Catal., 2004, 278, 11–23 CrossRef.
- R. Grabowski, J. Słoczynski, M. Sliwa, D. Mucha, R. Socha, M. Lachowska and J. Skrzypek, ACS Catal., 2011, 1, 266–278 CrossRef CAS.
- A. Wang, J. Li and T. Zhang, Nat. Rev. Chem, 2018, 2, 65–81 CrossRef CAS.
- H. Li, L. Wang, Y. Dai, Z. Pu, Z. Lao, Y. Chen, M. Wang, X. Zheng, J. Zhu and W. Zhang, Nat. Nanotechnol., 2018, 13, 411–417 CrossRef CAS PubMed.
- K. W. Ting, T. Toyao, S. H. Siddiki and K.-i. Shimizu, ACS Catal., 2019, 9, 3685–3693 CrossRef CAS.
- Z. Han, C. Tang, J. Wang, L. Li and C. Li, J. Catal., 2021, 394, 236–244 CrossRef CAS.
- W. Zhang, L. Wang, H. Liu, Y. Hao, H. Li, M. U. Khan and J. Zeng, Nano Lett., 2017, 17, 788–793 CrossRef CAS PubMed.
- Y. Yin, B. Hu, X. Li, X. Zhou, X. Hong and G. Liu, Appl. Catal., B, 2018, 234, 143–152 CrossRef CAS.
- M. S. Frei, C. Mondelli, R. García-Muelas, K. S. Kley, B. Puértolas, N. López, O. V. Safonova, J. A. Stewart, D. Curulla Ferré and J. Pérez-Ramírez, Nat. Commun., 2019, 10, 3377 CrossRef PubMed.
- T. T. N. Vu, P. Fongarland, L. Vanoye, F. Bornette, G. Postole, A. Desgagnés and M. C. Iliuta, Ind. Eng. Chem. Res., 2022, 61, 15085–15102 CrossRef CAS.
- F. Lin, X. Jiang, N. Boreriboon, Z. Wang, C. Song and K. Cen, Appl. Catal., A, 2019, 585, 117210 CrossRef.
- S. Wang, Q. Li, Y. Xin, S. Hu, X. Guo, Y. Zhang, L. Zhang, B. Chen, W. Zhang and L. Wang, Nanoscale, 2023, 15, 6999–7005 RSC.
- J. Guo, Z. Wang, T. Gao and Z. Wang, Chem. Eng. J., 2024, 483, 149370 CrossRef CAS.
- J. Song, S. Liu, C. Yang, G. Wang, H. Tian, Z.-j. Zhao, R. Mu and J. Gong, Appl. Catal., B, 2020, 263, 118367 CrossRef CAS.
- M. Zabilskiy, V. L. Sushkevich, M. A. Newton, F. Krumeich, M. Nachtegaal and J. A. van Bokhoven, Angew. Chem. Int. Ed., 2021, 133, 17190–17196 CrossRef.
- F. Jiang, S. Wang, B. Liu, J. Liu, L. Wang, Y. Xiao, Y. Xu and X. Liu, ACS Catal., 2020, 10, 11493–11509 CrossRef CAS.
- A. S. Malik, S. F. Zaman, A. A. Al-Zahrani, M. A. Daous, H. Driss and L. A. Petrov, Appl. Catal., 2018, 560, 42–53 CrossRef CAS.
- X. Jiang, N. Koizumi, X. Guo and C. Song, Appl. Catal., B, 2015, 170, 173–185 CrossRef.
- X.-L. Liang, X. Dong, G.-D. Lin and H.-B. Zhang, Appl. Catal., B, 2009, 88, 315–322 CrossRef CAS.
- T. Fujitani, M. Saito, Y. Kanai, T. Watanabe, J. Nakamura and T. Uchijima, Appl. Catal., A, 1995, 125, L199–L202 CrossRef CAS.
- K. Lee, U. Anjum, T. P. Araújo, C. Mondelli, Q. He, S. Furukawa, J. Pérez-Ramírez, S. M. Kozlov and N. Yan, Appl. Catal., B, 2022, 304, 120994 CrossRef CAS.
- K. Sun, N. Rui, Z. Zhang, Z. Sun, Q. Ge and C.-J. Liu, Green Chem., 2020, 22, 5059–5066 RSC.
- Z. Han, C. Tang, J. Wang, L. Li and C. Li, J. Catal., 2021, 394, 236–244 CrossRef CAS.
- Z. Lu, J. Wang, K. Sun, S. Xiong, Z. Zhang and C.-j. Liu, GreenChE, 2022, 3, 165–170 Search PubMed.
- E. S. Gutterød, A. Lazzarini, T. Fjermestad, G. Kaur, M. Manzoli, S. Bordiga, S. Svelle, K. P. Lillerud, E. Skúlason and S. Øien-Ødegaard, J. Am. Chem. Soc., 2019, 142, 999–1009 CrossRef PubMed.
- Y. Hartadi, D. Widmann and R. J. Behm, ChemSusChem, 2015, 8, 456–465 CrossRef CAS PubMed.
- L. H. Vieira, L. F. Rasteiro, C. S. Santana, G. L. Catuzo, A. H. da Silva, J. M. Assaf and E. M. Assaf, ChemCatChem, 2023, 15, e202300493 CrossRef CAS.
- Z. Lu, K. Sun, J. Wang, Z. Zhang and C. Liu, Catalysts, 2020, 10, 1360 CrossRef CAS.
- A. Rezvani, A. M. Abdel-Mageed, T. Ishida, T. Murayama, M. Parlinska-Wojtan and R. J. r. Behm, ACS Catal., 2020, 10, 3580–3594 CrossRef CAS.
- N. Rui, F. Zhang, K. Sun, Z. Liu, W. Xu, E. Stavitski, S. D. Senanayake, J. A. Rodriguez and C.-J. Liu, ACS Catal., 2020, 10, 11307–11317 CrossRef CAS.
- M. J. Blom, W. P. van Swaaij, G. Mul and S. R. Kersten, ACS Catal., 2021, 11, 6883–6891 CrossRef CAS.
- L. Lu, X. Sun, J. Ma, D. Yang, H. Wu, B. Zhang, J. Zhang and B. Han, Angew. Chem., 2018, 130, 14345–14349 CrossRef.
- W. Zhang, Q. Qin, L. Dai, R. Qin, X. Zhao, X. Chen, D. Ou, J. Chen, T. T. Chuong and B. Wu, Angew. Chem. Int. Ed., 2018, 57, 9475–9479 CrossRef CAS PubMed.
- G. Brisard, A. P. M. Camargo, F. C. Nart and T. Iwasita, Electrochem. Commun., 2001, 3, 603–607 CrossRef CAS.
- S. Shironita, K. Karasuda, K. Sato and M. Umeda, J. Power Sources, 2013, 240, 404–410 CrossRef CAS.
- Q.-L. Tang, Q.-J. Hong and Z.-P. Liu, J. Catal., 2009, 263, 114–122 CrossRef CAS.
- D. G. Olson, K. Tsuji and I. Shiraishi, Fuel Process. Technol., 2000, 65, 393–405 CrossRef.
- M. Saito, T. Fujitani, M. Takeuchi and T. Watanabe, Appl. Catal., A, 1996, 138, 311–318 CrossRef CAS.
- M. Mikkelsen, M. Jørgensen and F. C. Krebs, Energy Environ. Sci., 2010, 3, 43–81 RSC.
- M. Ng, V. Jovic, G. I. Waterhouse and J. Kennedy, Emerg. Mater. Res., 2023, 6, 1097–1115 CrossRef CAS.
- X. Cui, S. K. Kær and M. P. Nielsen, Fuel, 2022, 307, 121924 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2025 |