Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Green and efficient one-pot synthesis of the bio-based platform molecule 4-hydroxymethyl-2-furfural on a multigram scale†
Kubilay
Ceyhan
,
Mattis
Rottmann
and
Harald
Gröger
 *
*
Chair of Industrial Organic Chemistry and Biotechnology, Faculty of Chemistry, Bielefeld University, Universitätsstraße 25, 33615 Bielefeld, Germany. E-mail: harald.groeger@uni-bielefeld.de
First published on 13th January 2025
Abstract
This study outlines a detailed process development of dendroketose preparation and its conversion to the bio-based platform molecule 4-hydroxymethyl-2-furfural (4-HMF) through a dehydration reaction in water as a green and sustainable solvent. Initially, dendroketose was synthesized via a monoaldol reaction of dihydroxyacetone (DHA) as a glycerin- or CO-based product, simply using NaOH as a catalyst at various DHA concentrations. We successfully demonstrate preparation of a high loading of dendroketose, resulting in an ecological factor (E-factor, EF) of EF = 1. For the subsequent dehydration of dendroketose to 4-HMF, an optimized process was designed after evaluating various solvents and catalysts. Saturated aqueous NaCl solution offered the highest 4-HMF:5-HMF reaction selectivity of 95%. Optimal conditions for the 4-HMF synthesis were determined as 0.25 M HCl, 80 °C, 100 g per L dendroketose, and a reaction time of 120 minutes, achieving an 80% selectivity towards the formation of total HMF. In addition, scale-up experiments on an elevated lab scale of 100 g dendroketose in combination with a tailor-made reactor set-up for smooth product removal confirmed the identified preferred process conditions, leading to an 89% reaction yield over nine cycles, with an isolated 4-HMF yield of 76%, a purity of 92% and an EF = 0.67. These results also underline the potential of this process and reactor set-up for efficient and scalable 4-HMF production, with further optimization opportunities identified in salt selection, catalyst loadings, and process control strategies.
Sustainability spotlightAn efficient and sustainable process for the biobased chemical 4-hydroxymethyl-2-furfural (4-HMF) was developed. We optimized the synthesis of the substrate dendroketose (running at 1000 g per L) and improved the process for 4-HMF through optimization of multiple reaction parameters. Our process was already carried out at an elevated lab scale with a substrate amount of 100 g in combination with a tailor-made reactor set-up, leading to 89% reaction yield over nine cycles and 76% yield. Environmental metrices of our 4-HMF process were also determined, revealing an outstanding E-factor of only 0.67. Besides opening up a perspective for technical production of 4-HMF, our work addresses particularly the UN sustainable development goals “Industry, innovation, and infrastructure (SDG 10)” and “Climate action (SDG 13)”. |
Introduction
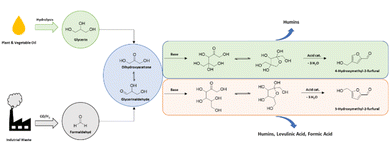
In recent years, the plant biomass-based compound 5-hydroxymethyl-2-furfural (5-HMF) has garnered significant attention in the framework of the green transformation of the chemical industry. This multifunctional aromatic compound is considered a key platform chemical for replacing petroleum-based drugs, fuels, and organic materials and its oxidized derivative 2,5-furandicarboxylic acid was ranked by the national renewable energy laboratories (NREL) to be one of the top 12 renewable feedstocks in 2004.1 While the production of 5-HMF appears straightforward through Brønsted or Lewis acid-catalyzed triple dehydration of fructose, the state of the art is that both the selective synthesis of 5-HMF and the extraction and purification processes are so challenging that large-scale production of 5-HMF is still done to a limited extent.2,3 A major limitation in the production of 5-HMF is the high waste generation due to humification reactions, which result from cross-polymerization reactions between the sugar feedstock and the synthesized 5-HMF alongside with its degradation products. These reactions produce “humin,” a black polymeric solid that is in some modifications soluble and in others insoluble in water and most organic solvents, making the overall 5-HMF synthesis economically difficult on a large scale.3,4a–c However, recent developments in industrial 5-HMF production show interesting advances. For instance, AVA Biochem's hydrothermal carbonization process (which is primarily a biocoal production process), developed in 2014, was announced with an annual production capacity of 20 tons of 5-HMF with the expectation to double the production capacity by the end of the same year.5 On the current website of AVA-Biochem from 2024 it is reported that the company is producing 5-HMF for the specialty chemical market, as well as “technical grade” 5-HMF for bulk application.6 Another significant effort in the production of a 5-HMF derivative comes from the Dutch company Avantium, which has developed a production process for FDCA on a plant scale.7 Here, instead of 5-HMF Avantium produces 5-methoxymethyl-2-furfural (5-MMF) as a key intermediate due to the challenges posed by 5-HMF's high polarity and reactivity.7 Since 5-HMF contains a hydroxymethyl group and an aldehyde functionality, dissolving 5-HMF in common apolar solvents for extraction purposes is difficult.7 Purifying 5-HMF is especially problematic due to its instability, which can lead to the formation of humins or its reaction with acidic water to form levulinic acid. It is noteworthy that many researchers have focused on synthetic and process optimization approaches to maximize 5-HMF reaction selectivity and yield. The most promising results have been achieved using very polar solvents such as THF,8 DMSO,9 DMAc,10 or deep eutectic solvents (DES)11 during the sugar dehydration reaction, resulting in HMF reaction selectivity exceeding 99%.12 Nevertheless, purifying the aromatic compound from such high-boiling or water-miscible organic solvents is highly energy-intensive and, thus, considered questionable in terms of sustainability.While much attention has been focused on 5-HMF, another intriguing derivative, in detail its regioisomer 4-hydroxymethyl-2-furfural (4-HMF), deserves consideration. This compound can be synthesized through the simple mono-aldol reaction of dihydroxyacetone (DHA), which is available from the renewable resource glycerol or industrial waste carbon monoxide, followed by a Brønsted acid-catalyzed triple dehydration of the resulting sugar molecule 2,4-bis(hydroxymethyl)tetrahydrofuran-2,3,4-triol, which is further named dendroketose.13 Dihydroxyacetone itself can be produced from CO being available from industrial waste streams, or through enzymatic or anodic oxidation of bio-based glycerol, making it an attractive, sustainable, and inexpensive starting material (Scheme 1).14,15 Selective oxidation of glycerol can take place via various reaction pathways, including microbial fermentation with Gluconobacter oxydans.14 This method represents an industrial application of microorganisms that has been extensively investigated from a microbiological perspective.16
Notably, Deng et al. made significant progress by synthesizing 4-HMF from formalin, subsequently hydrogenating it to 2,4-dimethylfuran and further to C9–C18 alkanes for biofuel applications. They reported a high HMF selectivity of 93% using DMSO as a solvent and Amberlyst®15 (Amb15) as the acidic ion exchange resin catalyst, at a substrate loading of 200 g per L (0.5 g scale synthesis).17 Later, in 2016, the same group described an optimized process for selective 4-HMF synthesis and subsequent isolation from the DMSO-based solution on a 400 mg scale (100 g per L), achieving an 80% HPLC yield and an 80% isolated yield through column chromatography, or 60% when purified by distillation.13 The 4-HMF process developed by the Deng group was successfully demonstrated in a flow setup, showcasing the potential for a scale up into industrially relevant scales.13 Furthermore, they synthesized important derivatives of 4-HMF such as 2,4-furandicarboxylic acid or 2,4-dihydroxymethylfuran, which are suitable for the production of bioplastics as already demonstrated for their 5-HMF-based counterparts 2,5-furandicarboxylic acid and bishydroxymethylfuran.13 Inspired by the Deng group's work, we aimed to further optimize the 4-HMF synthesis process by excluding DMSO and using water as a green and sustainable solvent, although it has to be mentioned that a water/MIBK-based biphasic system was already introduced by Deng et al. in 2013 resulting in an impressive 4-HMF yield of 80% at a 99% dendroketose conversion using a self-synthesized tantalum phosphate catalyst (reaction scale 2.4 g, dendroketose loading 100 g per L, catalyst loading 10 g per L, with an organic to aqueous ratio of 1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, and a reaction temperature of 180 °C at 50 bar N2 pressure).13 Our approach aims to simplify this synthesis process by facilitating product removal via straightforward liquid–liquid extraction, thereby avoiding the energy-intensive DMSO distillation. We focus on using inexpensive, readily available catalysts and conducting the reaction under mild conditions, at low temperatures and atmospheric pressure. This methodology is designed with scalability in mind, enabling the reaction to be performed efficiently on a multigram scale. Additionally, our goal has been to develop a feasible, cost-effective, and reproducible process for the selective and sustainable synthesis of 4-HMF. This study focuses on synthesizing 4-HMF in water, followed by an in situ product removal process to enhance the yield in combination with a tailor-made reactor set-up. Like 5-HMF, 4-HMF is a highly versatile compound that can be further derivatized into valuable chemicals such as biofuels, pharmaceuticals, lubricants, and plastics.18
1, and a reaction temperature of 180 °C at 50 bar N2 pressure).13 Our approach aims to simplify this synthesis process by facilitating product removal via straightforward liquid–liquid extraction, thereby avoiding the energy-intensive DMSO distillation. We focus on using inexpensive, readily available catalysts and conducting the reaction under mild conditions, at low temperatures and atmospheric pressure. This methodology is designed with scalability in mind, enabling the reaction to be performed efficiently on a multigram scale. Additionally, our goal has been to develop a feasible, cost-effective, and reproducible process for the selective and sustainable synthesis of 4-HMF. This study focuses on synthesizing 4-HMF in water, followed by an in situ product removal process to enhance the yield in combination with a tailor-made reactor set-up. Like 5-HMF, 4-HMF is a highly versatile compound that can be further derivatized into valuable chemicals such as biofuels, pharmaceuticals, lubricants, and plastics.18
Results and discussion
Dendroketose preparation
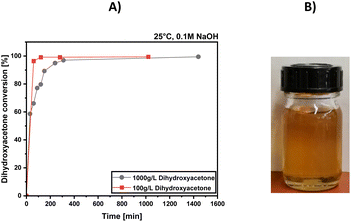
For the preparation of dendroketose, we adapted the method of the Deng group by using 0.1 M NaOH solution for the mono-aldol reaction of two molecules of DHA. The synthesis was performed at 100 g per L and 1000 g per L DHA loading. Fig. 1A) shows the DHA conversion over time for both experiments, revealing that the reaction is faster performed at lower DHA concentration which can be rationalized by the relative concentrations between DHA and the catalyst sodium hydroxide. For example, for a 100 g per L DHA loading, the total catalyst loading of NaOH is calculated to be 8.3 mol%, while for a 1000 g per L DHA loading, it is 0.9 mol%. Indeed, the reaction can be performed faster, when for higher DHA loadings the NaOH concentration is adapted, but for a later neutralization step this would mean a higher amount of salt production. When calculating the ecological factor (E-factor; EF = mass (waste)/mass (product)) for both reactions, the 100 g per L reaction achieves an undesired EF = 10, while the 1000 g per L reaction achieves an EF = 1, when the water is removed for substance isolation. When the water is not removed, as it will be demonstrated in the last section of this work, the E-factor is calculated to be EF = 0.01 for the 1000 g per L reaction with the assumption that the produced NaCl is treated as waste (which will not be the case as demonstrated in the further sections). The atom economy of the reaction is calculated to be AE = 100%. The reaction is performed at ambient conditions, and the reaction enthalpy is endothermic. For the work up procedure, the reaction water might be removed by distillation procedures. For substance characterization, we removed the water at 40 °C and 0.001 mbar, which lasted 2 h of distillation time to remove 100 mL of water. The total energy consumption for this process was calculated to be 1.04 kW h, which is related to the production of 374 g of CO2 per 100 g of product and an estimated energy cost of 0.19 EUR per 100 g of produced dendroketose (see ESI† for further details). The 1H-NMR and 13C-NMR of the synthesized dendroketose is presented in the ESI† of this work. According to both spectra successful production of dendroketose is concluded, which is present as its 5-membered ring furanose form, since no evidence was found for a substance with keto-functionality. Dendroketose appears as a yellowish oil and was isolated with a purity of 92%, while 8% is attributed to the produced NaCl salt during the neutralization step.Solvent screening for the selective preparation of 4-HMF
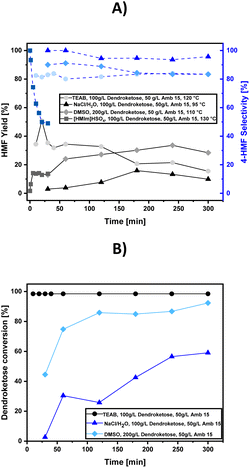
To optimize the preparation of 4-HMF starting from the aldol product of DHA, we investigated the most promising synthesis conditions already established for producing 5-HMF from D-fructose or similar C6-sugars. We initially adopted the method of Yu et al.,19 who achieved an 82% yield of 5-HMF from sucrose using the ionic liquid [HMIm]HSO4, synthesized by simply adding sulfuric acid to N-methylimidazole (NMI). This yield is remarkably high for sucrose. Another efficient synthesis strategy was reported by Guo et al.,20 who used a tetraethylammonium bromide (TEAB)/CrCl3 system to obtain a high 90% yield of 5-HMF starting from D-fructose and 76% from D-glucose. We also explored the method of the Deng group,13 who synthesized 4-HMF with yields close to 80% using DMSO as a solvent and Amb15 as an immobilized acidic catalyst. Additionally, we were inspired by the Yousatit group,21 who achieved a 56% yield of 5-HMF with 82% sugar conversion in an aqueous NaCl/THF solution; however, we omitted THF and focused on synthesizing 4-HMF solely in an aqueous NaCl solution. To ensure comparability across all synthetic strategies, we consistently used Amb15 as a reusable strong acid catalyst, conducted all reactions at 95 °C, and monitored the 4-HMF yield and dendroketose conversion to assess the reaction progress and the 4-HMF selectivity, which are shown in Fig. 2A and 2B.For example, the dehydration reaction of dendroketose, produced from the aldol reaction of two molecules of DHA, proceeds most rapidly when a deep eutectic solvent such as [HMIm]HSO4 or TEAB is used as the reaction medium. However, in [HMIm]HSO4, we observed a continuous decline in 4-HMF selectivity from the start, with a maximum yield of only 14% after 30 minutes, leading us to conclude that this reaction medium is not promising for further optimization. In contrast, using TEAB as the solvent, we achieved a maximum 4-HMF yield of 50% after 30 minutes, although the yield then steadily decreased, likely due to humin formation which could be observed from the black discoloration of the reaction solvent over time. The 4-HMF selectivity in the TEAB system was calculated to be 82%, with the remaining 18% attributed to the formation of 5-HMF. When using DMSO as the solvent, the reaction rate slowed down, reaching a maximum 4-HMF yield of 37% after 4 hours. The selectivity for 4-HMF decreased from 90% at the beginning of the reaction to 80% after 5 hours, with 5-HMF again identified as a by-product. In the dehydration reaction of dendroketose in saturated aqueous NaCl solution, a 4-HMF yield of only 17% was achieved after 3 hours, but this reaction exhibited the highest selectivity for 4-HMF being close to 95%, which would mean in a later purification process that the separation between 4-HMF and 5-HMF can be nearly omitted. Additionally, monitoring dendroketose conversion over time revealed nearly complete conversion in the TEAB system, 93% conversion in the DMSO system after 5 hours, but only 57% conversion in the NaCl solution over the same period. In conclusion, while the NaCl/H2O system offers the highest selectivity for 4-HMF, the incomplete reaction presented a challenge for further optimization to improve the overall 4-HMF yield.
Another key factor in choosing the NaCl/H2O system over the DES and DMSO systems is the energetic and toxicological advantages offered by the aqueous system. In the case of the DMSO system, isolating the product requires the distillation of the solvent. As reported by Deng et al.,13 this distillation process should preferably be carried out after basification of the reaction mixture, below 50 °C and under a high vacuum of at least 10−3 mbar for several hours to ensure complete removal of DMSO. For example, our laboratory vacuum oil pump consumes 0.18 kW of energy and to remove 1 L of DMSO at 50 °C would require approximately 16 h of distillation time. This would mean an energy consumption of 0.18 kW × 16 h = 2.88 kW h. Additionally, heating 1.1 kg of DMSO (cp = 2780 J (kg × °C) from 25 °C to 50 °C and maintaining that temperature for 16 hours with using the laboratory heating device (0.1 kW average power) requires approximately 1.64 kW h of energy. Thus, the total energy consumption for only DMSO distillation in the purification process amounts to around 4.52 kW h per kg of solvent which is related to the production of 52 g of 4-HMF with respect to our best achieved synthesis yield of 37% at a dendroketose loading of 200 g per L.
On the other hand, the DES system, which includes TEAB, involves less favorable halogen chemistry, as the salt is synthesized from toxic bromoethane (a known CMR – carcinogenic, mutagenic, or reprotoxic substance) and triethylamine, which is an inhalation toxin. TEAB itself is a respiratory and skin irritant.
In contrast, the aqueous NaCl solution uses non-toxic sodium chloride, and the product can be efficiently extracted using low-boiling solvents like methyl tetrahydrofuran (MTHF), a bio-based THF alternative. MTHF can be regenerated in a closed-loop process, further reducing environmental and energy costs.
However, since the aqueous system currently achieves a low HMF yield of only 17%, the goal is to optimize process parameters such as salt additives, synthesis temperature, catalyst type and dendroketose concentration, as well as the reaction control which is needed to enhance yields. These adjustments aim to improve HMF selectivity and yield, ensuring a process with minimized waste generation and maximum productivity.
Salt screening
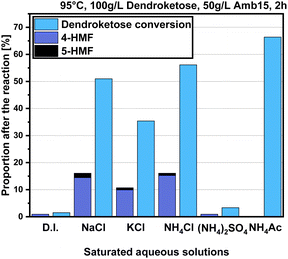
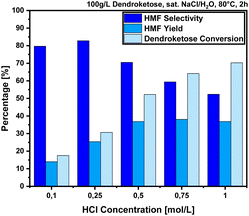
The first optimization step in the saturated salt/H2O system consisted of a screening of different salt systems while varying the cations and anions to observe their impacts on the reaction. For example, we screened also KCl and NH4Cl next to NaCl, thus varying the nature of cations, and on the other side we screened (NH4)2SO4 and NH4Ac to get an insight into the effect of the anion. We chose readily available and cost-efficient salt additives to make the overall synthesis economically valuable. For comparison, the reaction was also conducted in deionized water (D.I.) and the results of these experiments, which were conducted at 95 °C and with an Amb15 catalyst loading of 50 g per L at a reaction time of 2 h, are provided in Fig. 3.The results presented in Fig. 3 show that the addition of salt can significantly influence the dehydration reaction leading to 4-HMF formation. For instance, when the reaction was conducted in deionized (D.I.) water, the 4-HMF yield was surprisingly low with just 3% and a dendroketose conversion of only 5%. However, under the same conditions, the addition of NaCl increased the 4-HMF yield to 15% and the dendroketose conversion to 51%. Using a saturated KCl solution resulted in a reduction of the 4-HMF yield to 10% and a dendroketose conversion to 35%. With a saturated NH4Cl solution, the 4-HMF yield was like that of NaCl at 15%, but the 4-HMF:5-HMF ratio and dendroketose conversion were significantly higher. Interestingly, when the anion was changed from Cl− to SO42− or acetate, the 4-HMF yields dropped significantly, and in case of NH4Ac, no 4-HMF was detected at all, despite the consumption of dendroketose. For further optimization, we selected NaCl as the salt additive of choice due to its lower cost, greater availability, and lower toxicity compared to ammonium-based salts.
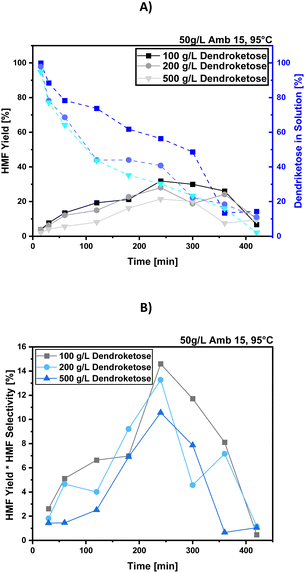
Screening of different dendroketose concentrations for the synthesis of 4-HMF
The next step in optimizing our 4-HMF-synthesis involved screening different dendroketose concentrations to determine the ideal sugar loading that balances both optimal space-time yields and overall HMF selectivities. We tested dendroketose loadings across a range from 10 g per L to 500 g per L (see ESI† for comprehensive data). For clarity reasons, we present the results for 100 g per L, 200 g per L, and 500 g per L dendroketose loadings, illustrating the HMF yield over time in Fig. 4A and yield-related selectivities in Fig. 4B. As before, we maintained constant unoptimized parameters such as a reaction temperature of 95 °C, and 50 g per L Amb15 catalyst.In Fig. 4A, which illustrates the time course of HMF yield in solution and dendroketose conversion, it becomes evident that higher sugar loadings, such as 200 g per L or 500 g per L, result in a more rapid consumption of the sugar molecules, but the HMF yield remains relatively low. Typically, the HMF yield decreases over time as the sugar consumption curve intersects with the HMF production curve, indicating that side reactions are becoming more dominant. For the 100 g per L dendroketose loading, the highest HMF yield of 32% is observed after 240 minutes of reaction time. However, when analyzing yield-related selectivities (HMF yield × HMF selectivity), which are shown in Fig. 4B, the 100 g per L sugar loading delivers the best results in terms of yield and overall HMF selectivity, while the process running at a 200 g per L sugar loading gives similar results at higher substance concentrations, thus making it more advantageous from an industrial production perspective. The results suggests that further optimization, particularly by screening reaction temperatures at 100 g per L and 200 g per L dendroketose loadings, could further enhance the process.
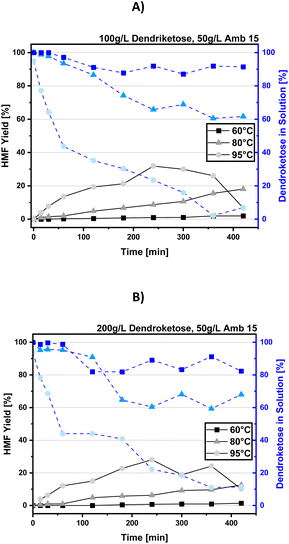
Temperature screening
The reaction temperature represents an important parameter when it comes to the dehydration reaction of sugar molecules. In case of 5-HMF-production, the reaction temperature is a key criterium to determine dehydration velocity, but if the temperature is chosen too high, side reactions such as humification will dominate leading to non-satisfactory selectivity. For 5-HMF, typically reaction temperatures between 100 °C to 200 °C are chosen,22 but in our case of the 4-HMF process reaction temperature is limited to the boiling temperature of the aqueous NaCl system at 1013 mbar and, thus, we investigated the reaction at temperatures of 60 °C, 80 °C, and 95 °C. When employing a pressurized system, the maximal reaction temperature can be increased, but this requires special authorized equipment and appropriate safety measures. As discussed in the section before, we focused to study the temperature effect on the dendroketose dehydration at 100 g per L and 200 g per L loadings. The results on the corresponding 4-HMF yields and dendroketose conversion are summarized in Fig. 5A and 5B.The reaction temperature turned out as a critical factor in the dehydration of dendroketose to 4-HMF, as clearly shown in Fig. 5A and B. For instance, at 60 °C, almost no 4-HMF is produced over 420 minutes, although dendroketose consumption reaches nearly 10% at a 100 g per L sugar loading and 20% at a 200 g per L sugar loading. When the temperature is increased to 95 °C, both 4-HMF yield and dendroketose conversion rate rise significantly, with maximum yields of 32% and 28% observed after 240 minutes for 100 g per L and 200 g per L sugar loadings, respectively. At 80 °C, the reaction is slower than at 95 °C but faster than at 60 °C, with a more linear dendroketose conversion rate. Notably, during the first two hours at 80 °C and a 100 g per L sugar loading, a high 4-HMF selectivity is observed. With this positive result in hand, at a dendroketose substrate loading of 100 g per L and temperatures of 95 °C and 80 °C, optimization of the catalyst amount was carried out.
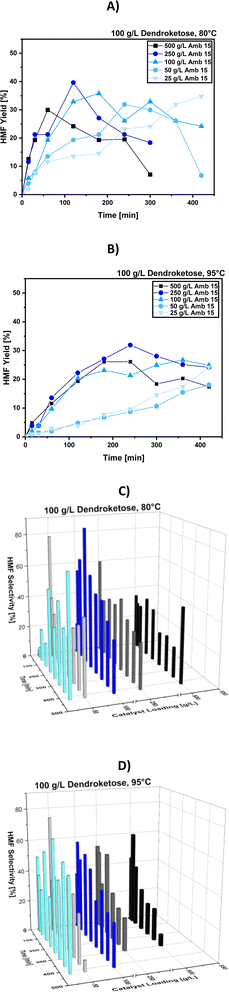
Screening of different catalyst loadings
The next step in our optimization process focuses on adjusting the amount of catalyst used, specifically the ion exchange resin Amberlyst 15. Amberlyst 15 (Amb15) is a strong acidic solid catalyst known for its effectiveness in the dehydration of C6-sugar molecules to produce 5-HMF, and in our case, 4-HMF. Being a heterogeneous catalyst, a key advantage is its easy removal from the reaction system and its potential for recyclability. Numerous studies have demonstrated Amb15's reusability in reactions forming both 5-HMF and 4-HMF across various solvents such as ionic liquids,23 water-based systems,24 and DMSO.24,25We tested varying amounts of Amb15, ranging from 25 g per L to 500 g per L, to assess its impact on the reaction. We then measured the resulting 4-HMF yields and selectivities at reaction temperatures of 80 °C and 95 °C over a time frame of 420 min, as shown in Fig. 6.
In general, increasing the amount of catalyst accelerates the reaction rate, as illustrated in Fig. 6A and B, with the reaction progress steadily increasing from 25 g per L to 500 g per L Amb15 loading. However, at 80 °C, the overall HMF selectivity appears to be higher compared to the reaction at 95 °C. Specifically, at 95 °C, HMF selectivity is high when using lower catalyst amounts, such as 25 g per L Amb15, where a maximum selectivity of 60% is achieved after 240 minutes. However, the corresponding HMF yield is only 10%. As the catalyst amount increases, HMF yields improve, but selectivity decreases. This indicates that at 95 °C, while selectivity peaks early in the reaction, the yields remain too low.
In contrast, at 80 °C the same trend of decreased selectivity with increasing catalyst amounts is observed. However, with 100 g per L Amb15, the highest overall HMF selectivity of 80% is achieved, along with a corresponding yield of 33%. Since both yield and selectivity decrease over time due to the formation of soluble and insoluble oligo- or polymeric side-products, we propose a strategy to remove HMF from the system via liquid–liquid extraction. We expected that by isolating the HMF, it is prevented from further participating in undesired reactions. This approach suggests the following optimal reaction conditions: 80 °C, 120 minutes, and 100 g per L Amb15, followed by HMF extraction. The remaining dendroketose in the aqueous phase can then be dehydrated in subsequent cycles. However, before scaling up the reaction and engineering the process, the final parameter requiring optimization is the catalyst type.
Catalyst type
Next to Amb15, we also evaluated alternative heterogeneous solid catalysts, including Dowex50, Amberlyst16, Fulcat22F, and Fulcat22B (for details, see ESI†). Amb15 consistently provided the best HMF yields and selectivity. However, despite its effectiveness, recycling studies in our saturated NaCl/H2O system (for details, see ESI†) revealed that Amb15 became inactivated after the first recycling cycle and was no longer able to catalyze the dehydration of dendroketose to 4-HMF. During the recycling process, we washed the solid catalyst with the reaction solvent before reusing it in the subsequent cycle. Upon measuring the pH of the solution with fresh Amb15, we found it to be 1, indicating a proton (H+) exchange with sodium ions (Na+) from the resin. This suggests that, in essence, aqueous HCl is the real catalyzing component in this system. This hypothesis was confirmed by a control experiment (results are available in the ESI† of this work), where we compared the dehydration of dendroketose using (1) fresh Am15 beads and its reuse in the 1st recycling cycle after washing it with the reaction solvent NaCl/H2O, and (2) the NaCl aqueous solution of fresh Amb15 beads, which was incubated in the named solvent for 2 h at 80 °C without dendroketose. Accordingly, when the reaction solution, which was previously incubated with Amb15 beads, was used for the dehydration reaction of dendroketose without the presence of Amb15, we found an HMF yield of 33%, while the same reaction gave an HMF yield of 3% when ‘recycled’ Amb15 beads were used. This finding proves that the ion exchange resin does not act as a heterogeneous catalyst in our reaction solvent, but releases HCl into the solution, which catalyzes the dehydration reaction as a homogeneous catalyst. From this point on, we screened several homogeneous Brønsted acid catalysts such as HCl, H2SO4, trifluoromethanesulfonic acid or Lewis acid catalysts like several metal triflates (see ESI† for further details), but the utilization of HCl as homogeneous Brønsted acid catalyst delivered best results. For identifying optimal conditions, we screened HCl concentrations between 0.1 M to 1 M (Fig. 7).It was found that both 4-HMF yields and selectivity decrease as the HCl concentration increases, a trend previously observed when using the Amb15 catalyst. The optimal HCl concentration under the given conditions was determined to be 0.25 M, as this concentration resulted in 83% selectivity towards 4-HMF with a 31% yield. A similar result was obtained when the reaction was carried out with 100 g per L Amb15. Using the resin's acid number, we calculated the theoretical amount of HCl that could be released during the ion exchange reaction. Taking into account its 50% water retention capacity, this amount was estimated to be 0.24 M HCl.26 Finally, a control test to determine if a 0.25 M HCl solution in deionized water would yield the same reaction result was carried out. As shown in the ESI (see Fig. S7†), the addition of NaCl significantly enhances the dehydration reaction of dendroketose demonstrating the crucial role of the salt addition in this reaction.
Optimization of the reaction process
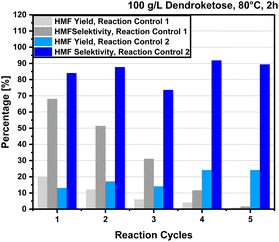
Through our optimization process, we have achieved a maximum selectivity of 80% for 4-HMF synthesis using a dendroketose loading of 100 g per L, an HCl concentration of 0.25 M, a reaction temperature of 80 °C, and a reaction time of 120 minutes. Under these conditions, we obtain a maximum yield of 30% HMF when the reaction is performed on a 1 g scale. As discussed above, our strategy to further improve the HMF yield involves isolating the product from the reaction mixture after 2 hours, since extended reaction times decrease both yield and selectivity. Therefore, we stop the reaction after 120 minutes, at which point the HMF selectivity is 80%, and extract the product using 2-methyltetrahydrofuran (MTHF) as the solvent. From our data we are aware that after 2 hours the dendroketose is not fully consumed. When the reaction mixture is reheated to 80 °C for a second dehydration cycle, the dendroketose concentration will be below 100 g per L, leading to a gradual decline in both product yield and selectivity with each cycle. To maintain consistent reaction control, one strategy is to replenish the consumed dendroketose after each cycle to keep the concentration at 100 g per L. To evaluate the impact of these two approaches, we performed the reaction on a 10 g scale using two methods: (1) starting with a dendroketose loading of 100 g per L and continuing the reaction in multiple cycles until all dendroketose is consumed, and (2) maintaining a 100 g per L dendroketose loading in every cycle, allowing the reaction to proceed without interruption. The results of both methods, which are designated as ‘reaction control 1’ and ‘reaction control 2’, are summarized in Fig. 8. The experiment was conducted over five reaction cycles for both control variants. In ‘reaction control 1’, the initial amount of dendroketose was fully consumed at the end of the cycles, while in ‘reaction control 2’, the dendroketose concentration was maintained at 100 g per L throughout. In ‘reaction control 1′, where the dendroketose concentration gradually declined, the HMF yield peaked at 20% in the first cycle (100 g per L dendroketose) with a reaction selectivity of 68%. However, both yield and selectivity steadily decreased in subsequent cycles, resulting in an average HMF yield of 43% and an average selectivity of 33% after five cycles. In contrast, for ‘reaction control 2’, where dendroketose was replenished to 100 g per L after each cycle, HMF yields remained relatively stable between 10% and 20%, with reaction selectivity consistently ranging between 70% and 90% (Fig. 8). The average HMF yield for this method was calculated to be 92%, with an average selectivity of 85%. These favorable results from ‘reaction control 2’ encouraged us to further scale up the reaction to an elevated 100 g lab scale.Multigram preparation under optimized conditions
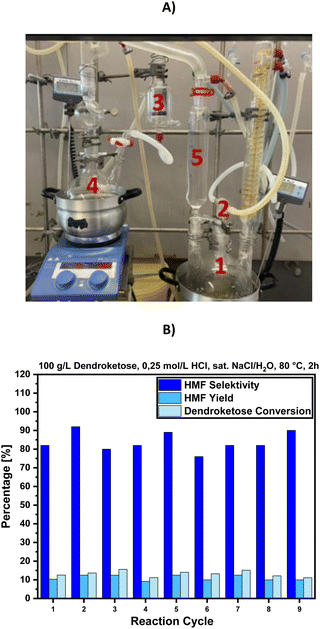
This section presents the synthesis of 4-HMF on a 100 g scale, aimed at validating the robustness of the developed process under optimized reaction parameters, including the optimal solvent, dendroketose concentration, temperature, reaction time, catalyst, and reaction control. For this purpose, we designed a customized reaction apparatus to stop the reaction at the optimal reaction time, extract the produced 4-HMF prior to the occurrence of side reactions, and replenish the consumed dendroketose after each cycle. The apparatus, shown in Fig. 9A, consists of five components: (1) reaction zone, (2) pump, (3) filtration, (4) product concentration, and (5) solvent reservoir. In the reaction zone, dehydration of dendroketose takes place using saturated NaCl/H2O as the reaction medium and 0.25 M HCl as a homogeneous catalyst. After 2 hours at 80 °C, the extraction solvent, MTHF, is added, initiating the first of three extraction cycles. Using the neckpiece of a Wulf bottle, the organic solvent is drawn from the reaction zone into the filtration zone, where it passes through activated carbon filtration. From there, it moves to the vacuum distillation zone, where MTHF is distilled at 40 °C and 150 mbar into the solvent reservoir. Once the reservoir collects 250 mL of MTHF, the next extraction cycle begins. Fig. 9B presents the results of the multigram synthesis of 4-HMF. In each cycle, the HMF yield consistently reached 10%, with reaction selectivity ranging between 75% and 90%.Approximately 15% of dendroketose was consumed per cycle, and fresh dendroketose was added prior to the start of the next cycle. Using this approach, we achieved a total reaction yield of 89% related to the total amount of dendroketose that was consumed across nine reaction cycles (109 g consumed dendroketose in total). The average HMF selectivity was calculated to be 84%. After removing the solvent MTHF, the isolated yield of 4-HMF was determined to be 76% (58 g of 4-HMF were determined by weighing), with a purity of 92%, and 8% attributed to the side product 5-HMF (Fig. 10). The mentioned yields were calculated as follows: yield [%] = moles of HMF produced/maximal moles of HMF that can be formed from the reacted amount of dendroketose. The final product appeared as a dark reddish viscous oil. The E-factor for the multigram reaction was determined by mass analysis to be EF = 0.67, as the organic solvent was recycled in a closed-loop process. This calculation assumes uninterrupted reaction cycles, with the NaCl/H2O reaction solvent continuously reused as demonstrated during this work for the nine reaction cycles (see ESI† for further details). The total energy consumption of this reaction is calculated with the energy requirement that is needed to heat the sat. NaCl solution (cp = 2.97 kJ (kg × °C) from 25 °C to 80 °C in the first cycle and from 60 °C to 80 °C in the further cycles, and to hold it at that temperature for 2 h (see ESI† for further details). Accordingly, the heating up operation will require a total energy amount of 0.18 kW h. The energy requirement to hold 80 °C for 2 h of reaction time is calculated to be 0.2 kW h × 9 cycles = 1.8 kW h. The energy consumption for 15 min of MTHF distillation, which is required to recover 0.25 L of the solvent, is calculated to be 0.05 kW h × 9 cycles × 3× extraction per cycle = 1.22 kW h for the pump, and additional 0.68 kW h to hold the distillation temperature of 40 °C for 9 cycles and 3× extraction procedure with the used laboratory heating device. Therefore, the total amount of energy that is required to produce 58 g of 4-HMF is calculated to be 3.88 kW h.
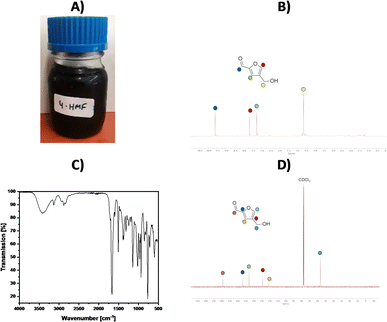 | ||
| Fig. 10 (A) Physical appearance of the isolated 4-HMF, (B) 1H-NMR spectrum of the isolated 4-HMF, (C) ATR-FTIR spectrum of the isolated 4-HMF, (D) 13C-NMR spectrum of the isolated 4-HMF. | ||
Sustainability metrics
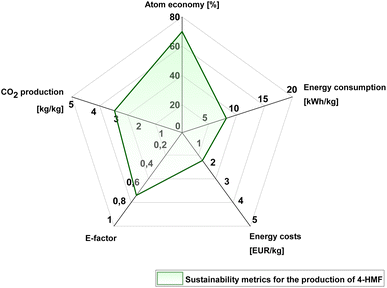
The optimized multigram scale 4-HMF synthesis process has been evaluated by means of sustainability metrics including E-factor, total energy consumption, estimated product costs, atom ecomomy and total CO2 emissions (Fig. 11). During the whole process, which includes 9 reaction cycles, 209 g dendroketose was needed to conduct the reaction. Accordingly, 109 g took part in the dehydration reaction within the 9 reaction cycles, and the residual 100 g were kept in loop to stabilize the whole process. The total yield of 4-HMF was determined to be 58 g (76%) and the total dry amount of solid humin was determined to be 13.7 g after 9 reaction cycles. The total loss of the biobased organic solvent MTHF was determined to be 24.7 g which results in an overall excellent E-factor of EF = 0.67. This desirable E-factor is calculated with the assumption that the reaction is conducted in a non-interrupted mode, because the saturated NaCl solution can be used for further dendroketose dehydration cycles. In comparison to a typical 5-HMF process, the E-factor of only 0.67 for this 4-HMF process is remarkably low, thus being highly attractive and underlining the sustainability of the 4-HMF production process from the perspective of a decreased waste generation. Furthermore, it justifies to focus in further research on this aromatic compound 4-HMF as a biorenewable platform-chemical with potential for bulk production.The total energy amount that was needed to produce 58 g of 4-HMF was calculated to be 3.88 kW h with respect to the energy requirements for heating and distillation procedures. This would correspond to a total energy requirement of approximately 66.90 kW h for the production of 1 kg of 4-HMF by means of this process being conducted at a 1 L synthesis scale. With the current energy costs of 0.181 EUR per kW per h in Germany,27 the production of 4-HMF with the described procedure would cost 12.11 EUR per kg from the energy cost perspective. In Germany, the amount of released CO2 per generated kW h of energy is estimated to be 360 g kW per h in 2023.28 For our process this would mean a generation of 24.1 kg CO2 per generated kg of 4-HMF. Indeed, the calculated amount of 66.90 kW h per kg is considered to be high for a compound that is intended to be produced on bulk industrial scale. On the other side, the current calculations are based on our lab-scale experiments, and we are confident that the energy requirements can be significantly reduced when upscaling the reaction. Energy costs for heating and distillation will be reduced significantly when producing 1 kg of product in just one cycle, instead of 153 cycles (which would be the case in the 1 L scale experiment). For example, to produce 1 kg of 4-HMF with our synthesis strategy in only one cycle, one would need a synthesis-scale of 125 L. The total energy requirement on a 125 L scale is estimated to be only 8.61 kW h, and accordingly the production costs will be reduced to only 1.56 EUR/kg, while the corresponding CO2 production reduces to only 3.1 kg kg−1 (for details, see ESI†). It should be added that our estimation is made based on the assumption that product yield and selectivity (and with it also the E-factor) remain the same as in the 1 L scale reaction. Note that our evaluation of production costs and CO2 emissions was based solely on an energy perspective. The method used to produce DHA from glycerol—whether through anodic oxidation or biocatalytic oxidation—can significantly impact both the CO2 emissions and the product price, potentially altering the calculated values by a factor of 5 to 10. Therefore, the origin of the glycerol feedstock (whether bio-based, fossil-based, or CO-based) can have a substantial effect on the sustainability metrics of the process.
However, also employing more energy-efficient devices, integrating heat exchangers for better energy circulation and recovery, and utilizing advanced extraction technologies (e.g., stronger extraction agents or membrane-assisted pervaporation techniques) can reduce energy requirements. Additionally, incorporating solar energy could significantly reduce dependence on fossil energy sources and can further reduce the carbon footprint of the whole process. The atom economy for the dendroketose dehydration reaction is calculated to be 70%.
Conclusions
In summary, we successfully optimized the preparation of 4-HMF from dendroketose through a series of reactions and process enhancements. Based on a literature-known protocol, initially dendroketose preparation using 0.1 M NaOH has been optimized, demonstrating that the reaction can be performed at a high substrate loading of 1000 g per L. With this optimized synthesis of dendroketose in hand, we then explored various synthetic strategies for its dehydration under formation of the desired target compound 4-HMF. Our results showed that when using [HMIm]HSO4 and TEAB as reaction media, 4-HMF reaction was connected with selectivity concerns due to substantial extent of side reactions such as 5-HMF and humin formation. In contrast, using an aqueous saturated NaCl solution as a solvent provided the highest 4-HMF:5-HMF selectivity of 95%, with an acceptable 17% yield. Through salt screening, we identified NaCl as the optimal additive, improving both conversion and yield while being economically accessible. Further optimization focused on dendroketose concentration, temperature, catalyst amount, and type of catalyst. The optimal conditions were determined to be 100 g per L dendroketose, 0.25 M HCl, 80 °C, and a reaction time of 120 minutes, resulting in a maximum 4-HMF yield of 30% with 80% selectivity. Furthermore, our findings indicated that controlling dendroketose loading across multiple reaction cycles significantly improves the yield, with ‘reaction control 2’ maintaining a 100 g per L dendroketose concentration yielding a stable average yield of 92% over five cycles. In the final scale-up experiment on a 100 g scale, we validated our optimized parameters, achieving a total reaction yield of 89% over nine reaction cycles with an average HMF selectivity of 84%. The isolated yield of 4-HMF was 76%, with a purity of 92%, making this process highly efficient and attractive in terms of future technical applications. The calculation of the E-factor for the multigram reaction turned out to give a favorable value of 0.67, underlining the environmental and economic viability of this optimized synthesis pathway for 4-HMF.Experimental section (materials and methods)
Chemicals
2-Methyltetrahydrofuran (>99%, Roth), acetonitrile (>99.9%, Honeywell), Amberlyst 15 (Thermo Scientific), ammonium acetate (98.2%, VWR Chemicals), ammoniumchloride (98%, Roth), ammonium sulfate (99.5%, Thermo Scientific), calciumchloride (99%, Supelco), deuteriumoxide (99.9%, Deutero), dihydroxyaceton (98%, BLD Pharm), potassium chloride (>99%, VWR Chemicals), magnesium sulfate pentahydrate (>99%, Roth), sodium chloride (99.8%, VWR Chemicals), 1 M hydrochloric acid solution (Fisher), tetraethylammonium bromide (98%, Thermo Scientific), N-methylimidazole (>99%, Roth), sulfuric acid (97%, Bielefeld University), 1 M sodium hydroxide solution (Fisher) were purchased from the suppliers given in parentheses.Preparation and isolation of dendroketose
100 g of DHA (1.11 mol) and 100 mL of 0.1 M NaOH were stirred in a one-necked flask at room temperature for 22 hours. The pH was then adjusted to 7 using 1 M HCl. An orange, transparent solution was obtained. The water was then removed under reduced pressure. Yield: >99%, Purity: 92%.1H NMR (500 MHz, D2O) δ [ppm]: 4.19–3.41 (m, 7H).
13C NMR (126 MHz, D2O) δ [ppm]: 103.15, 82.39, 77.24, 77.19, 63.86, 61.75.
ATR-FTIR ν [cm−1]: 3014–3666, 2913, 2887, 1003.
Preparation of 4-HMF on a 100 g scale
In a 2 L three-necked flask, DHA (100 g, 1.11 mol) and 100 mL NaOH (0.1 M) were stirred at room temperature for 22 hours. The pH was then adjusted to 7 with 1 M HCl. 40 g NaCl, 237.5 mL HCl (1 M) and 600 mL saturated NaCl/H2O solution were added to the yellow solution. The mixture was stirred at an oil bath temperature of 80 °C for 2 hours. The mixture was then extracted with 3 × 250 mL of MTHF. Using a vacuum pump, the organic phase was passed through a CaCl2/activated charcoal filter (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 w/w) and the MTHF was distilled under reduced pressure and re-used, thus being retained in the system loop. After the determination of the dendroketose consumption (see ESI† for further details) an amount of sugar being equivalent to that consumed in the previous cycle was re-added to the aqueous phase and the next cycle was started. The extract was dried with 100 g MgSO4·5H2O, filtered, and was then distilled off completely under reduced pressure. 4-HMF was obtained as a dark reddish oil. Isolated yield: 76%.
1 w/w) and the MTHF was distilled under reduced pressure and re-used, thus being retained in the system loop. After the determination of the dendroketose consumption (see ESI† for further details) an amount of sugar being equivalent to that consumed in the previous cycle was re-added to the aqueous phase and the next cycle was started. The extract was dried with 100 g MgSO4·5H2O, filtered, and was then distilled off completely under reduced pressure. 4-HMF was obtained as a dark reddish oil. Isolated yield: 76%.
1H-NMR (500 MHz, CDCl3) δ [ppm]: 9.61 (s, 1H), 7.66 (s, 1H), 7.26 (s, 1H), 4.62 (s, 2H).
13C-NMR (126 MHz, CDCl3) δ [ppm]: 178.15, 153.29, 145.31, 128.28, 120.59, 57.60.
ATR-FTIR ν [cm−1]: 3157–3600, 3125, 2885, 1666, 1508, 1145, 1018, 763.
EI-MS [m/z]: 126[M]·+, 97 [M-HCO]+.
Data availability
The data supporting this article have been included in this work and as part of the ESI.†Author contributions
K. C. established the process concept. M. R. performed the experimental work. K. C. and M. R. performed data analysis and data interpretation. H. G. supervised the work. The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors acknowledge Bielefeld University for the financial funding of this work.References
- G. P. T. Werpy, Top Value Added Chemicals From Biomass. Volume I: Results of Screening for Potential Candidates from Sugars and Synthesis Gas, 2004 Search PubMed.
- A. A. Rosatella, S. P. Simeonov, R. F. M. Frade and C. A. M. Afonso, Green Chem., 2011, 13, 754 RSC.
- K. I. Galkin and V. P. Ananikov, ChemSusChem, 2019, 12, 2976 CrossRef CAS PubMed.
- (a) G. Almendros, J. Sanz and L. Sobrados, Sci. Total Environ., 1989, 81–82, 91 CrossRef; (b) K. I. Galkin and V. P. Ananikov, ChemistryOpen, 2020, 9, 1135 CrossRef CAS PubMed; (c) M. Sajid, B. Hanif, R. Bano and G. Bari, Academia Letters, 2022, 5658 Search PubMed.
- T. Kläusli, Green Process. Synth., 2014, 3, 235 CrossRef.
- AVA Biochem AG, High-quality, Sustainable Chemicals, 2024, https://ava-biochem.com/our-products/, accessed on 19.10.2024 Search PubMed.
- E. de Jong, H. R. A. Visser, A. S. Dias, C. Harvey and G.-J. M. Gruter, Polymers, 2022, 14, 943 CrossRef CAS PubMed.
- (a) Y. Román-Leshkov and J. A. Dumesic, Top. Catal., 2009, 52, 297 CrossRef; (b) Q. Sun, S. Wang, B. Aguila, X. Meng, S. Ma and F.-S. Xiao, Nat. Commun., 2018, 9, 3236 CrossRef PubMed.
- (a) K. Shimizu, R. Uozumi and A. Satsuma, Catal. Commun., 2009, 10, 1849 CrossRef CAS; (b) A. Chinnappan, A. H. Jadhav, H. Kim and W.-J. Chung, Chem. Eng. J., 2014, 237, 95 CrossRef CAS.
- J. B. Binder and R. T. Raines, J. Am. Chem. Soc., 2009, 131, 1979 CrossRef CAS PubMed.
- S. Simeonov, Org. Synth., 2016, 93, 29 CrossRef CAS.
- S. K. R. Patil, J. Heltzel and C. R. F. Lund, Energy Fuels, 2012, 26, 5281 CrossRef CAS.
- M.-S. Cui, J. Deng, X.-L. Li and Y. Fu, ACS Sustainable Chem. Eng., 2016, 4, 1707 CrossRef CAS.
- R. Ciriminna, G. Palmisano, C. Della Pina, M. Rossi and M. Pagliaro, Tetrahedron Lett., 2006, 47, 6993 CrossRef CAS.
- A. M. Bahmanpour, A. Hoadley, S. H. Mushrif and A. Tanksale, ACS Sustainable Chem. Eng., 2016, 4, 3970 CrossRef CAS.
- N. Izuo, K. Nabe and S. Yamada, J. Ferment. Technol., 1980, 58, 221 CAS.
- J. Deng, T. Pan, Q. Xu, M.-Y. Chen, Y. Zhang, Q.-X. Guo and Y. Fu, Sci. Rep., 2013, 3, 1244 CrossRef PubMed.
- Y. Wang, C. A. Brown and R. Chen, AIMS Microbiol., 2018, 4, 261 CAS.
- S.-B. Yu, H.-J. Zang, X.-L. Yang, M.-C. Zhang, R.-R. Xie and P.-F. Yu, Chin. Chem. Lett., 2017, 28, 1479 CrossRef CAS.
- X. Guo, H. Zhu, Y. Si, H. Ban, X. Lv, Y. Cheng, L. Wang and X. Li, ACS Sustainable Chem. Eng., 2022, 10, 14579 CrossRef CAS.
- S. Yousatit, R. Osuga, J. N. Kondo, T. Yokoi and C. Ngamcharussrivichai, Fuel, 2022, 311, 122577 CrossRef CAS.
- (a) Y. H. Seo and J.-I. Han, Food chemistry, 2014, 151, 207 CrossRef CAS PubMed; (b) F. Menegazzo, E. Ghedini and M. Signoretto, Molecules, 2018, 23, 2201 CrossRef PubMed; (c) L. Yan, N. Liu, Y. Wang, H. Machida and X. Qi, Bioresour. Technol., 2014, 173, 462 CrossRef CAS PubMed; (d) Y.-B. Yi, J.-W. Lee, S.-S. Hong, Y.-H. Choi and C.-H. Chung, J. Ind. Eng. Chem., 2011, 17, 6 CrossRef CAS.
- X. Qi, M. Watanabe, T. M. Aida and J. R. L. Smith, Green Chem., 2009, 11, 1327 RSC.
- P. P. Upare, D. W. Hwang, Y. K. Hwang, U.-H. Lee, D.-Y. Hong and J.-S. Chang, Green Chem., 2015, 17, 3310 RSC.
- (a) G. Sampath and S. Kannan, Catal. Commun., 2013, 37, 41 CrossRef CAS; (b) K. Shimizu, R. Uozumi and A. Satsuma, Catal. Commun., 2009, 10, 1849 CrossRef CAS.
- Thermo Fisher Scientific, Amberlyst™ 15(H), Ion Exchange Resin, Product Specification, 2024 Search PubMed.
- Statistisches Bundesamt, https://www.destatis.de/DE/Themen/Wirtschaft/Preise/Publikationen/Energiepreise/energiepreisentwicklung-pdf-5619001.html, accessed on 19.10.2024 Search PubMed.
- Umweltbundesamt, zu finden unterhttps://www.umweltbundesamt.de/themen/co2-emissionen-pro-kilowattstunde-strom-2023, accessed on 19.10.2024 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4su00790e |
| This journal is © The Royal Society of Chemistry 2025 |