Open Access Article
Open Access ArticleOne-pot synthesis of fully biobased polyureas with excellent mechanical properties from epoxidized soybean oil†
Lei Zhanga and
Donglin Tang *ab
*ab
aDepartment of Polymer Materials Science Engineering, South China University of Technology, Guangzhou 510640, China. E-mail: msdltang@scut.edu.cn
bGuangdong Provincial Key Laboratory of Luminescence from Molecular Aggregates (South China University of Technology), Guangzhou 510640, China
First published on 15th May 2025
Abstract
Owing to their highly flexible structure, polymers based on epoxidized soybean oil (ESO) usually exhibit poor mechanical properties. This work was aimed at improving their mechanical properties by incorporating a ureido bond into the backbone of such polymers. Polyureas (PUrs) were successfully designed and prepared from urea, 1,10-decanediamine (DDA) and ESO via a one-pot strategy. Urea first reacted with DDA to form an oligourea, which was then polymerized with ESO. The content of ureido function could be adjusted by simply changing the feeding ratio of urea/DDA and epoxy/ureido to tune the mechanical properties of the obtained PUrs. The one-pot PUrs exhibited comparable thermal and mechanical properties to those obtained via the two-pot synthetic method, in which ESO was polymerized with 1,10-decylene bisurea (DBU). By maintaining an equal amount of ureido and epoxy groups and changing the feeding ratio of urea/DDA from 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 3
1 to 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 and even to 4
2 and even to 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, the glass transition temperature of PUrs increased from 26.9 to 56.4 °C, as detected by the dynamic mechanical thermal analysis. Furthermore, the tensile strength increased from 8.2 to 17.7 MPa, while the elongation at break declined from 430% to 180%. The one-pot synthetic strategy for PUrs developed in this work is promising for the construction of high-performance vegetable oil-based sustainable polymers.
3, the glass transition temperature of PUrs increased from 26.9 to 56.4 °C, as detected by the dynamic mechanical thermal analysis. Furthermore, the tensile strength increased from 8.2 to 17.7 MPa, while the elongation at break declined from 430% to 180%. The one-pot synthetic strategy for PUrs developed in this work is promising for the construction of high-performance vegetable oil-based sustainable polymers.
Sustainability spotlightPlant oils are important biobased feedstocks for manufacturing polymer materials, and thus, polymers manufactured from plant oils are considered to contribute towards achieving sustainability. The most important issue limiting the application of plant oil-based polymers is their poor mechanical properties. The main goal of this work was to improve the mechanical properties of plant oil-based polymers. This work revealed that the mechanical properties can be greatly improved by incorporating ureido functions into the backbone of polymers via a one pot synthetic method using urea, diamine and epoxidized soybean oil. This work contributes to the UN SDG goal 12 (sustainable consumption and production). |
Introduction
Polyureas (PUrs) are polymers containing ureido groups in the backbone. Owing to the formation of double-dentate hydrogen bonding, PUrs usually exhibit relatively higher performance than polyurethanes, including higher tensile strength, higher tearing strength, and excellent chemical resistance.1 The conventional feedstocks used to synthesize PUrs, namely, isocyanates, are recognized to have high reactivity, especially with amines. However, these isocyanates are basically derived from petrochemical feedstocks, and generally, extremely hazardous synthetic procedures are required. Hence, researchers are currently making great efforts in exploring non-isocyanate routes for the preparation of PUrs, such as polymerization of CO2 with amines, polycondensation of carbamates with amines and polycondensation of urea with amines.2–4 However, when using the CO2 route, high pressure is required, and the molecular weight of the obtained PUrs is generally not high enough, limiting the further development of this method. When compared with the carbamate route, the urea route seems similar but could be more straightforward since the starting material for carbamate formation, dimethyl carbonate, is also derived from the alcoholysis of urea. Therefore, the urea route is believed to be more convenient and economically efficient. In our previous work,5 we developed a catalyst-free and solvent-free route towards linear PUrs with excellent mechanical properties via direct polycondensation of urea and aliphatic diamines.Considering the depletion of petroleum resources, the current focus is on using more sustainable biomass.6 Biomass resources, including cellulose, starch, proteins, and vegetable oils, are considered to be suitable feedstocks for the preparation of biobased polymers.7–9 Vegetable oils are abundant and of great variety, including castor oil, soybean oil, linseed oil and palm oil. Usually, there are multiple functional groups in vegetable oils, making them easy to modify.10,11 Owing to the presence of double bonds (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C) in their structure, vegetable oils have potential in the preparation of various thermosets via cationic, free radical, or olefin metathesis polymerizations.12 Soybean oil is nontoxic, inexpensive and abundant, and hence, it is currently considered to be the most attractive biobased feedstock for constructing biobased polymers.13,14 The application of soybean oil can be largely expanded via epoxidation to form epoxidized soybean oil (ESO),15 and the large number of epoxy groups in ESO makes it a suitable candidate in the production of renewable polymer materials.16,17 ESO is typically used to construct epoxy resins or polyols for further polyurethane synthesis.18 When reacted with CO2, ESO can also be converted into carbonated soybean oil, which is another desirable biobased feedstock for the synthesis of polyhydroxyurethane.19–25 However, the mechanical properties of polymers based on soybean oil and its derivatives are still not good enough and need to be improved.26–29 Several approaches have been tried to increase the mechanical properties of soybean oil-based polymers, including curing of ESO with polyamide oligomers,29,30 and introduction of a rigid structure (fumaropimaric acid or maleic anhydride) into the backbone.31–33
C) in their structure, vegetable oils have potential in the preparation of various thermosets via cationic, free radical, or olefin metathesis polymerizations.12 Soybean oil is nontoxic, inexpensive and abundant, and hence, it is currently considered to be the most attractive biobased feedstock for constructing biobased polymers.13,14 The application of soybean oil can be largely expanded via epoxidation to form epoxidized soybean oil (ESO),15 and the large number of epoxy groups in ESO makes it a suitable candidate in the production of renewable polymer materials.16,17 ESO is typically used to construct epoxy resins or polyols for further polyurethane synthesis.18 When reacted with CO2, ESO can also be converted into carbonated soybean oil, which is another desirable biobased feedstock for the synthesis of polyhydroxyurethane.19–25 However, the mechanical properties of polymers based on soybean oil and its derivatives are still not good enough and need to be improved.26–29 Several approaches have been tried to increase the mechanical properties of soybean oil-based polymers, including curing of ESO with polyamide oligomers,29,30 and introduction of a rigid structure (fumaropimaric acid or maleic anhydride) into the backbone.31–33
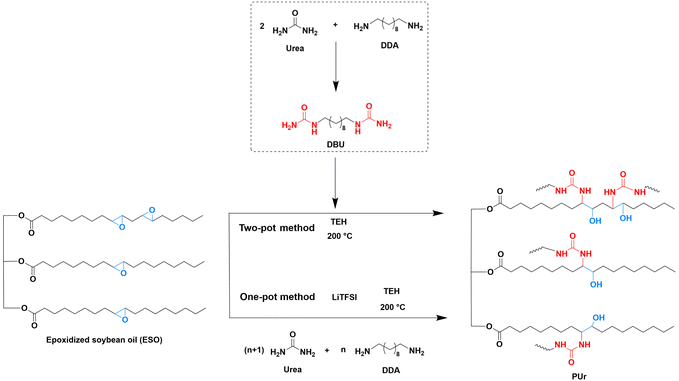
It is envisioned that incorporation of polyurea or oligourea blocks into soybean oil moieties would enhance the mechanical properties of the resulting polymers. Therefore, this study utilized the reaction between ureido and epoxy groups to synthesize ESO-based PUrs and tuned their mechanical properties by changing the content of ureido function. Urea, 1,10-diamine (DDA) and ESO were reacted in a one-pot in two steps. In the first step, urea and DDA were reacted to form oligourea, and the content of ureido function could be easily tuned by varying the feeding ratio of urea and DDA. Then, in the second step, ESO was added to react with the formed oligourea, resulting in the formation of PUrs. To decrease the melting point of oligourea and afford a homogeneous reaction mixture with ESO, lithium salt LiTFSI was added to assist the melting of oligourea. For comparison, a two-pot reaction was performed, starting from the synthesis and purification of 1,10-decylene bisurea (DBU). DBU is the product of urea and DDA at a feed ratio of 2/1. All the starting materials, including ESO, urea, and DDA, are biobased, and accordingly, the obtained polymers are fully biobased PUrs. Additionally, the structure and properties of the obtained PUrs were investigated. Thus, this work offers a promising strategy to enhance the performance of vegetable oil-based polymers, thereby extending their application scope.
Experimental section
Materials
Urea (99%), 1,10-decanediamine (DDA, 97%), tin(II) 2-ethylhexanoate (TEH, 95%), n-octylamine (99%) and lithium bis(trifluoromethane)sulfonimide (LiTFSI, 99%) were purchased from Shanghai Aladdin Biochemical Technology Co., Ltd (Shanghai, China). ESO (epoxy value = 6) and deuterated dimethyl sulfoxide (DMSO-d6) were purchased from Anhui Senrise Technology Co., Ltd (Anhui, China). All chemicals were used without further treatment.Synthesis of DBU
1,10-Decylene bisurea (DBU) was synthesized according to the method of Guerrero-Alburquerque.34 DDA (250 mmol, 43.08 g) and urea (750 mmol, 45.05 g) were added into a 500 mL single-neck flask equipped with a condenser. Deionized water (100 g) was added as a solvent. The reaction was carried out under stirring at 110 °C in an oil-bath for 12 h. A white solid product was collected, ultrasonicated in deionized water for 90 min, filtered and then dried under vacuum at 60 °C for 12 h. The isolated yield is 97%. The obtained final product is a white solid and it was characterized by 1H NMR (Fig. S1†).Two-pot synthesis of PUr
ESO (13.33 g, 50 mmol epoxy groups) and DBU (6.46 g, 25 mmol) were added into a reactor equipped with a mechanical stirrer. Under an argon atmosphere, the reactor was heated at 200 °C for 15 min to allow melting of DBU. Upon stirring, TEH (0.81 g, 2 mmol) was added into the reactor to start the polymerization. The viscosity of the mixture increased after reacting for about 30 min. The stirring was stopped to allow the curing of the product for another 5 h to obtain PUr1.0-TP (where 1.0 represents the molar ratio between the epoxy and ureido groups).One-pot synthesis of PUrs
Taking the synthesis of PUr1.0-3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 as an example, urea (4.50 g, 75 mmol), DDA (8.62 g, 50 mmol) and LiTFSI (2.87 g, 10 mmol, 20 mol% relative to DDA) were first added into a reactor equipped with a mechanical stirrer. The mixture was stirred and reacted at 200 °C for 1 h, under an argon atmosphere. ESO (13.33 g, 50 mmol epoxy groups) was then added dropwise under stirring, along with TEH (0.81 g, 2 mmol, 4 mol% relative to epoxy groups). The viscosity increased after 1 h and the stirring was stopped to allow the curing of the mixture for another 5 h to obtain PUr1.0-3
2 as an example, urea (4.50 g, 75 mmol), DDA (8.62 g, 50 mmol) and LiTFSI (2.87 g, 10 mmol, 20 mol% relative to DDA) were first added into a reactor equipped with a mechanical stirrer. The mixture was stirred and reacted at 200 °C for 1 h, under an argon atmosphere. ESO (13.33 g, 50 mmol epoxy groups) was then added dropwise under stirring, along with TEH (0.81 g, 2 mmol, 4 mol% relative to epoxy groups). The viscosity increased after 1 h and the stirring was stopped to allow the curing of the mixture for another 5 h to obtain PUr1.0-3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 (where 3
2 (where 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 represents the molar ratio between urea and DDA). PUr1.0-4
2 represents the molar ratio between urea and DDA). PUr1.0-4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, PUr0.8-3
3, PUr0.8-3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 and PUr1.0-2
2 and PUr1.0-2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 were synthesized with the same procedure but with different feeding amounts of urea, DDA and ESO (see Table 1).
1 were synthesized with the same procedure but with different feeding amounts of urea, DDA and ESO (see Table 1).
| Samples | REpoxy/Ureido | Urea (mmol) | DDA (mmol) | LiTFSI (mmol) | ESO (g) | TEH (mmol) |
|---|---|---|---|---|---|---|
PUr1.0-2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1.0 | 100 | 50 | 10 | 13.33 | 2 |
PUr1.0-3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
1.0 | 75 | 50 | 10 | 13.33 | 2 |
PUr1.0-4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
1.0 | 100 | 75 | 12 | 13.33 | 2 |
PUr0.8-3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
0.8 | 75 | 50 | 10 | 10.66 | 1.6 |
Characterization
Fourier transform infrared (FTIR) spectroscopy analysis was carried out on a Nicolet IS50 FTIR spectrometer (USA). After 32 scans at 25 °C, the spectra were obtained in a range of 4000–400 cm−1 with a resolution of 4 cm−1. 1H NMR tests were performed on a BRUKER AV III 500 (500 MHz) spectrometer (Switzerland) operating at 25 °C. DMSO-d6 containing 0.03% internal standard TMS was used as the solvent and the chemical shifts are reported in ppm. Solid state 13C NMR tests were performed on a Jeol ECZ-400R/M1 (400 MHz) spectrometer (Japan) operating at 25 °C with a rotation rate set at 5 kHz, the relaxation time set at 2 s, and 1000 repeat scans taken to finalize the results. Elemental analysis was carried out on a HITACHI Z-2000 atomic absorption spectrophotometer (Japan). Thermogravimetric analysis (TGA) was carried out using a Netzsch TG209 F3 analyzer (Germany). The heating rate was set as 20 °C min−1 and the N2 gas flow rate was 20 mL min−1. Differential scanning calorimetry (DSC) was executed using a Netzsch DSC 204 F1 calorimeter (Germany) under a N2 atmosphere with a flow rate of 40 mL min−1. For DSC scans, a heating rate of 20 °C min−1 and a cooling rate of 10 °C min−1 were set. Dynamic mechanical thermal analysis (DMA) was conducted on a Netzsch DMA 242 E Artemis dynamic mechanical analyzer (Germany). For each experiment, a sample cut into a rectangle shape (10 × 5 × 0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) mm3) was loaded on a tension-type fixture, and the loading mode was selected as multi-frequency strain. Strain amplitude and frequency were set at 0.1% and 1 Hz, respectively. The temperature was set ranging from −50 to 140 °C with a heating rate of 3 °C min−1. Mechanical properties were estimated by tensile tests. Samples for tensile tests were obtained by compression molding. Compression molding was carried out at 200 °C, the clamping pressure was 20 MPa and the compression time was set to be 15 min. The dimension of the mold was 60 × 60 × 0.5 mm3. Then film samples were cooled to room temperature in a cold compression mold with a clamping pressure of 20 MPa. Samples were cut into dumbbell-shaped tensile samples according to ISO 37 Type 4 standard. Tensile tests were performed in an Instron 5960 tester equipped with a load transducer of 10 kN. The tensile rate was set at 50 mm min−1, and the tests were performed at 25 °C. For each sample, the tests were repeated at least 5 times and the average values were calculated.
mm3) was loaded on a tension-type fixture, and the loading mode was selected as multi-frequency strain. Strain amplitude and frequency were set at 0.1% and 1 Hz, respectively. The temperature was set ranging from −50 to 140 °C with a heating rate of 3 °C min−1. Mechanical properties were estimated by tensile tests. Samples for tensile tests were obtained by compression molding. Compression molding was carried out at 200 °C, the clamping pressure was 20 MPa and the compression time was set to be 15 min. The dimension of the mold was 60 × 60 × 0.5 mm3. Then film samples were cooled to room temperature in a cold compression mold with a clamping pressure of 20 MPa. Samples were cut into dumbbell-shaped tensile samples according to ISO 37 Type 4 standard. Tensile tests were performed in an Instron 5960 tester equipped with a load transducer of 10 kN. The tensile rate was set at 50 mm min−1, and the tests were performed at 25 °C. For each sample, the tests were repeated at least 5 times and the average values were calculated.
Results and discussion
Synthesis of PUrs
The synthetic procedure for PUrs is shown in Scheme 1. It has been confirmed in our previous work35 that epoxy is able to react with the ureido group (pendant urea group, –NHCONH2). The chemical structures existing in the network of obtained products include urethane, ureido and carbonate groups. In our study here, reactions between bisurea and ESO were utilized to synthesize ESO-based PUrs. A model reaction was first conducted to confirm the reaction product of the ureido group with ESO. Octyl urea (1H NMR spectrum, see Fig. S2†), which was obtained from octylamine and urea, was used to react with ESO at 200 °C and the conversion was traced by 1H NMR (see Fig. S3†). There is no peak of urethane group (NHCOO, normally at 7.0 ppm) observed during the whole reaction process. Octyl urea was fully consumed within 60 min. Therefore, it is assumed that the polymer network formed from bisurea and ESO should be basically a PUr under such reaction conditions. PUrs can be synthesized in two ways. One is the two-pot method in which bisurea has to be synthesized and purified before reacting with ESO. The other is that the synthesis of both bisurea and polymers is performed in the same reactor, which is called a one-pot method. Compared with the two-pot method, it is more convenient to change the feed ratio of urea and DDA to achieve the adjustment of ureido content in the one-pot method. The change of ureido content is expected to tune the mechanical properties of the obtained PUrs. Oligourea was formed in the first step when DDA reacted with excess urea. The feeding ratio of urea to DDA was set to 2/1, 3/2 and 4/3 and the products were named PUr1.0-2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, PUr1.0-3
1, PUr1.0-3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 and PUr1.0-4
2 and PUr1.0-4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, respectively. Lithium ions are able to form coordination compounds together with ureas.36 For instance, urea and LiTFSI were applied to form deep eutectic solvents with a melting point as low as −33 °C.37 Thus, LiTFSI was added to reduce the melting temperature of the oligourea so as to keep the polymerization mixture in a homogeneous state. Otherwise rapid solidification would occur. After the completion of oligourea formation, ESO was directly added to allow the polymerization for another 6 h to obtain PUr products. PUr0.8-3
3, respectively. Lithium ions are able to form coordination compounds together with ureas.36 For instance, urea and LiTFSI were applied to form deep eutectic solvents with a melting point as low as −33 °C.37 Thus, LiTFSI was added to reduce the melting temperature of the oligourea so as to keep the polymerization mixture in a homogeneous state. Otherwise rapid solidification would occur. After the completion of oligourea formation, ESO was directly added to allow the polymerization for another 6 h to obtain PUr products. PUr0.8-3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 (the molar ratio between the epoxy and ureido groups equals 0.8) was also synthesized to investigate the effect of group ratio, to see if the formed β-hydroxy group further reacts with the ureido group.38 LiTFSI and TEH can be removed from PUrs by swelling in dimethylformamide. The yield of PUr is 77.9%. After purification, the remaining content of Li and Sn is found by elemental analysis to be 0.017% and 0.61%, respectively. In the two-pot route (PUr1.0-TP), DBU (1H NMR spectrum, see Fig. S1†) was synthesized and purified before reacting with ESO.
2 (the molar ratio between the epoxy and ureido groups equals 0.8) was also synthesized to investigate the effect of group ratio, to see if the formed β-hydroxy group further reacts with the ureido group.38 LiTFSI and TEH can be removed from PUrs by swelling in dimethylformamide. The yield of PUr is 77.9%. After purification, the remaining content of Li and Sn is found by elemental analysis to be 0.017% and 0.61%, respectively. In the two-pot route (PUr1.0-TP), DBU (1H NMR spectrum, see Fig. S1†) was synthesized and purified before reacting with ESO.
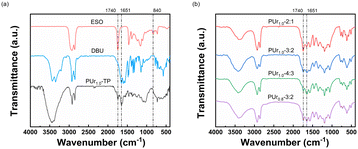
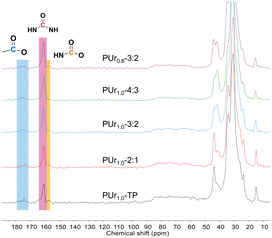
As observed from the FTIR spectrum of PUr1.0-TP (see Fig. 1a), the peak at 840 cm−1 attributed to the epoxy group (stretching of C–O–C) disappears after polymerization, while the carbonyl stretching signals of ester and ureido groups are observed at 1740 and 1651 cm−1, respectively. The signal of stretching vibrations of N–H in the ureido group and O–H in the β-hydroxy group can be found at 3400 cm−1. The FTIR spectra of PUrs obtained from one pot are similar to that of PUr1.0-TP (see Fig. 1b and S4†). Since the obtained PUr are gels, solid state 13C NMR spectra were used to further determine the chemical structures (see Fig. 2). The peak at 160.6 ppm is ascribed to polyurea, while the peak locating at 172–175 ppm represents the ester groups originating from ESO. Due to the decomposition of DBU, a little amount of isocyanate formed upon heating (Fig. S5†). Such a little amount of isocyanate must be able to react with OH function to yield urethane functions. So signals can be observed between 157 ppm and 160 ppm. However, the main structure is still ureido function. In conclusion, the results of FTIR and 13C NMR indicate the successful formation of PUrs from bisurea and ESO, either via one-pot or two-pot synthetic strategies.
Thermal properties
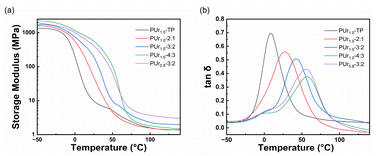
Thermogravimetric (TG) and derivative thermogravimetric (DTG) curves of all PUr samples are shown in Fig. 3a and b. T5% (the temperature corresponding to 5% mass loss) of all PUr samples are found to be similar at around 300 °C, which indicates rather good thermal stability. The two loss peaks observed in the DTG curves at 336 °C and 475 °C represent the decomposition of ester and urea groups and that of hydrocarbon chains, respectively. Only glass transition but no melting or crystallization peak is observed on the DSC curves (see Fig. 3c and d).Dynamic mechanical thermal analysis (DMA) curves are shown in Fig. 4. In the DMA curves for all samples, glass transition is obvious and the glass transition temperature (Tg) can be determined and the results are listed in Table 2. When the feeding ratio of urea to DDA was changed from 2/1 (PUr1.0-2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to 3/2 (PUr1.0-3
1) to 3/2 (PUr1.0-3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) and further to 4/3 (PUr1.0-4
2) and further to 4/3 (PUr1.0-4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3), the Tg value of the resulting PUr shifts from 26.9 to 41.2 and then to 56.4 °C. The rubbery plateau after the glass transition is clearly flat, which fits with the behaviors of crosslinking polymers. According to the theory of rubber elasticity, the crosslinking density (ν) of the elastomer can be determined by eqn (1).
3), the Tg value of the resulting PUr shifts from 26.9 to 41.2 and then to 56.4 °C. The rubbery plateau after the glass transition is clearly flat, which fits with the behaviors of crosslinking polymers. According to the theory of rubber elasticity, the crosslinking density (ν) of the elastomer can be determined by eqn (1).
 | (1) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, PUr1.0-3
1, PUr1.0-3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 and PUr1.0-4
2 and PUr1.0-4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, their crosslinking densities are 393.19, 300.21 and 267.72 mol m−3, respectively. The descending crosslinking density can be attributed to the increasing chain length of oligourea. The storage moduli in the glassy state for these three samples follow the same trend due to the increasing content of ureido function. When compared with PUr1.0-3
3, their crosslinking densities are 393.19, 300.21 and 267.72 mol m−3, respectively. The descending crosslinking density can be attributed to the increasing chain length of oligourea. The storage moduli in the glassy state for these three samples follow the same trend due to the increasing content of ureido function. When compared with PUr1.0-3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, PUr0.8-3
2, PUr0.8-3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 has a higher crosslinking density of 503.64 mol m−3, which might be due to the fact that β-hydroxy groups can further react with the ureido groups. PUr1.0-TP shows the highest crosslinking density of 845.08 mol m−3.
2 has a higher crosslinking density of 503.64 mol m−3, which might be due to the fact that β-hydroxy groups can further react with the ureido groups. PUr1.0-TP shows the highest crosslinking density of 845.08 mol m−3.
| Samples | Td,5% (°C) | Td,max (°C) | Tg,DSC (°C) | Tg,DMA (°C) | v (mol m−3) |
|---|---|---|---|---|---|
| PUr1.0-TP | 290.4 | 470.1 | 28.8 | 8.6 | 845.08 |
PUr1.0-2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
295.6 | 471.8 | 25.9 | 26.9 | 393.19 |
PUr1.0-3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
292.8 | 476.5 | 32.5 | 41.2 | 300.21 |
PUr1.0-4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
301.1 | 477.7 | 35.0 | 56.4 | 267.72 |
PUr0.8-3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
295.8 | 473.7 | 37.8 | 53.9 | 503.64 |
Mechanical properties
Typical stress–strain curves from tensile tests are shown in Fig. 5 and relative data are listed in Table S1.† The tensile strength and elongation at break of PUr1.0-TP are detected to be 5.4 MPa and 190%, respectively, which are higher when compared with most other polyurethanes based on carbonated soybean oil.20,22–25,40 All PUr samples obtained from one-pot procedures show superior mechanical properties to PUr1.0-TP. Increasing content of ureido function could significantly enhance the mechanical properties; for instance, higher tensile strength (from 8.2 to 17.7 MPa) and Young's modulus (from 7.8 to 125.0 MPa) can be achieved. It is understandable that the elongation at break value declines. When decreasing the ratio of epoxy/ureido (from 1.0 to 0.8), PUr0.8-3:2 shows higher tensile strength and Young's modulus than PUr1.0-3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2. A lower elongation at break is also achieved. Mechanical properties of the PUr samples can be easily tuned by controlling both the feeding ratio of epoxy/ureido and urea/DDA. The mechanical properties of PUrs are superior to those of most of the ESO-based polymers, of which tensile strength ranges from 0.5 to 7.5 MPa and elongation at break ranges from 50 to 230% (see Fig. S6†).20–25,41–43
2. A lower elongation at break is also achieved. Mechanical properties of the PUr samples can be easily tuned by controlling both the feeding ratio of epoxy/ureido and urea/DDA. The mechanical properties of PUrs are superior to those of most of the ESO-based polymers, of which tensile strength ranges from 0.5 to 7.5 MPa and elongation at break ranges from 50 to 230% (see Fig. S6†).20–25,41–43
Conclusions
Fully biobased PUr networks were successfully synthesized from urea, DDA and ESO via a facile one-pot procedure. It was proved that oligourea can be prepared via reacting urea and DDA before polymerization with ESO. The content of ureido function in the oligourea (or the length of oligourea) could be tuned easily in this one-pot procedure, and as a result, the properties of the obtained PUrs could be adjusted. All PUr samples exhibited good thermal stability, with a T5% of around 300 °C. When the feeding ratio of urea/DDA was changed from 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 3
1 to 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 and even to 4
2 and even to 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, the Tg of PUrs, as detected by DMA, increased from 26.9 to 56.4 °C since the density of ureido function increased. The tensile strength of the PUr samples increased from 8.2 to 17.7 MPa, while the elongation at break decreased from 430% to 180%. This work provides a prospective strategy for the construction of vegetable oil-based polymers with enhanced performances.
3, the Tg of PUrs, as detected by DMA, increased from 26.9 to 56.4 °C since the density of ureido function increased. The tensile strength of the PUr samples increased from 8.2 to 17.7 MPa, while the elongation at break decreased from 430% to 180%. This work provides a prospective strategy for the construction of vegetable oil-based polymers with enhanced performances.
Data availability
Data for this article are available at https://doi.org/10.1039/D5SU00095E.Author contributions
The author contribution is as follows: investigation, formal analysis, and writing – original draft (L. Zhang); supervision, writing – original draft, writing – review and editing (D. Tang).Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors gratefully acknowledge the Fund of Guangdong Provincial Key Laboratory of Luminescence from Molecular Aggregates (South China University of Technology, No. 2019B030301003).Notes and references
- B. Shojaei, M. Najafi, A. Yazdanbakhsh, M. Abtahi and C. Zhang, Polym. Adv. Technol., 2021, 32, 2797–2812 CrossRef CAS.
- C. Y. Wu, J. Y. Wang, P. J. Chang, H. Y. Cheng, Y. C. Yu, Z. J. Wu, D. W. Dong and F. Y. Zhao, Phys. Chem. Chem. Phys., 2012, 14, 464–468 RSC.
- D. Tang, D.-J. Mulder, B. A. J. Noordover and C. E. Koning, Macromol. Rapid Commun., 2011, 32, 1379–1385 CrossRef CAS PubMed.
- D. Montarnal, P. Cordier, C. Soulié-Ziakovic, F. Tournilhac and L. Leibler, J. Polym. Sci., Part A: Polym. Chem., 2008, 46, 7925–7936 CrossRef CAS.
- C. Lin, K. Xie and D. Tang, J. Appl. Polym. Sci., 2022, 139, e52513 CrossRef CAS.
- A. Gandini, Macromolecules, 2008, 41, 9491–9504 CrossRef CAS.
- S. Sahoo, S. Mohanty and S. K. Nayak, J. Macromol. Sci., Part A: Pure Appl. Chem., 2018, 55, 36–48 CrossRef CAS.
- B. Nohra, L. Candy, J.-F. Blanco, C. Guerin, Y. Raoul and Z. Mouloungui, Macromolecules, 2013, 46, 3771–3792 CrossRef CAS.
- X.-L. Zhao, P.-X. Tian, Y.-D. Li and J.-B. Zeng, Green Chem., 2022, 24, 4363–4387 RSC.
- M. A. Sawpan, J. Polym. Res., 2018, 25, 184 CrossRef.
- C. Q. Zhang, T. F. Garrison, S. A. Madbouly and M. R. Kessler, Prog. Polym. Sci., 2017, 71, 91–143 CrossRef CAS.
- Y. Lu and R. C. Larock, ChemSusChem, 2009, 2, 136–147 CrossRef CAS PubMed.
- C. K. Williams and M. A. Hillmyer, Polym. Rev., 2008, 48, 1–10 CrossRef CAS.
- D. P. Pfister, Y. Xia and R. C. Larock, ChemSusChem, 2011, 4, 703–717 CrossRef CAS PubMed.
- D. Swern, G. N. Billen, T. W. Findley and J. T. Scanlan, J. Am. Chem. Soc., 1945, 67, 1786–1789 CrossRef CAS.
- S. Chu and A. Majumdar, Nature, 2012, 488, 294–303 CrossRef CAS PubMed.
- G. Lligadas, J. C. Ronda, M. Galià and V. Cádiz, Biomacromolecules, 2010, 11, 2825–2835 CrossRef CAS PubMed.
- S. Kondaveeti, P. Patel, F. M. de Souza and R. K. Gupta, J. Polym. Environ., 2024, 32, 5873–5887 CrossRef CAS.
- H. Tomita, F. Sanda and T. Endo, J. Polym. Sci., Part A: Polym. Chem., 2001, 39, 4091–4100 CrossRef CAS.
- X. Yang, S. Wang, X. Liu, Z. Huang, X. Huang, X. Xu, H. Liu, D. Wang and S. Shang, Green Chem., 2021, 23, 6349–6355 RSC.
- S. Samanta, S. Selvakumar, J. Bahr, D. S. Wickramaratne, M. Sibi and B. J. Chisholm, ACS Sustainable Chem. Eng., 2016, 4, 6551–6561 CrossRef CAS.
- X. Liu, X. Yang, S. Wang, S. Wang, Z. Wang, S. Liu, X. Xu, H. Liu and Z. Song, ACS Sustainable Chem. Eng., 2021, 9, 4175–4184 CrossRef CAS.
- S. Jalilian and H. Yeganeh, Polym. Bull., 2015, 72, 1379–1392 CrossRef CAS.
- S. Hu, X. Chen and J. M. Torkelson, ACS Sustainable Chem. Eng., 2019, 7, 10025–10034 CrossRef CAS.
- X. Yang, C. Ren, X. Liu, P. Sun, X. Xu, H. Liu, M. Shen, S. Shang and Z. Song, Mater. Chem. Front., 2021, 5, 6160–6170 RSC.
- X.-Y. Jian, X.-P. An, Y.-D. Li, J.-H. Chen, M. Wang and J.-B. Zeng, Macromolecules, 2017, 50, 5729–5738 CrossRef CAS.
- D. Yang, X. Peng, L. Zhong, X. Cao, W. Chen, X. Zhang, S. Liu and R. Sun, Carbohydr. Polym., 2014, 103, 198–206 CrossRef CAS PubMed.
- A. Li and K. Li, ACS Sustainable Chem. Eng., 2014, 2, 2090–2096 CrossRef CAS.
- X. Y. Jian, X. P. An, Y. D. Li, J. H. Chen, M. Wang and J. B. Zeng, Macromolecules, 2017, 50, 5729–5738 CrossRef CAS.
- L. Poussard, J. Mariage, B. Grignard, C. Detrembleur, C. Jérôme, C. Calberg, B. Heinrichs, J. De Winter, P. Gerbaux, J. M. Raquez, L. Bonnaud and P. Dubois, Macromolecules, 2016, 49, 2162–2171 CrossRef CAS.
- X. X. Yang, L. Z. Guo, X. Xu, S. B. Shang and H. Liu, Mater. Des., 2020, 186, 108248 CrossRef CAS.
- J. M. España, L. Sánchez-Nacher, T. Boronat, V. Fombuena and R. Balart, J. Am. Oil Chem. Soc., 2012, 89, 2067–2075 CrossRef.
- F. I. Altuna, L. H. Espósito, R. A. Ruseckaite and P. M. Stefani, J. Appl. Polym. Sci., 2011, 120, 789–798 CrossRef CAS.
- N. Guerrero-Alburquerque, S. Zhao, D. Rentsch, M. M. Koebel, M. Lattuada and W. J. Malfait, Polymers, 2021, 13, 1583 CrossRef CAS.
- K. Xie, D. Tang and G. Zhang, Macromol. Chem. Phys., 2022, 223, 2100452 CrossRef CAS.
- X. Chen, K. D. Fulfer, K. T. Woodard and D. G. Kuroda, J. Phys. Chem. B, 2019, 123, 9889–9898 CrossRef CAS.
- A. Nandy and J. Smiatek, Phys. Chem. Chem. Phys., 2019, 21, 12279–12287 RSC.
- Y. Yang, L. Zhang, F. Meng and D. Tang, Macromol. Chem. Phys., 2024, 225, 2300401 CrossRef CAS.
- W. Hu, Z. Ren, J. Li, E. Askounis, Z. Xie and Q. Pei, Adv. Funct. Mater., 2015, 25, 4827–4836 CrossRef CAS.
- I. Javni, D. P. Hong and Z. S. Petrović, J. Appl. Polym. Sci., 2012, 128, 566–571 CrossRef.
- G. Seychal, C. Ocando, L. Bonnaud, J. De Winter, B. Grignard, C. Detrembleur, H. Sardon, N. Aramburu and J.-M. Raquez, ACS Appl. Polym. Mater., 2023, 5, 5567–5581 CrossRef CAS.
- J. Li, B. Ju and S. Zhang, Ind. Crops Prod., 2023, 205, 117466 CrossRef CAS.
- M. Safarpour, A. Zych, M. Najafi, G. Tedeschi, L. Ceseracciu, L. Marini, G. Perotto and A. Athanassiou, J. Appl. Polym. Sci., 2024, 141, e55527 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5su00095e |
| This journal is © The Royal Society of Chemistry 2025 |