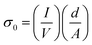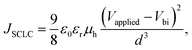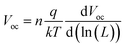Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Highly efficient organic solar cells enabled by ultraviolet-ozone treated molybdenum oxide hole transport layers†
Apostolos Panagiotopoulos a,
George Kakavelakis
a,
George Kakavelakis *b,
Kyriakos Almpanidis
*b,
Kyriakos Almpanidis a,
Leslie Askewa,
Dimitar I. Kutsarova and
S. Ravi P. Silva
a,
Leslie Askewa,
Dimitar I. Kutsarova and
S. Ravi P. Silva *a
*a
aAdvanced Technology Institute, School of Computer Science and Electronic Engineering, University of Surrey, Guildford, Surrey GU2 7XH, UK. E-mail: s.silva@surrey.ac.uk; kakavelakis@hmu.gr
bDepartment of Electronic Engineering, School of Engineering, Hellenic Mediterranean University, Romanou 3, Chalepa, GR-73100, Chania, Crete, Greece
First published on 20th February 2025
Abstract
The application of ultraviolet ozone (UV-Ozone) treatment of thermally evaporated molybdenum oxide (MoOx) as a hole transport layer (HTL) in non-fullerene acceptor (NFA)-organic solar cells (OSCs) has markedly improved the charge carrier transport. As a result, we report the power conversion efficiency (PCE) of PM6:Y6-based OSCs has been improved from 14.26% for pristine to 15.06% for UV-Ozone-treated devices. This PCE enhancement is attributed to increased hole mobility, more balanced mobilities ratio and higher direct current (DC) conductivity. Additionally, the formation of a more favourable interface between MoOx and the PM6:Y6 due to the UV-Ozone exposure, resulted in longer charge carrier lifetimes. Light soaking experiments at 55 °C in a nitrogen environment demonstrated superior operational stability with pristine and UV-Ozone-treated MoOx, retaining 58% and 65% of their initial PCE after 100 hours, respectively. This stands in contrast to devices based on PEDOT:PSS that deteriorated to 23% of their initial PCE after half the time period. This strategy is an enabler towards simultaneous improvement in performance and stability compared to the control PEDOT:PSS-based cells, presenting high efficiency but significantly lower lifetime stability. The broad applicability of UV-Ozone treatment of thermally evaporated MoOx HTLs was further validated through the fabrication of OSCs with a PM6:L8-BO photoactive layer, achieving a peak PCE value of 16.85%. These findings indicate significant advancements in the use of transition metal oxides in NFA-based OSCs and highlight the potential for new device architectures for organic electronics.
Introduction
In recent years, intense research to develop novel materials used for organic solar cells (OSCs) has resulted in power conversion efficiency (PCE) values of over 19%.1–3 Among the emerging photovoltaic technology candidates, OSCs have shown great potential for a plethora of applications where high power-per-weight (PPW) is the key requirement.4 Despite the successful deployment of novel electron transporting layers (ETLs)5–10 and bulk-heterojunction (BHJ) photoactive layers,2,11,12 the progress of hole transporting layers (HTLs) remains limited. To date, poly(3,4-ethylenedioxythiophene):poly(styrenesulfonate) (PEDOT:PSS) and carbon based derivatives13–15 remain the commonly used materials for OSCs with a standard (p–i–n) architecture.16,17 However, the acidic nature of PEDOT:PSS (pH = 1) and its hygroscopicity18 are known for reducing the long-term operational stability of OSCs which commonly rely on indium tin oxide (ITO) as the transparent electrode.19,20 Hence, a stable and efficient HTL alternative is needed for the progressing commercialization of NFA-based OSCs.21Based on the potential for superior environmental stability,22–25 transition metal oxides (TMOs) such as molybdenum oxide (MoOx),26 nickel oxide (NiOx),27 tungsten oxide (WO3)28 and vanadium oxide (V2O5)29 have been successfully used as replacements for PEDOT:PSS in OSCs. Among the TMOs, MoOx has received much attention due to its high optical transmittance in the visible wavelengths, suitable work function (WF), and its versatile easy processing with vacuum- or solution deposition techniques.30,31 Even though evaporated MoOx has already been studied as an HTL for fullerene-based OSCs,32 it has not been deployed successfully in non-fullerene acceptor (NFA)-based systems yet. Today a handful of reports show the use of MoOx as HTL in NFA OSCs, however there are still areas for development on the findings of these studies. More specifically, the work conducted by Brinkmann et al.33 demonstrated a 16.5% PCE on binary OSCs where evaporated MoOx was used as an HTL. In the report, the PCE was measured for devices with an active area of 0.017 cm2, which is smaller than the standard device areas shown in the literature (∼0.1 cm2) and accepted by most international centres for standardization (NREL, Fraunhofer etc.). Furthermore, studies presented by Yaozhao Li et al.30 and Wisnu Hadmojo et al.34 showed poor reproducibility when evaporated MoOx was used as the HTL and with approximately 20% PCE loss compared to the reference samples based on PEDOT:PSS. The authors observed lower PCEs of the used pristine MoOx films as HTLs compared to PEDOT:PSS in NFA-based OSCs and ascribed this to inferior charge transport and collection probabilities at the HTL/ITO interface. This inferior charge transport/collection behaviour is directly related to a poor hole mobility and an unfavourable work function of the MoOx at the interface with the ITO transparent electrode compared to its PEDOT:PSS counterpart.
Herein, we report a facile universal post-deposition treatment on MoOx films that leads to increased charge carrier mobilities, more balanced mobility ratio and improved energetical alignment at the TMO/active layer interface for NFA-based OSCs. In this respect, we investigate the influence of different HTLs on binary PM6:Y6 BHJ-based OSCs. Devices utilizing the UV-Ozone treated MoOx HTL demonstrate improved PCE values peaking at 15.06% compared to the pristine MoOx HTL with a maximum PCE of 14.26%, respectively. This identified enhancement is ascribed to an improvement in the hole mobility and mobilities ratio with a more favourable energy level alignment between the treated MoOx HTL/ITO anode electrode upon the ultraviolet ozone (UV-Ozone treatment). Furthermore, stability studies indicate that OSCs containing PEDOT:PSS experience a rapid performance degradation to 23% of their initial PCE after 51 hours of continuous light soaking at 55 °C in nitrogen atmosphere. By replacing PEDOT:PSS with pristine and UV-Ozone-treated MoOx we observed a significantly reduced device degradation, which provides for more stable interfaces, thus maintaining ∼58% and 65% of their initial PCE after 100 hours. We also explored the applicability of UV-Ozone treated MoOx HTL by employing a state-of-the-art binary photoactive blend PM6:L8-BO and showed that the devices achieved PCE values of 16.85%. Our study sheds light on the mechanisms underlying the performance and mainly the stability of MoOx-based devices, which is the key parameter towards the commercialization of OSCs.
Experimental
Materials
All chemicals used in this paper were obtained commercially and used without further purification. The traditional hole conductive polymer poly(3,4-ethylenedioxythiophene):poly(styrenesulfonate) (PEDOT:PSS Al4083) was obtained from (Ossila, UK) and molybdenum(VI) oxide from (Sigma-Aldrich, UK). The polymer donor poly[(2,6-(4,8-bis(5-(2-ethylhexyl-3-fluoro)thiophen-2-yl)-benzo[1,2-b:4,5-b′]dithiophene))-alt-(5,5-(1′,3′-di-2-thienyl-5′,7′-bis(2-ethylhexyl)benzo[1′,2′-c:4′,5′c′]dithiophene-4,8-dione))] (PM6), the non-fullerene acceptors 2,2′-((2Z,2′Z)-((12,13-bis(2-ethylhexyl)-3,9-diundecyl-12,13-dihydro-[1,2,5]thiadiazolo[3,4-e]thieno[2′′,3′′:4′,5′]thieno[2′,3′:4,5]pyrrolo[3,2-g]thieno[2′,3′:4,5]thieno[3,2-b]indole-2,10-diyl)bis(methanylylidene))bis(5,6-difluoro-3-oxo-2,3-dihydro-1H-indene-2,1-diylidene))dimalononitrile (Y6) and 2,2′-((2Z,2′Z)-((12,13-bis(2-ethylhexyl)-3,9-(2-butyloctyl)-12,13-dihydro-[1,2,5]thiadiazolo[3,4-e]thieno[2′′,3′′:4′,5′]thieno[2′,3′:4,5]pyrrolo[3,2-g]thieno[2′,3′:4,5]thieno[3,2-b]indole-2,10-diyl)bis(methanylylidene))bis(5,6-difluoro-3-oxo-2,3-dihydro-1H-indene-2,1-diylidene))dimalononitrile (L8-BO) were purchased from (Solarmer Inc). Also, (poly((2,7-bis(2-ethylhexyl)-1,2,3,6,7,8-hexahydro-1,3,6,8-tetraoxobenzo[lmn][3,8]phenanthroline-4,9-diyl)-2,5-thiophenediyl(9,9-bis(3-(dimethylamino)propyl)-9H-fluorene-2,7-diyl)-2,5-thiophenediyl)) (PNDIT-F3N) was purchased from (Solarmer Inc, China). 1-Chloronaphthalene (CN) and 1,4 diiodobenzene (DIB) were obtained from (Sigma-Aldrich, UK) and (Merck, UK) respectively. Chloroform (CF) and methanol (MeOH) were purchased from (Thermofisher, UK) and (Sigma-Aldrich, UK) respectively.Substrate cleaning and preparation
Patterned indium tin oxide (ITO)-coated glass substrates purchased from Hunan Xiangcheng Ltd (China) (20 mm × 20 mm with a thickness of 1.1 mm and a sheet resistance < 15 Ω sq−1) were first cleaned by sonicating in a 2% v/v Hellmanex in water solution for 20 min. The substrates were then rinsed with deionized water and sonicated in water for a further 15 min. Thereafter, they were sequentially cleaned in acetone, 2-propanol, and methanol in an ultrasonic bath at ≈40 °C for 15 min each and blow-dried with nitrogen. Before coating the substrates were subjected to an UV-Ozone process (Jetlight Company In. MODEL 24) for 15 min before fabrication. On top of the precleaned substrates a ∼25 nm-thick PEDOT:PSS thin film was deposited onto the indium tin oxide surface by spin-coating and baked at 150 °C for 15 min. For the molybdenum-based devices the treated ITO substrates were loaded to an evaporator (Moorfield) placed outside the glove box and the precursor of molybdenum(VI) oxide was thermally evaporated at low rates to obtain a ∼5 nm-thick MoOx thin film. Then the samples were transferred again for UV-Ozone (Jetlight Company In. MODEL 24) for 2.5 min exposure before fabrication. The solutions of PM6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Y6 (1
Y6 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2 w/w, 16.5 mg ml−1 in total), in chloroform with 1-chloronaphthalene (0.5% v/v) and PM6
1.2 w/w, 16.5 mg ml−1 in total), in chloroform with 1-chloronaphthalene (0.5% v/v) and PM6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) L8-BO (1
L8-BO (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2 w/w, 16.5 mg ml−1 in total) in chloroform with 1,4-diiodobenzene as a solid additive (the content of 1,4-diiodobenzene is 50% of the total mass of donor and acceptor) were advance and then spin-coated on top of the PEDOT:PSS layer. The prepared films were treated with thermal annealing at 100 °C for 10 min for the PM6:Y6 cells and 85 °C for 5 min for the PM6:L8BO respectively. After cooling to room temperature, a ∼5 nm-thick PNDIT-F3N (0.5 mg ml−1 in methanol with 0.5% acetic acid, v/v) was spin-coated on the top of the active layer. Then, the samples were transferred into the evaporating chamber Angstrom EvoVac system inside the glove box and a 100 nm-thick silver (Ag) layer was thermally evaporated on the PNDIT-F3N layer.
1.2 w/w, 16.5 mg ml−1 in total) in chloroform with 1,4-diiodobenzene as a solid additive (the content of 1,4-diiodobenzene is 50% of the total mass of donor and acceptor) were advance and then spin-coated on top of the PEDOT:PSS layer. The prepared films were treated with thermal annealing at 100 °C for 10 min for the PM6:Y6 cells and 85 °C for 5 min for the PM6:L8BO respectively. After cooling to room temperature, a ∼5 nm-thick PNDIT-F3N (0.5 mg ml−1 in methanol with 0.5% acetic acid, v/v) was spin-coated on the top of the active layer. Then, the samples were transferred into the evaporating chamber Angstrom EvoVac system inside the glove box and a 100 nm-thick silver (Ag) layer was thermally evaporated on the PNDIT-F3N layer.
Current (I)–voltage (V) characteristics
I–V characteristics of the fabricated solar cells were evaluated using an Enlitech SS-F5-3A (Class 3A) solar simulator with a Keysight 2901A source measure unit acting as the electrical load. The calibration of the simulator was carried out using a KG-5 filtered Si diode. A mask with 0.09 cm2 aperture area was used to define the active area of the device. The physical area of the device (the overlap between the top and bottom electrodes) was approximately 0.25 cm2. All devices were measured without any encapsulation under ambient conditions at a temperature of ∼25 °C and relative humidity of 30–35%, with a light intensity of 100 mW cm−2 (AM1.5G), calibrated using a reference cell purchased from Fraunhofer ISE CalLab (ISE001/013-2018).External quantum efficiency (EQE), internal quantum efficiency (IQE)
EQE measurements of the fabricated devices were carried out using a Bentham PVE300 system. All measurements were carried out under ambient conditions. The monochromatic light intensity was calibrated by a traceable silicon reference detector (300–1100 nm) from the national metrology institute (NMI). All devices were measured without any encapsulation under ambient conditions at a temperature of ∼25 °C and a relative humidity of 30–35%. For each device, a 0.16 cm2 mask was used during the measurement to ensure the probing beam (size is 2.2 mm × 2.2 mm) fully inside the electrode area. The IQE was calculated by the equation: IQE = EQE/(1 − R). The reflectance spectra were performed on a Varian Cary 5000 UV-vis-NIR spectrophotometer. In Reflectance mode the measurements were obtained by fitting the spectral and diffuse reflectance accessory (integrated sphere).Light soaking testing
For light soaking stability test, samples were illuminated in nitrogen chamber at a temperature of ∼55 °C, light intensity of equivalent of one-sun (100 mW cm−2) using LED 6500 K light source. All devices were measured without any encapsulation under ambient conditions during the various time points during the test.Contact angle
The contact angles were measured using a contact angle analyzer (Drop shape analyzer-DSA25, KRŰSS GmbH). DI water dropped on the surface of the samples and measured in the air under room temperature.Ultraviolet-visible (UV-vis) absorption, transmittance spectra
The transmittance and absorption spectra were performed on a Varian Cary 5000 UV-vis-NIR spectrophotometer.Atomic force microscopy (AFM)
AFM images were obtained using Bruker Dimension Edge in tapping mode with scanning area size of 50 μm x 50 μm for each sample.Photoluminescence (PL)
For the PL spectra a Horiba Xplora Plus used to obtain the spectra with the use of a 532 nm laser at 50×/0.5 mag/numerical aperture and 1200 grating.Results and discussion
Transmittance and absorbance properties
The thickness of the MoOx layer is vital for fabricating highly efficient OSCs as it affects the optical transmittance and electrical properties (charge transport and extraction) of the layer.32 Therefore, we optimized the MoOx layer thickness by fabricating OSCs with MoOx thicknesses from 2.5 nm, 5 nm, 7.5 nm, and 10 nm (for thicknesses > 5 nm we observed a loss of performance), (see ESI S5†). Transmittance spectra, as shown in Fig. 1(a) indicates that the ITO/MoOx (5 nm thick) anode exhibited higher transparency compared to PEDOT:PSS (∼30 nm) in the wavelength ranges 460 to 620 nm and 740 to 1000 nm. At shorter wavelengths below 440 nm, the PEDOT:PSS deposited on top of ITO is more transmissive than bare ITO due to refractive index matching.35 Such an anti-reflection effect is attributed to the optical interference between the organic layer PEDOT:PSS and inorganic ITO layer due to large refractive index (n) difference.36 However, as shown (ESI S1 and S2†) by the absorption spectra of the photoactive blends PM6:Y6 and PM6:L8-BO, this increased transmission does not contribute much towards the photocurrent generation, due to the limited number of solar photons absorbed by the blends at this wavelengths of the solar spectrum.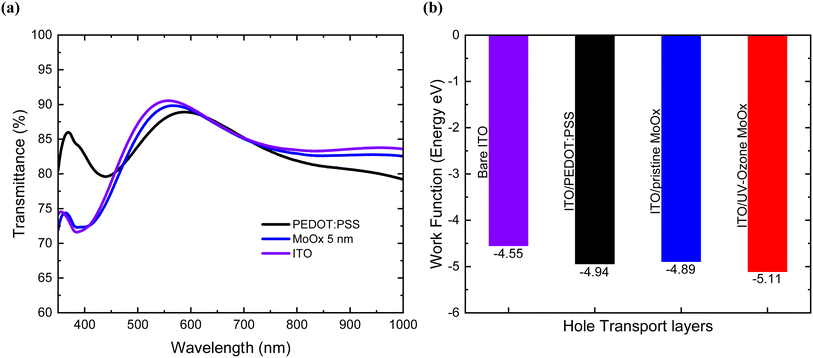 | ||
| Fig. 1 (a) Transmittance spectra of ITO, ITO/pristine MoOx, ITO/PEDOT:PSS, (b) KP measurements values of the different HTLs on top of ITO. | ||
Despite their high transmittance in the visible wavelengths, MoOx films show poor electrical characteristics, and an unfavourable work function value compared to organic semiconductors such as PEDOT:PSS.37 In the present study we use UV-Ozone treatment to modify and improve the electrical and interfacial properties of MoOx films. UV-Ozone treatment is widely used in the field of electronics and optoelectronics for cleaning and modifying purposes with the use of photo-sensitized oxidation process.38 It is known that molecular oxygen excited by ultraviolet light can dissociate to form atomic oxygen; each atomic oxygen combines with a molecular oxygen to form an ozone molecule. Ozone (O3) has one more oxygen atom than the atmospheric oxygen. As a result, this third loosely-bonded oxygen atom can effectively destroy contaminants, which is key to its strong oxidizing properties.32,39–41 Also, applying UV-Ozone treatment to ITO is expected to improve OSCs performance by increasing the hole-extraction efficiency.41
Kelvin probe measurements
Firstly, kelvin probe (KP) measurements were performed to determine the WF before and after the deposition of HTL layers on top of the ITO. For each sample, three measurements across the surface of the sample were carried out in order to evaluate the uniformity of the deposited HTL layers and the corresponding WF reproducibility.42 For the KP the contact potential difference (CPD) between a sample and a tip was calibrated. The WF of the sample can then be determined by the WF of the tip calibrated against a known surface. In our case, freshly cleaved Highly Order Pyrolytic Graphite (HOPG) was used to calibrate the tip WF. The HOPG WF is 4.48 eV as confirmed in the literature through ultraviolet photoelectron spectroscopy (UPS).43 To convert the contact potential difference measurement to work function, the following equations are used to determine the tip and sample WF values:| WF of tip = WF of test sample − CPD (measured) |
| WF of sample = WF of Tip + CPD |
Generally, for thick layers of MoOx the predominant oxidation state is Mo6+ while for thinner MoOx layers it has been observed that closer to the interface, additional oxidation states of Mo5+, Mo4+, and Mo2+ arise, possibly due to an increased number of oxygen vacancies.33 As expected from literature during the process of UV-Ozone treatment process of MoOx films, the vacant sites are filled with oxygen atoms (O3 can oxidize large quantities of Mo5+ to Mo6+ oxidation state) and as a result the MoOx films become nearly stoichiometric.32,44 Furthermore, an increased proportion of Mo6+ in MoOx films leads to a higher work function. Our measurements also showed a clear increase of 0.2 eV (in absolute values) of MoOx WF from (−4.89 ± 0.02 eV)45,46 to (−5.11 ± 0.03 eV) after the UV-Ozone treatment was confirmed directly using KP measurements as shown in Fig. 1(b). Additionally, previous studies of ITO MTO/Ag/MTO multilayer transparent electrode demonstrate that in the case of the pristine MoOx films, Mo6+ cations adjacent to the oxygen vacancies (loss of oxygen atom from their respective position in the crystal lattice) within the MoOx lattice, undergo reduction to Mo5+ oxidation state by gaining a free electron in the conduction band. On the contrary, following the application of UV-Ozone treatment on MoOx films the reduction of oxygen vacancies during this process limits the generation of excess electrons, leading to an increase in the WF.44 This downshift of the WF of treated MoOx samples is highly desired as it will lead to a more preferable energetic alignment with PM6 organic semiconductor used as a polymer donor in the OSC devices. This energy level alignment is expected to facilitate better hole extraction and collection in the devices using treated MoOx HTLs. Also, the obtained WF values for the bare ITO were measured at (−4.55 ± 0.05 eV) and in the case of ITO/PEDOT:PSS at (−4.94 ± 0.02 eV), respectively.
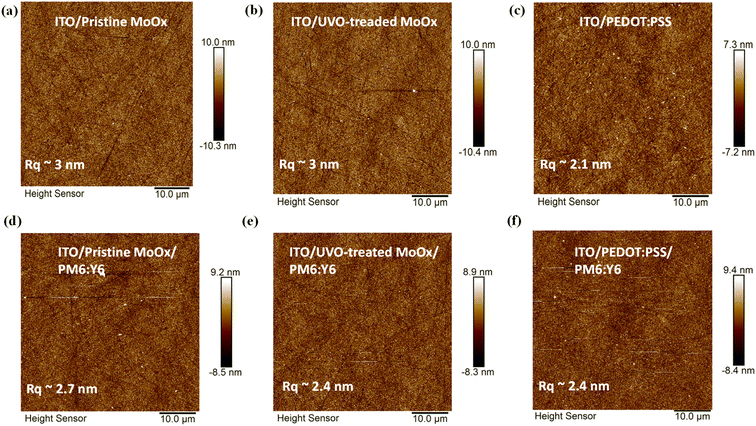
Atomic force microscopy
Tapping-mode Atomic Force Microscopy (AFM) was used to determine the surface topography of the anode films. The ITO/pristine MoOx and ITO/UV-Ozone-treated MoOx, as shown in Fig. 2, demonstrate root-mean-square surface roughness (Rq) values of (3.0 ± 0.02 nm). In comparison, the ITO/PEDOT:PSS sample exhibited an Rq value of (2.1 ± 0.01 nm). We also investigated the morphology of PM6:Y6 films deposited on top of the different HTLs. There is a known relation between the charge transport properties within the bulk-heterojunction and the nanoscale phase separation of the donor and acceptor materials.47 The AFM height images reveal Rq values of (2.7 ± 0.01 nm), (2.4 ± 0.02 nm), and (2.4 ± 0.01 nm) for the PM6:Y6 layers deposited on top of pristine MoOx, UV-Ozone-MoOx, and PEDOT:PSS, respectively. Thus, we can postulate the UV-Ozone-treatment of MoOx does not have an impact on the deposition and the surface morphology of the photoactive layer, which is essential for the efficient operation of the device. On the other hand, we observe a slight increase in Rq for the untreated MoOx-based samples, which might a reason for the inferior HTL/Active layer interface of OSC reported in the prior art.Contact angle measurements
The effect of the UV-Ozone treatment on the surface energy of MoOx layers was further analysed with the contact angle measurement technique. As shown in Fig. 3, DI water was drop casted on top of the films to measure the contact angle formed between the droplet and the HTL layer (see Table 1). The ITO/PEDOT:PSS films exhibited contact angle values between (13 ± 1)° to (16 ± 2°) whereas the pristine MoOx showed an increased contact angle of (67 ± 2°) to (68 ± 2°). In the case of UV-Ozone-treated MoOx films, a significant reduction in the contact angles of the DI water droplet was observed, resulting to an angle below the detectivity limit of the camera.44 Since UV-Ozone treatment synergistically introduces oxygen (increases the oxygen content), effectively removing hydrocarbon contaminants from the MoOx surface and change a of stoichiometry eventually increases its surface energy. Thus, after applying UV-Ozone treatment, the MoOx surface is more hydrophilic for the BHJ deposition, which facilitates an improved material wetting and surface contact with the PM6:Y6 layer, as confirmed by the AFM measurements and the lower Rq values.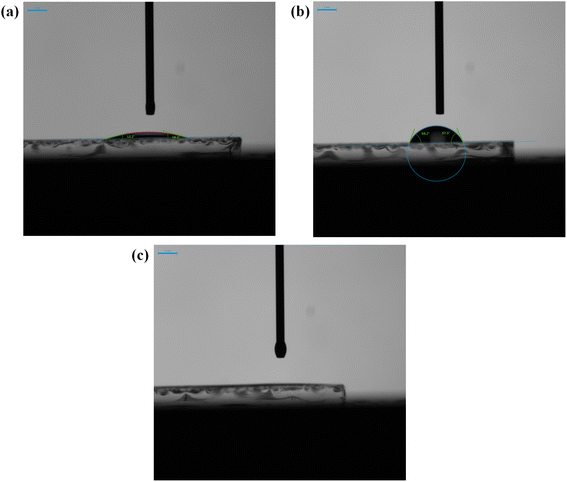 | ||
| Fig. 3 Contact angle images of (a) ITO/PEDOT:PSS, (b) ITO/pristine MoOx and (c) ITO, ITO/UV-Ozone-treated MoOx films. | ||
| Sample | H2O contact angle |
|---|---|
| ITO/PEDOT:PSS | (13 ± 1°)–(16 ± 2°) |
| ITO/pristine MoOx | (67 ± 2°)–(68 ± 2°) |
| ITO/UV-Ozone-treated MoOx | — |
Photoluminescence (PL)
Besides the surface and work function properties, we also performed electrical and optical characterization to explore the potential of UVO-treated MoOx over pristine MoOx and PEDOT:PSS. The experimental conditions used to optimize the MoOx as HTL onto ITO (e.g., thickness and UV-Ozone exposure time of evaporated MoOx films) are shown in (ESI S3 and S4†). The optimum conditions for the thickness and UV-Ozone post-treatment exposure time of the evaporated MoOx, revealed it to be 5 nm and 2.5 minutes respectively.Based on the absorbance spectra of the active layer cast on top of the pristine and UV-Ozone-treated MoOx HTL, there is negligible difference with a slightly enhanced absorbance strength at wavelengths 350, 610, and 800 nm as shown in Fig. 4(a). To get an insight into the charge extraction properties of the photogenerated carriers from the BHJ active layer to the different HTLs, the steady-state photoluminescence (PL) spectra were measured as illustrated in Fig. 4(b). It is evident that an increased PL quenching at peak (∼15%) for the UV-Ozone-treated MoOx relative to the pristine MoOx, proving that the treatment has enhanced the rate of carrier extraction at the HTL/PM6:Y6 interface.48
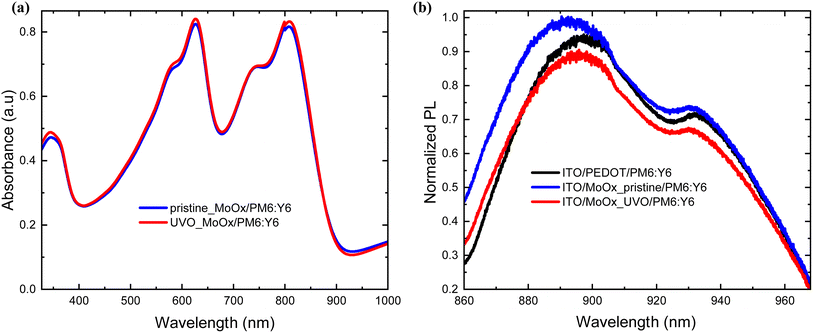 | ||
| Fig. 4 (a) Absorbance of PM6:Y6 on both pristine and UV-Ozone-treated MoOx films, (b) normalized PL intensity of PM6:Y6 BHJ system with the various HTLs. | ||
Photovoltaic performance
To understand the effect of the UV-Ozone treatment on the device performance, p–i–n (normal) OSCs were fabricated incorporating the different HTLs. The representative J–V curves obtained from the champion cells are shown in Fig. 5(a), with a summary of the device photovoltaic parameters provided in Table 2. The statistical analysis of experimental data obtained from 8 individual cells is furthermore shown in ESI S6.†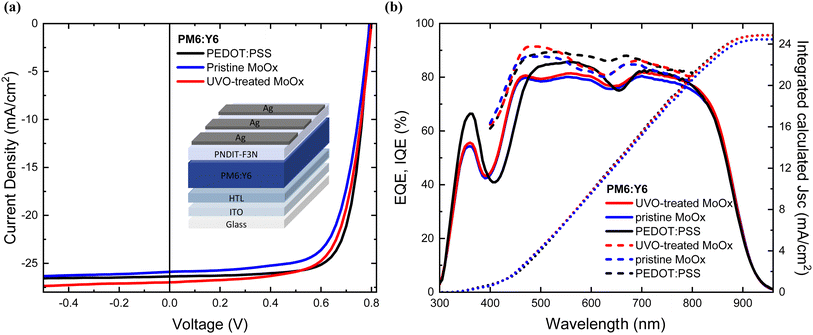 | ||
| Fig. 5 (a) J–V curves of champion cells PM6:Y6 solar cells incorporating different HTLs, (b) EQE and IQE spectra of PM6:L8BO solar cells incorporating different HTLs. | ||
| HTL | Voc mean (Voc hero) (V) | Jsc mean (Jsc hero) (mA cm−2) | Jsc EQE (mA cm−2) | FF mean (FFhero) | PCE mean (PCEhero) (%) |
|---|---|---|---|---|---|
| PEDOT:PSS | 0.80 (0.80) | 26.20 (26.37) | 24.88 | 71.75 (74.87) | 15.10 (15.60) |
| Pristine-MoOx | 0.80 (0.80) | 26.05 (25.88) | 24.46 | 67.13 (69.40) | 14.10 (14.26) |
| UVO-treated MoOx | 0.79 (0.80) | 26.90 (26.97) | 24.90 | 69.12 (69.77) | 14.73 (15.06) |
As summarised in Table 2, the PEDOT:PSS-based OSCs demonstrated a maximum PCE of 15.60% on 0.09 cm2 active area, which is comparable to the published results for PM6:Y6 based OSCs (15.70%) with a standard device architecture.49 Cells with pristine MoOx exhibit a maximum PCE of 14.26% with Jsc of 25.88 mA cm−2, an open circuit voltage (Voc) of 0.8 V, and a fill factor (FF) of 69.40. Remarkably, 2.5 minutes of UV-Ozone exposure of MoOx improve the device performance with a maximum PCE of 15.06% was measured. This is due to an increased Jsc of 26.97 mA cm−2 and an FF of 69.77, compared to the reference device with a pristine MoOx.
Thus, we observe a significant improvement in PCE of 5.5% compared to untreated MoOx OSCs and a PCE comparable to the PEDOT:PSS-based devices. The Jsc improvement is also confirmed by the calculated EQE photocurrent densities, which follow a similar trend to those obtained from the J–V curves as shown in Table 2. To further validate the improvement achieved through UV-Ozone treatment, the internal quantum efficiency (IQE) of each OSC was assessed. The MoOx cells subjected to treatment exhibited higher internal photon-to-electron conversion efficiency in the wavelength range of 450–620 nm and 680–800 nm compared to the untreated MoOx-based cells Fig. 5(b), ultimately leading to higher FF and increased Jsc.11 This improvement can be further supported by the reduced charge interface recombination and enhanced charge extraction, as evidenced by the transient analysis later in this case study. The UV-Ozone treated MoOx-based cells exhibited a maximum values of 92% at 480 nm in comparison to the pristine cells which showed a maximum values of 88% at 450 nm respectively. The higher average IQE spectrum for the treated cells indicates that a larger number of absorbed photons is actually converted into electrons, which are subsequently collected at the corresponding electrodes.
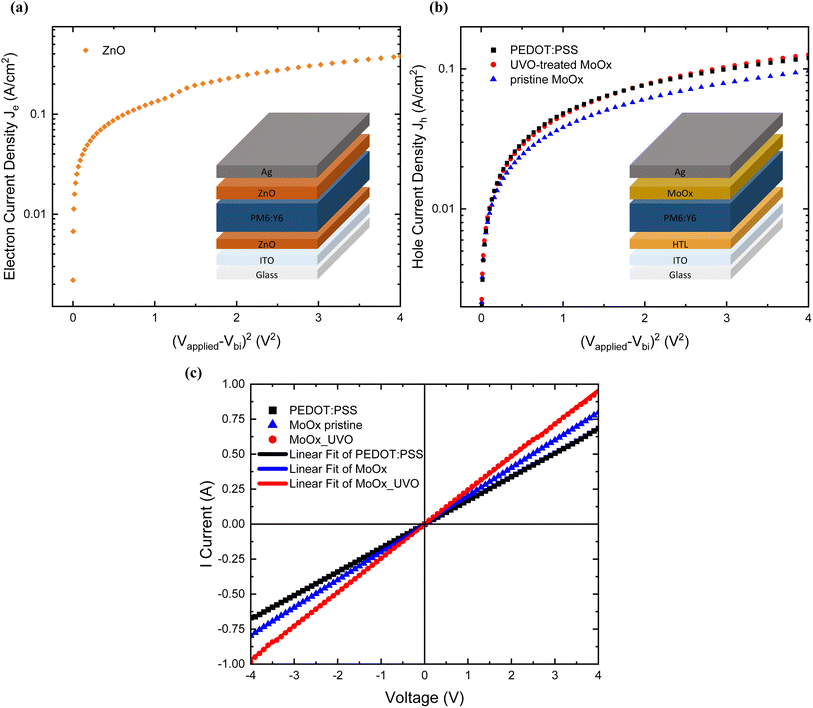
DC conductivity of the different HTLs
To further understand the origin of the PCE enhancement for the UV-Ozone-treated MoOx samples compared to the pristine MoOx, glass-ITO/HTL/Ag-based devices were fabricated in order to get an insight on the electrical conductivity of the different HTL. Fig. 6(c) depicts the I–V characteristics for devices with PEDOT:PSS, pristine MoOx and UV-Ozone-treated MoOx used as HTLs. The direct current (DC) conductivity (σ0) can be determined from the slope of the I–V plot using the following equation:where A is the physical active area of the samples (0.25 cm2), and d is the thickness of different HTL layers. The thickness of pristine MoOx and UV-Ozone-treated films was 5 nm, and in the case of PEDOT:PSS 30 nm. The value for the conductivity of PEDOT:PSS was calculated to 2.20 ± 0.11 × 10−5 S cm−1, whereas the DC conductivity of pristine MoOx and UVO-treated showed values of 0.40 ± 0.17 × 10−7 S cm−1 and 0.48 ± 0.20 × 10−7 S cm−1 respectively and in line with the literature reports (DC conductivity region 10−13 ≤ σ0 ≤ 10−4).50 This implies that the UV-Ozone treatment leads to an enhancement of σ0 by 20%. The significance of this increase lies in addressing the primary concern associated with MoOx-based HTL in OSCs, which is their relatively poor electrical performance stemming from inherently low conductivity. This improvement further corroborates the increased Jsc observed alongside the reduced PL intensity in these devices. Additionally, the lower conductivity of the MoOx-based samples compared to PEDOT:PSS, justifies the need for significantly thinner (5 nm) MoOx films compared to (30 nm) PEDOT:PSS for optimal device operation (the lower the conductivity the thinner the HTL needs to be to prevent change accumulation).51,52
Charge carrier mobility measurements
The evaluation of hole and electron transport properties of the OSCs was conducted through the fabrication of structures tailored for hole-only devices (HOD) and electron-only devices (EOD), as shown in Table 3. The following architectures fabricated for this purpose including ITO/HTL/PM6:Y6/MoOx/Ag and ITO/ZnO/PM6:Y6/ZnO/Ag respectively. The hole mobility values for each HTL (PEDOT:PSS, pristine or UV-Ozone MoOx) and the electron mobility for (ZnO) were calculated based on the Mott–Gurney equation respectively:53in which εr is the relative dielectric constant, ε0 is the permittivity of free space, μh or μe is the hole and electron mobility respectively, Vapplied is the applied voltage, Vbi is the built-in potential, and d is the thickness of the active layer.
| Sample | μh (cm2 V−1 s−1) | μe (cm2 V−1 s−1) | Ratio (μh/μe) |
|---|---|---|---|
| PEDOT:PSS | (1.98 ± 0.01) × 10−4 | (1.95 ± 0.01) × 10−4 | 1.01 |
| UV-ozone-treated MoOx | (2.01 ± 0.01) × 10−4 | (1.95 ± 0.02) × 10−4 | 1.03 |
| Pristine MoOx | (1.85 ± 0.01) × 10−4 | (1.95 ± 0.01) × 10−4 | 0.95 |
The hole mobility for the reference OSCs based on PEDOT:PSS was 1.98 ± 0.01 cm2 V−1 s−1, in line with literature reports.54 On the other hand, the hole mobility for the pristine MoOx HTL films was calculated at 1.85 ± 0.01 × 10−4 cm2 V−1 s−1, while the UV-Ozone-treated MoOx HTL films demonstrated a 10% enhancement compared to the pristine MoOx HTL films with value of 2.01 ± 0.01 cm2 V−1 s−1. The higher device hole charge carrier mobility in UVO-treated MoOx films most likely originates from the observed improved conductivity and enhanced wettability for the PM6:Y6 layer on top of the UV-Ozone treated MoOx as shown by the DC conductivity and contact angle measurements, respectively. This results also is in excellent agreement with the observed enhanced rate of carrier extraction at the HTL/PM6:Y6 interface shown by the PL analysis. In addition, the calculated μe values for the PM6:Y6 blend were reproducible 1.95 ± 0.02 × 10−4 cm2 V−1 s−1 and consistent with the literature.54 The balanced ratio between the charge carrier mobility (μh/μe close to 1) is of paramount importance for obtaining high Jsc and FF.55 The ratio plays a vital role in mitigating charge accumulation within the device.56 An imbalance in the mobility of charge carriers results in the formation of a positive space charge at the photoanode, which consequently causes the trapping of electrons near the back electrode in the BHJ OSCs.53,57 Thus, the cell utilizing PEDOT:PSS displayed a μh/μe ratio of 1.01, signifying the highest level of mobility balance among the three device types. On the other hand, the UV-Ozone MoOx cell demonstrated a considerably more balanced mobility ratio of 1.03 than the device with the untreated MoOx with a ratio 0.95 which reduces the charge accumulation effect leading to improved Jsc and FF values.58
Charge carrier lifetime
To get an additional insight on the operation of our OSCs, we performed electrochemical impedance spectroscopy (EIS) measurements which provides important insights related to the charge extraction and lifetime.59 EIS measurements were performed to examine the transient behaviour in the various OSCs fabricated in this work. Thus, the measurements were obtained, in dark conditions from 1 Hz to 1 MHz, with DC bias equal to the Voc for each device.60 The Nyquist plots and the fitting model shown in Fig. 7(a) were fitted by using the equivalent circuit model61,62 shown in Fig. 7(b) while the relevant data for each HTL type are summarized in Table 4.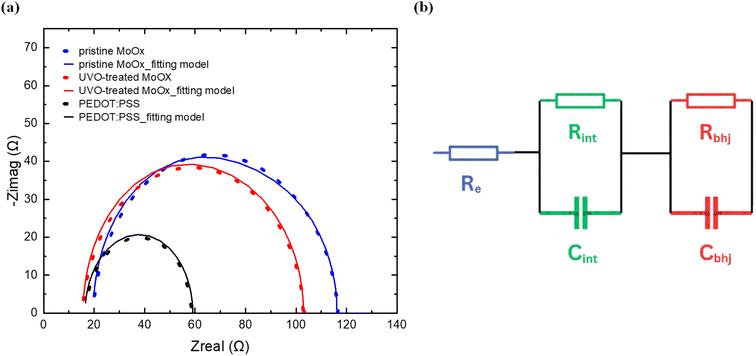 | ||
| Fig. 7 (a) Nyquist plots of the EIS data with the equivalent fitting models for various HTL-based OSCs. (b) The equivalent circuit model for EIS data fitting the different OSCs. | ||
| Sample | Re (Ω) | Rint (Ω) | Cint (nF) | Rbhj (Ω) | Cbhj (nF) | τ (μs) |
|---|---|---|---|---|---|---|
| Pristine MoOx | 19.60 | 33.87 | 285 | 62.79 | 41.98 | 2.63 |
| UV-ozone-treated MoOx | 15.42 | 29.79 | 270.7 | 57.56 | 50.83 | 2.87 |
| PEDOT:PSS | 16.40 | 20.86 | 95.30 | 21.8 | 149.3 | 3.30 |
The Re corresponds to electrode resistance (ITO and Ag); Rint and Cint in parallel correspond to the interface layer's resistance and capacitance, and Rbhj and Cbhj in parallel correspond to the resistance and capacitance of the bulk heterojunction, respectively. The performance of the OSCs can be correlated by analyzing the Rint of the interface layer in conjunction with the average carrier transition lifetime (τ).62–64 The reduced Rint values for the UV-Ozone-treated MoOx cells (29.79 Ω) further confirms its enhanced interface conductivity compared to the pristine MoOx (33.87 Ω) which supports our findings regarding the higher FF values obtained in UV-Ozone-treated MoOx cells.65 Moreover the τ values for each HTL type were calculated based on the following equation:
| τ = Rbhj·Cbhj |
The τ values for the pristine, the treated MoOx and the PEDOT:PSS were calculated as 2.63 μs, 2.87 μs and 3.30 μs respectively. The longer τ values for the treated MoOx-based OSCs compared to the pristine is associated with a reduced the trap-assisted recombination,66 which is also confirmed by the PL measurements (15% PL quenching at peak for the UV-Ozone-treated samples compared to the pristine). Overall the EIS findings underscore the benefits of UV-Ozone treatment on MoOx, which contributes to reduced charge recombination and improved charge extraction to the electrodes compared with the pristine MoOx, ultimately enhancing the FF and Jsc.67
Exciton generation
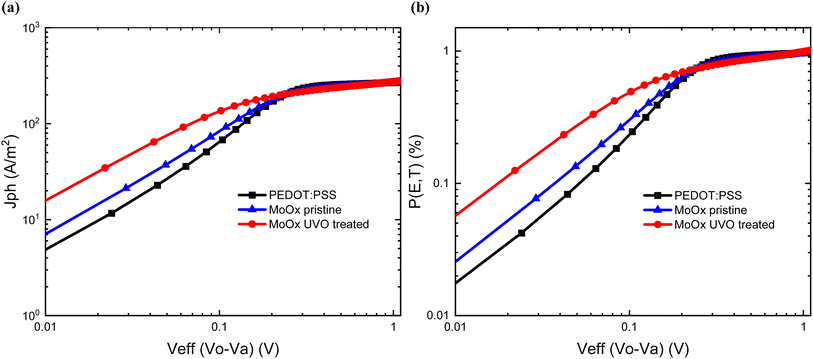
To gain a better insight into the exciton generation and dissociation processes, the dependence of the photocurrent densities (Jph) of the cells with the different HTLs was plotted versus the effective voltage (Veff), from which the maximum exciton generation rate (Gmax) and charge collection probabilities P(E, T) were calculated. Jph is determined as Jph = JL − JD, where JL and JD are the current densities under illumination and dark conditions, respectively. Veff is determined as Veff = V0 − Va, where V0 is the voltage at the point of Jph = 0, and Va is the applied bias voltage. If we assume that the saturated current density (Jsat) is defined by the total quantity of the absorbed photons and all the photogenerated excitons are dissociated to free charge carriers at higher voltage (>1 V), then Gmax can be extracted by the formula Jsat = qGmaxL.53,68 The values of Gmax, as calculated from Fig. 8(a) were 1.66 × 1028 s−1 m−3 (267 A m−2) for the PEDOT:PSS-based cells, 1.64 × 1028 s−1 m−3 (263 A m−2) for the UV-Ozone-treated MoOx-based cells and 1.63 × 1028 s−1 m−3 (261 A m−2) for the pristine MoOx-based cells. Fundamentally, the Gmax is correlated to the maximum absorption of incident photons.69,70 The almost unchanged values of Gmax suggest that the overall exciton generation upon samples based on PEDOT:PSS and MoOx is approximately the same. This can further be supported by the transmission spectra of the different HTLs and the nearly identical absorbance spectra strength of PM6:Y6 on top of both pristine and UV-Ozone-treated MoOx films, as demonstrated in Fig. 1(a) and 4(a).Charge collection probabilities
On the other hand, the charge collection probability, P(E, T), can be calculated from the ratio of Jph/Jsat.71 Under short circuit conditions for the reference PEDOT:PSS cells, the P(E, T) was estimated at (96 ± 1%), while the samples based on pristine and UV-Ozone-treated MoOx devices exhibited values of (95 ± 1%) and (93 ± 2%), respectively Fig. 8(b). The increased P(E, T) values of samples PEDOT:PSS and UV-Ozone-treated MoOx suggested more efficient charge collection compared to the pristine MoOx cells. These results can be further correlated by the higher values of FF as presented by the statistical analysis distribution of the devices (ESI S6†) in combination with the improved device hole charge carrier mobility and the DC conductivity as shown earlier. Thus, it is more evidence that the UV-Ozone treatment has a beneficial effect on the device performance of OSCs, making MoOx a candidate for HTL in efficient OSCs.Light soaking stability measurements
We also investigated the stability of the optimized PM6:Y6 OSCs based on the highest PCE HTLs by performing light-soaking measurements, which is important in the commercialization of NFA-based OSCs. The devices were characterized under nitrogen conditions while being exposed to continuous white colour (6500 K) light-emitting diode (LED) source with light intensity equivalent to ∼1 sun (100 mW cm−2) at 55 °C. The normalized figures of merit (i.e. normalized PCE, FF, Jsc and Voc) of the cells plotted as a function of light-soaking time are presented in Fig. 9. After 100 hours of continuous light soaking, the UV-Ozone-treated and the pristine MoOx-based device retained ∼65% and 58% of their initial PCE respectively, while the performance of the PEDOT:PSS-based counterpart deteriorated rapidly to 23% of its initial PCE after only 51 hours. Thus, both MoOx HTLs are fundamentally more stable than the PEDOT:PSS counterpart, while the efficiency improvement of treated cells compared to pristine originates from the UV-Ozone treatment process. This striking difference in the Fig. 9(c) (dependence of FF with time), explains well the significance of our work towards stable OSCs. In particular, stabilised FF over time of both MoOx-based cells imply stable and intact interface.72 In our results we observe that the control samples' (PEDOT:PSS) FF decrease very fast and is the main reason for the fast drop of the PCE. On the contrary, our target devices (UV-Ozone-treated and pristine MoOx devices) present a much firmer FF trend over time and is the key reason for the more stable PCE with time. Although preliminary, the results indicate that ITO/UVO-treated MoOx could significantly enhance the lifetime stability and operation of state-of-the-art OSCs (the key issue of OSCs towards the commercialisation) as shown in ESI Fig. S7(a)–(c)† from the representative time depended J–V curves.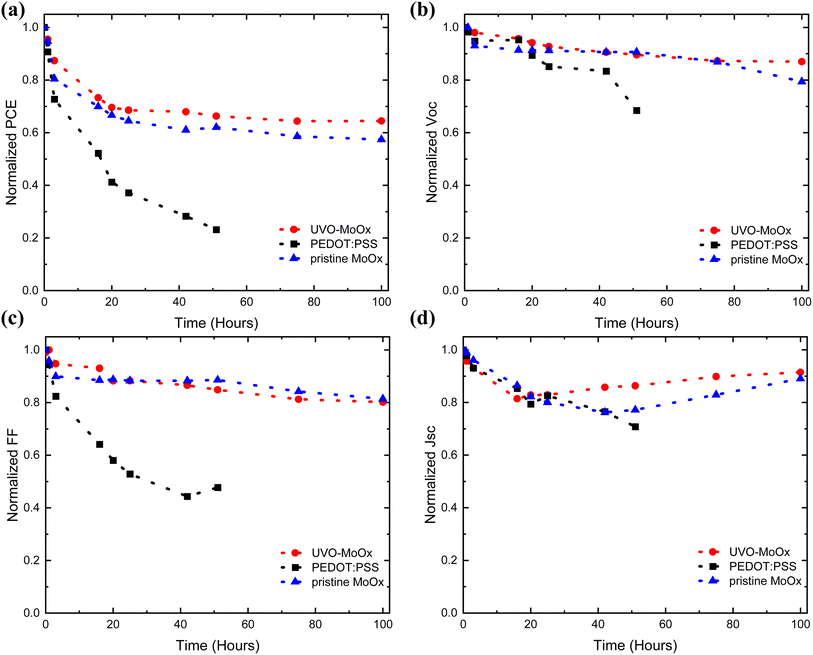 | ||
| Fig. 9 Normalized (a) PCE, (b) Voc, (c) FF and (d) Jsc over time graphs of light soaking test of PM6:Y6 solar cells based on UVO-treated MoOx and pristine MoOx versus PEDOT:PSS. | ||
Light depended measurements
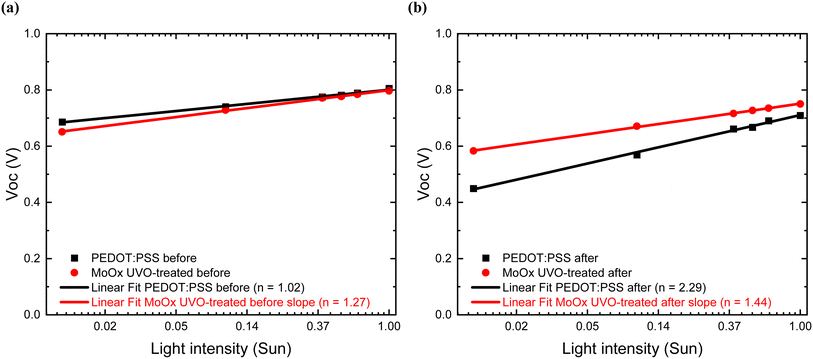
Next, we performed J–V light-intensity-dependence measurements to understand the impact of light-soaking on PEDOT:PSS-based and UV-Ozone-treated MoOx-based OSCs since both architectures present the highest PCE values in this case study. Measuring and tracking the Voc versus the light intensity can be used to extract the light ideality factor (n). The n is a measure of whether the recombination type is Shockley–Read–Hall (SRH (n = 2) or bimolecular (n = 1).70 The n values in each case were calculated based on the Shockley equation (under the assumption that the photocurrent scales linearly with the light intensity and photocurrent/saturation current ≫ 1:where, n is the light ideality factor, k is the Boltzmann constant, T is the temperature, and q is the unit charge.
The dependence of Voc versus the light intensity before the light soaking test (ESI S10(a) and S11(a)†) for PEDOT:PSS-based and UV-Ozone-treated MoOx-based devices presented slope values of n 1.02 and 1.27, respectively, as shown in Fig. 10(a). The lower n value of devices with PEDOT:PSS compared to the UVO-treated MoOx matches with the higher initial PCE of the devices before the stability test. Interestingly, a higher slope value, which leads to an inferior ideality factor, was observed after 51 hours in the case of PEDOT:PSS-based devices with an increase of 124% and n value of 2.29 (practically the PEDOT:PSS-based devices are fully degraded after 51 hours of exposure in the stability test) (see ESI S10(b)†). On the other hand, the UV-Ozone-treated MoOx-based cells (ESI S11(b)†), demonstrated a significantly lower increase of just 13% and an n value of 1.44, implying that these devices are fully functional and only slightly degraded. This suggests that more severe trap-assisted recombination occurs on PEDOT:PSS-based cells as the devices degrade,73,74 in contrast to the UV-Ozone-treated MoOx-based cells, demonstrating their better stability under light-soaking conditions. The significant increase of the ideality factor in PEDOT:PSS-based devices can further be supported by the rapid decrease of the FF factor over time since the FF can be described as the interplay between recombination and charge extraction processes in solar cells.75,76 More specifically, for the PEDOT:PSS cells, after 51 hours of light soaking, the FF reached values less than 50% of its initial value, while the UV-Ozone-treated MoOx cells retained almost 85% of its initial value. Considering that the main function of photovoltaic cells is to provide power to a load, it highlighted that the UV-Ozone-treated device exhibited longer stable power output values after 100 hours of light soaking (see ESI S8(a) and (b)†).
Broader applicability
Finally, we investigate the broader applicability of the UV-Ozone treatment on thermally evaporated MoOx HTL in another highly efficient BHJ system. For this purpose, we selected PM6:L8BO with PCE values beyond 17%, as reported in the literature.2,77 The results of the UV-Ozone treatment on MoOx demonstrated an equal impact on the PM6:L8BO devices, as observed in the case of the PM6:Y6 BHJ system. Representative J–V curves obtained from the champion cells using different HTLs are shown in Fig. 11(a), a summary of the champion data and mean device photovoltaic parameters is provided in Table 5. The statistical analysis of the devices from 5 individual cells (see ESI S9†).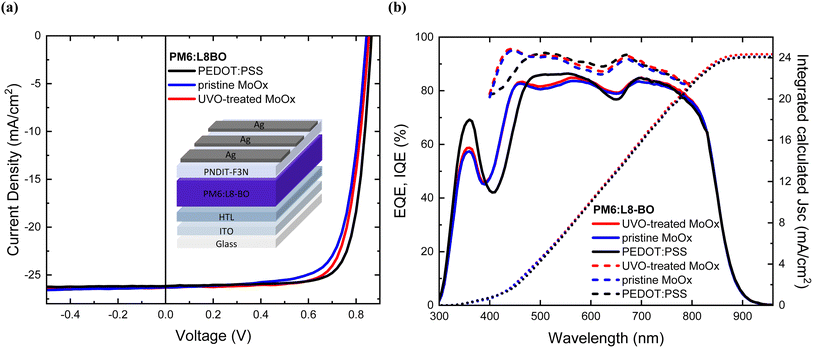 | ||
| Fig. 11 (a) J–V curves of champion cells PM6:L8BO solar cells incorporating different HTLs, (b) EQE and IQE spectra of PM6:L8BO solar cells incorporating different HTLs. | ||
| HTL | Voc mean (Voc hero) (V) | Jsc mean (Jsc hero) (mA cm−2) | Jsc EQE (mA cm−2) | FF mean (FFhero) | PCE mean (PCEhero) (%) |
|---|---|---|---|---|---|
| PEDOT:PSS | 0.86 (0.86) | 25.54 (26.17) | 24.10 | 76.82 (77.10) | 17.0 (17.35) |
| Pristine-MoOx | 0.85 (0.85) | 25.90 (26.28) | 24.12 | 72.37 (72.60) | 15.93 (16.21) |
| UVO-treated MoOx | 0.85 (0.86) | 26.05 (26.31) | 24.32 | 74.28 (74.90) | 16.47 (16.85) |
The PEDOT:PSS-based PM6:L8BO demonstrated a PCE of 17.35% with a Voc of 0.86 V, Jsc 26.17 mA cm−2 and an FF of 77.10 in line with the literature. On the other hand, pristine MoOx cells exhibited a maximum efficiency of 16.21% with Jsc of 26.28 mA cm−2, Voc of 0.85 V, and FF of 72.60. Notably, the UV-Ozone-treatment enhanced the performance of the pristine MoOx, demonstrating a maximum PCE of 16.85%, accompanied by a Jsc of 26.31 mA cm−2, an improved FF of 74.90 and Voc of 0.85 V. As a result, a noteworthy PCE increase of 3.95% compared to the pristine MoOx-based OSCs and the highest reported value to the best our knowledge for MoOx-based binary OSCs in the literature with an active area of ∼0.1 cm2.
From the EQE spectra as shown in Fig. 11(b), the calculated photocurrent densities follow similar trend (within ±7% for all devices difference) to those measured from the J–V curves with a solar simulator. The photocurrent values extracted from EQE spectra are consistent with the results based on the transmission spectra Fig. 1(b) demonstrated earlier. Similarly, the IQE measurements indicate that the UV-Ozone-treated MoOx-based devices exhibit a more efficient internal photon-to-electron conversion compared to the pristine MoOx-based OSCs. The average IQE value for these treated cells is 90%, with peak values reaching 96% and 93% at wavelengths of 450 nm and 665 nm, respectively. In contrast, the pristine MoOx-based cells show an average IQE value of 88%, with maximum values of 95% and 91% at the same wavelengths. The above results are in full agreement with findings mentioned earlier (IQE of PM6:Y6-based cells) that a larger number of absorbed photons is successfully converted into electrons, that contribute to current generation in the case of the UV-Ozone-treated MoOx-based cells in contrast with the pristine-based devices.
Conclusions
This study presents a comprehensive strategy aimed at enhancing the electrical and physical characteristics of MoOx-based HTL utilized in OSCs. The strategy involves a post-UV-Ozone treatment of the MoOx HTL, rendering it particularly beneficial for applications beyond photovoltaic technologies. Initially, we optimized both the thickness of the MoOx layer and the exposure time of the UV-Ozone treatment. Our findings suggest that an optimal MoOx thickness of 5 nm requires only 2.5 minutes of UV-Ozone exposure to achieve a notable enhancement in the electrical properties of the film. This treatment resulted in a 0.2 eV increase (in absolute values) in the work function of MoOx, attributed to alterations in the oxidation states of the material. Consequently, we observed an increase in hole mobilities, more balanced mobility ratios and an improvement in DC conductivity compared to pristine MoOx films. Additionally, the hydrophilicity of the MoOx film surface was markedly enhanced post-treatment, leading to better wetting of the subsequently applied photoactive layer. EIS in conjunction with IQE, charge collection probabilities and the PL characterisation revealed an improved interface between the UV-Ozone-treated MoOx and the photoactive layer, as indicated by longer charge carrier lifetimes, improved charge collection with a substantial 15% PL quenching, respectively. Furthermore, devices incorporating pristine and UV-Ozone-treated MoOx based OSCs exhibited significantly improved operational stability, maintaining approximately 58% and 65% of their initial performance after 100 hours, respectively. In contrast, devices based on PEDOT:PSS experienced rapid degradation, dropping to 23% of their pre-aging performance after 51 hours of light exposure at 55 °C, ultimately leading to complete failure. The enhanced stability of the high efficiency UV-Ozone-treated MoOx-based devices is attributed to minimal changes in the ideality factor and their ability to sustain high fill factor values (85% of initial values) after 100 hours. This approach serves as a catalyst for simultaneous high performance and stability relative to the control PEDOT:PSS-based cells, which demonstrate high efficiency but markedly shorter lifetime stability. Ultimately, the study highlighted the broad applicability of the UV-Ozone-treated MoOx HTL in OSCs that employ the highly efficient PM6:L8BO BHJ system. This system achieved a PCE of 16.85%, representing the highest efficiency recorded to date for binary OSCs utilizing metal oxide-based HTLs. Our findings underscore the potential for tuning the physicochemical characteristics of metal transition oxides to drive future innovations in device architecture and advanced surface engineering strategies.Data availability
The data supporting this article have been included as part of the ESI.†Author contributions
A. P. and G. K. conceived the idea. A. P., G. K. and S. R. P. S discussed, planned the content, analysed the literature. A. P. designed, fabricated and characterized the devices. A. L. assisted with the Kelvin Probe measurements. K. A. performed the EIS measurements and analyzed the data. A. P., G. K. contributed to the relevant data analysis and the revision of the first draft. A. P., G. K., K. A., D. K. and S. R. P. S., contributed to the preparation of the manuscript. S. R. P. S. supervised the project. All authors contributed to manuscript preparation, revision and approved its submission for publication.Conflicts of interest
There are no conflicts to declare.Acknowledgements
A. P. acknowledge the funding from UKRI (iCase/UKRI) studentship grant number is EP/T517616. A. P. and S. R. P. S. gratefully acknowledge the support of QinetiQ and MUSICODE H2020, the European collaborative research and innovation project led by multi-disciplinary consortium. G. K. gratefully acknowledge the support from the Hellenic Mediterranean University and the Department of Electronics Engineering.References
- NREL, Best Research Cell Efficiency Chart, https://www.nrel.gov/pv/assets/pdfs/best-research-cell-efficiencies.
- L. Zhu, M. Zhang, J. Xu, C. Li, J. Yan, G. Zhou, W. Zhong, T. Hao, J. Song, X. Xue, Z. Zhou, R. Zeng, H. Zhu, C. C. Chen, R. C. I. MacKenzie, Y. Zou, J. Nelson, Y. Zhang, Y. Sun and F. Liu, Nat. Mater., 2022, 21, 656–663 CrossRef CAS PubMed.
- R. Sun, Y. Wu, X. Yang, Y. Gao, Z. Chen, K. Li, J. Qiao, T. Wang, J. Guo, C. Liu, X. Hao, H. Zhu and J. Min, Adv. Mater., 2022, 34, 2110147 CrossRef CAS PubMed.
- A. Panagiotopoulos, T. Maksudov, G. Kakavelakis, G. Perrakis, E. A. Alharbi, D. Kutsarov, F. H. Isikgor, S. Alfihed, K. Petridis, M. Kafesaki, S. R. P. Silva, T. D. Anthopoulos and M. Graetzel, Appl. Phys. Rev., 2023, 10, 41303 CAS.
- A. Seitkhan, M. Neophytou, M. Kirkus, E. Abou-Hamad, M. N. Hedhili, E. Yengel, Y. Firdaus, H. Faber, Y. Lin, L. Tsetseris, I. McCulloch and T. D. Anthopoulos, Adv. Funct. Mater., 2019, 29, 1905810 CrossRef CAS.
- W. Y. Tan, R. Wang, M. Li, G. Liu, P. Chen, X. C. Li, S. M. Lu, H. L. Zhu, Q. M. Peng, X. H. Zhu, W. Chen, W. C. H. Choy, F. Li, J. Peng and Y. Cao, Adv. Funct. Mater., 2014, 24, 6540–6547 CrossRef CAS.
- Z.-G. Zhang, B. Qi, Z. Jin, D. Chi, Z. Qi, Y. Li and J. Wang, Energy Environ. Sci., 1966–1973, 2014, 10.1039/c4ee00022f.
- F. Huang, H. Wu, D. Wang, W. Yang and Y. Cao, Chem. Mater., 2004, 16, 708–716 CrossRef CAS.
- C. E. Small, S. Chen, J. Subbiah, C. M. Amb, S. W. Tsang, T. H. Lai, J. R. Reynolds and F. So, Nat. Photonics, 2011, 6, 115–120 CrossRef.
- K. D. G. I. Jayawardena, R. Rhodes, K. K. Gandhi, M. R. R. Prabhath, G. D. M. R. Dabera, M. J. Beliatis, L. J. Rozanski, S. J. Henley and S. R. P. Silva, J. Mater. Chem. A, 2013, 1, 9922–9927 Search PubMed.
- Y. Lin, Y. Firdaus, M. I. Nugraha, F. Liu, S. Karuthedath, A. H. Emwas, W. Zhang, A. Seitkhan, M. Neophytou, H. Faber, E. Yengel, I. McCulloch, L. Tsetseris, F. Laquai and T. D. Anthopoulos, Adv. Sci., 2020, 1903419, DOI:10.1002/ADVS.201903419.
- G. Cai, Z. Chen, X. Xia, Y. Li, J. Wang, H. Liu, P. P. Sun, C. Li, R. Ma, Y. Zhou, W. Chi, J. Zhang, H. Zhu, J. Xu, H. Yan, X. Zhan and X. Lu, Adv. Sci., 2022, 2200578, DOI:10.1002/ADVS.202200578.
- R. A. Hatton, N. P. Blanchard, L. W. Tan, G. Latini, F. Cacialli and S. R. P. Silva, Org. Electron., 2009, 10, 388–395 CrossRef CAS.
- C. T. G. Smith, R. W. Rhodes, M. J. Beliatis, K. D. G. Imalka Jayawardena, L. J. Rozanski, C. A. Mills and S. R. P. Silva, Appl. Phys. Lett., 2014, 105, 73304 CrossRef.
- G. D. M. R. Dabera, K. D. G. I. Jayawardena, M. R. R. Prabhath, I. Yahya, Y. Y. Tan, N. A. Nismy, H. Shiozawa, M. Sauer, G. Ruiz-Soria, P. Ayala, V. Stolojan, A. A. D. T. Adikaari, P. D. Jarowski, T. Pichler and S. R. P. Silva, ACS Nano, 2013, 7, 556–565 CrossRef CAS PubMed.
- Z. Zheng, Q. Hu, S. Zhang, D. Zhang, J. Wang, S. Xie, R. Wang, Y. Qin, W. Li, L. Hong, N. Liang, F. Liu, Y. Zhang, Z. Wei, Z. Tang, T. P. Russell, J. Hou and H. Zhou, Adv. Mater., 2018, 1801801, DOI:10.1002/ADMA.201801801.
- M. Zeng, X. Wang, R. Ma, W. Zhu, Y. Li, Z. Chen, J. Zhou, W. Li, T. Liu, Z. He, H. Yan, F. Huang and Y. Cao, Adv. Energy Mater., 2020, 10, 2000743 CrossRef CAS.
- Y. Wang, J. Han, L. Cai, N. Li, Z. Li and F. Zhu, J. Mater. Chem. A, 2020, 8, 21255–21264, 10.1039/d0ta08018g.
- M. P. De Jong, L. J. Van Ijzendoorn and M. J. A. De Voigt, Appl. Phys. Lett., 2000, 77, 2255–2257 CrossRef CAS.
- N. Wijeyasinghe, A. Regoutz, F. Eisner, T. Du, L. Tsetseris, Y. H. Lin, H. Faber, P. Pattanasattayavong, J. Li, F. Yan, M. A. McLachlan, D. J. Payne, M. Heeney and T. D. Anthopoulos, Adv. Funct. Mater., 2017, 27, 1701818 CrossRef.
- Z. Li, H. Huang, X. Zeng, B. Deng, C. Li, C. Gao, G. Zhang, S. Li and C. Xie, Org. Electron., 2024, 133, 107104 CrossRef CAS.
- C. Girotto, E. Voroshazi, D. Cheyns, P. Heremans and B. P. Rand, ACS Appl. Mater. Interfaces, 2011, 3, 3244–3247 CrossRef CAS PubMed.
- W. Feng, C. Song, X. Hu, S. Liu, R. Yi, X. Yang, H. Yan and X. Hou, ACS Appl. Mater. Interfaces, 2018, 10, 9 Search PubMed.
- J. Meyer, S. Hamwi, M. Kröger, W. Kowalsky, T. Riedl and A. Kahn, Adv. Mater., 2012, 24, 5408–5427 CrossRef CAS PubMed.
- D. I. Kutsarov, E. New, F. Bausi, A. Zoladek-Lemanczyk, F. A. Castro and S. R. P. Silva, Sol. Energy Mater. Sol. Cells, 2017, 161, 388–396 CrossRef CAS.
- K. E. Lee, L. Liu and T. L. Kelly, J. Phys. Chem. C, 27735–27741, 2014, DOI:10.1021/jp508972v.
- M. D. Irwin, D. B. Buchholz, A. W. Hains, R. P. H. Chang and T. J. Marks, Proc. Natl. Acad. Sci. U. S. A., 2008, 105, 2783–2787 CrossRef CAS.
- R. Remya, P. T. G. Gayathri and B. Deb, Mater. Chem. Phys., 2020, 255, 123584 CrossRef CAS.
- R. Miclette Lamarche, A. Gasonoo, A. Hoff, R. Chernikov, G. C. Welch and S. Trudel, Chem. Mater., 2023, 35, 2353–2362 CrossRef CAS.
- Y. Li, P. Li, M. Qu, F. Liu, B. Wei and G. Chen, Nanotechnology, 2023, 34, 285205, DOI:10.1088/1361-6528/acccfc.
- C. Seiichiro Murase, Y. Yang, S. Murase and Y. Yang, Adv. Mater., 2012, 24, 2459–2462 CrossRef PubMed.
- F. Cheng, G. Fang, X. Fan, H. Huang, Q. Zheng, P. Qin, H. Lei and Y. Li, Sol. Energy Mater. Sol. Cells, 2013, 110, 63–68 CrossRef CAS.
- K. O. Brinkmann, T. Becker, F. Zimmermann, C. Kreusel, T. Gahlmann, M. Theisen, T. Haeger, S. Olthof, C. Tückmantel, M. Günster, T. Maschwitz, F. Göbelsmann, C. Koch, D. Hertel, P. Caprioglio, F. Peña-Camargo, L. Perdigón-Toro, A. Al-Ashouri, L. Merten, A. Hinderhofer, L. Gomell, S. Zhang, F. Schreiber, S. Albrecht, K. Meerholz, D. Neher, M. Stolterfoht and T. Riedl, Nature, 2022, 280, DOI:10.1038/s41586-022-04455-0.
- W. Hadmojo, F. Isikgor, Y. Lin, Z. Ling, Q. He, H. Faber, E. Yengel, R. Ali, A. Samad, R. Enggar Anugrah Ardhi, S. Jeong, H. Young Woo, U. Schwingenschlögl, M. Heeney, T. Anthopoulos, W. Tantyo Hadmojo, F. H. Isikgor, S. Young Jeong, T. D. Anthopoulos and C. Authors, Energy Environ. Mater., 2024, 7, e12712 CrossRef CAS.
- Y. Wang, Z. Shi, H. Liu, F. Wang, Y. Bai, X. Bian, B. Zhang, T. Hayat, A. Alsaedi and Z. Tan, Polymers, 2017, 9, 571 CrossRef PubMed.
- X. Hou, Q. Li, T. Cheng, L. Yu, F. Wang, J. Lin, S. Dai, Y. Li and Z. Tan., J. Mater. Chem. A, 2015, 3, 18727–18734, 10.1039/c5ta03967c.
- W. J. Dong, G. H. Jung and J. L. Lee, Sol. Energy Mater. Sol. Cells, 2013, 116, 94–101 CrossRef CAS.
- W. Huang, H. Fan, X. Zhuang and J. Yu, Nanoscale Res. Lett., 2014, 9, 1–8 CrossRef CAS PubMed.
- J. R. Vig, J. Vac. Sci. Technol., A, 1985, 3, 1027–1034 CrossRef CAS.
- K. Sugiyama, H. Ishii, Y. Ouchi and K. Seki, J. Appl. Phys., 2000, 87, 295–298 CrossRef CAS.
- A. W. Hains, J. Liu, A. B. F. Martinson, M. D. Irwin and T. J. Marks, Adv. Funct. Mater., 2010, 20, 595–606 CrossRef CAS.
- Hardware: Series 10 Software: Series 11 KP manual 2021 Non-scanning Kelvin probe system manual: For software series 11 and hardware version KP020 (Win 10) Search PubMed.
- C. Melios, A. Centeno, A. Zurutuza, V. Panchal, C. E. Giusca, S. Spencer, S. R. P. Silva and O. Kazakova, Carbon, 2016, 103, 273–280 CrossRef CAS.
- P. C. Kao, C. J. Hsieh, Z. H. Chen and S. H. Chen, Sol. Energy Mater. Sol. Cells, 2018, 186, 131–141 CrossRef CAS.
- C. Lattyak, K. Gehrke and M. Vehse, J. Phys. Chem. C, 2022, 126, 13929–13935 CrossRef CAS.
- X. Wu, D. Zhang, B. Liu, Y. Wang, X. Wang, Q. Liu, D. Gao, N. Wang, B. Li, L. Wang, Z. Yu, X. Li, S. Xiao, N. Li, M. Stolterfoht, Y. H. Lin, S. Yang, X. C. Zeng and Z. Zhu, Adv. Mater., 2024, 2410692 CrossRef CAS PubMed.
- H. Bin, K. Datta, J. Wang, T. P. A. Van Der Pol, J. Li, M. M. Wienk and R. A. J. Janssen, ACS Appl. Mater. Interfaces, 2022, 14, 16497–16504 CrossRef CAS PubMed.
- J. Wu, H. Cha, T. Du, Y. Dong, W. Xu, C. T. Lin and J. R. Durrant, Adv. Mater., 2022, 2101833, DOI:10.1002/ADMA.202101833.
- J. Yuan, Y. Zhang, L. Zhou, G. Zhang, H.-L. Yip, T.-K. Lau, X. Lu, C. Zhu, H. Peng, P. A. Johnson, M. Leclerc, Y. Cao, J. Ulanski, Y. Li and Y. Zou, Joule, 2019, 3, 1140–1151 CrossRef CAS.
- A. Domínguez, C. Ramos, A. Dutt, G. Santana, Y. Kudriavtsev and O. de Melo, Mater. Lett., 2022, 318, 132171 CrossRef.
- B. Paci, G. Kakavelakis, A. Generosi, J. Wright, C. Ferrero, E. Stratakis and E. Kymakis, Sol. Energy Mater. Sol. Cell., 2017, 159, 617–624, DOI:10.1016/j.solmat.2016.01.003.
- B. Paci, G. Kakavelakis, A. Generosi, V. Rossi Albertini, J. P. Wright, C. Ferrero, D. Konios, E. Stratakis and E. Kymakis, RSC Adv., 2015, 5, 106930–106940 RSC.
- G. Kakavelakis, A. E. D. R. Castillo, V. Pellegrini, A. Ansaldo, P. Tzourmpakis, R. Brescia, M. Prato, E. Stratakis, E. Kymakis and F. Bonaccorso, ACS Nano, 2017, 11(4), 3517–3531, DOI:10.1021/acsnano.7b00323.
- R. Yu, H. Yao, Y. Cui, L. Hong, C. He, J. Hou, R. Yu, H. Yao, Y. Cui, L. Hong, C. He and J. Hou, Adv. Mater., 2019, 31, 1902302 CrossRef PubMed.
- C. Xie, X. Zeng, C. Li, X. Sun, S. Liang, H. Huang, B. Deng, X. Wen, G. Zhang, P. You, C. Yang, Y. Han, S. Li, G. Lu, H. Hu, N. Li and Y. Chen, Energy Environ. Sci., 2024, 17, 2441–2452 RSC.
- C. M. Proctor, J. A. Love, T.-Q. Nguyen, C. M. Proctor, J. A. Love and T.-Q. Nguyen, Adv. Mater., 2014, 26, 5957–5961 CrossRef CAS PubMed.
- P. Robaeys, F. Bonaccorso, E. Bourgeois, J. D'Haen, W. Dierckx, W. Dexters, D. Spoltore, J. Drijkoningen, J. Liesenborgs, A. Lombardo, A. C. Ferrari, F. Van Reeth, K. Haenen, J. V. Manca and M. Nesladek, Appl. Phys. Lett., 2014, 105, 83306 CrossRef.
- C. Xie, X. Zeng, C. Li, X. Sun, S. Liang, H. Huang, B. Deng, X. Wen, G. Zhang, P. You, C. Yang, Y. Han, S. Li, G. Lu, H. Hu, N. Li and Y. Chen, Energy Environ. Sci., 2024, 17, 2441–2452 RSC.
- G. Garcia-Belmonte, A. Munar, E. M. Barea, J. Bisquert, I. Ugarte and R. Pacios, Org. Electron., 2008, 9, 847–851 CrossRef CAS.
- E. Von Hauff, J. Phys. Chem. C, 2019, 123, 11329–11346 CrossRef CAS.
- E.-P. Yao, C.-C. Chen, J. Gao, Y. Liu, Q. Chen, M. Cai, W.-C. Hsu, Z. Hong, G. Li and Y. Yang, Sol. Energy Mater. Sol. Cell., 2014, 130, 20–26, DOI:10.1016/j.solmat.2014.05.049.
- Y. Lin, A. Magomedov, Y. Firdaus, D. Kaltsas, A. El-Labban, H. Faber, D. R. Naphade, E. Yengel, X. Zheng, E. Yarali, N. Chaturvedi, K. Loganathan, D. Gkeka, S. H. AlShammari, O. M. Bakr, F. Laquai, L. Tsetseris, V. Getautis and T. D. Anthopoulos, ChemSusChem, 2021, 14, 3569–3578 Search PubMed.
- S. Braun, W. R. Salaneck and M. Fahlman, Adv. Mater., 2009, 21, 1450–1472 CrossRef CAS.
- C. Xie, T. Heumüller, W. Gruber, X. Tang, A. Classen, I. Schuldes, M. Bidwell, A. Späth, R. H. Fink, T. Unruh, I. McCulloch, N. Li and C. J. Brabec, Nat. Commun., 2018, 9, 1–11 CrossRef PubMed.
- C. Wöpke, C. Göhler, M. Saladina, X. Du, L. Nian, C. Greve, C. Zhu, K. M. Yallum, Y. J. Hofstetter, D. Becker-Koch, N. Li, T. Heumüller, I. Milekhin, D. R. T. Zahn, C. J. Brabec, N. Banerji, Y. Vaynzof, E. M. Herzig, R. C. I. MacKenzie and C. Deibel, Nat. Commun., 2022, 13, 1–8 Search PubMed.
- A. C. Hurd, A. Alotaibi, A. Patterson, O. Alqahtani, J. Doyle, B. Akira, A. C. Authors Ally Hurd and B. Akira Collins, Macalester Journal of Physics and Astronomy, 2020, 9 Search PubMed.
- C. M. Proctor, M. Kuik and T. Q. Nguyen, Prog. Polym. Sci., 2013, 38, 1941–1960 CrossRef CAS.
- G. Kakavelakis, I. Vangelidis, A. Heuer-Jungemann, A. G. Kanaras, E. Lidorikis, E. Stratakis and E. Kymakis, Adv. Energy Mater., 2016, 1501640, DOI:10.1002/AENM.201501640.
- C. G. Shuttle, R. Hamilton, B. C. O'Regan, J. Nelson and J. R. Durrant, Proc. Natl. Acad. Sci. U. S. A., 2010, 107, 16448–16452 CrossRef CAS PubMed.
- V. D. Mihailetchi, L. J. A. Koster, J. C. Hummelen and P. W. M. Blom, Phys. Rev. Lett., 2004, 93, 216601, DOI:10.1103/PhysRevLett.93.216601.
- J.-L. Wu, F.-C. Chen, Y.-S. Hsiao, F.-C. Chien, P. Chen, C.-H. Kuo, M. H. Huang and C.-S. Hsu, ACS Nano, 2011, 5, 959–967, DOI:10.1021/nn102295p.
- L. Ciammaruchi, R. Oliveira, A. Charas, Tulus, E. Von Hauff, G. Polino, F. Brunetti, R. Hansson, E. Moons, M. Krassas, G. Kakavelakis, E. Kymakis, J. G. Sánchez, J. Ferre-Borrull, L. F. Marsal, S. Züfle, D. Fluhr, R. Roesch, T. Faber, U. S. Schubert, H. Hoppe, K. Bakker, S. Veenstra, G. Zanotti, E. A. Katz, P. Apilo, B. Romero, T. A. Tumay, E. Parlak, L. M. Stagno, V. Turkovic, H. G. Rubahn, M. Madsen, V. Kažukauskas, D. M. Tanenbaum, S. Shanmugam and Y. Galagan, J. Mater. Res., 2018, 33, 1909–1924 Search PubMed.
- C. M. Proctor and T.-Q. Nguyen, Appl. Phys. Lett., 2015, 106, 83301 CrossRef.
- D. Neher, J. Kniepert, A. Elimelech and L. J. A. Koster, Sci. Rep., 2016, 6, 1–9 CrossRef PubMed.
- C. Wöpke, C. Göhler, M. Saladina, X. Du, L. Nian, C. Greve, C. Zhu, K. M. Yallum, Y. J. Hofstetter, D. Becker-Koch, N. Li, T. Heumüller, I. Milekhin, D. R. T. Zahn, C. J. Brabec, N. Banerji, Y. Vaynzof, E. M. Herzig, R. C. I. MacKenzie and C. Deibel, Nat. Commun., 2022, 13, 4475 CrossRef PubMed.
- D. Bartesaghi, I. D. C. Pérez, J. Kniepert, S. Roland, M. Turbiez, D. Neher and L. J. A. Koster, Nat. Commun., 2015, 6, 1–10 Search PubMed.
- X. Zeng, T. Xu, H. Chen, B. Deng, Q. Yan, X. Wen, Z. Li, H. Zeng, C. Gao, Y. Xiao, J. Liao, H. Liu, B. He, P. Han, G. Zhang, S. Li, Y. Chen and C. Xie, Energy Environ. Sci., 2024, 17, 9383–9393 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4ta07795d |
| This journal is © The Royal Society of Chemistry 2025 |