Open Access Article
Open Access ArticleChallenges and mitigation strategies for general failure and degradation in polymer electrolyte membrane-based fuel cells and electrolysers
Malavika Arun
a,
Sarbjit Giddey
 b,
Paul Joseph
*a and
Dattatray S. Dhawale
b,
Paul Joseph
*a and
Dattatray S. Dhawale
 *b
*b
aInstitute for Sustainable Industries and Liveable Cities, Victoria University, P. O. Box 14428, Melbourne, VIC 8001, Australia. E-mail: paul.joseph@vu.edu.au
bCSIRO Energy, Private Bag 10, Victoria, Clayton South 3169, Australia. E-mail: dattatray.dhawale@csiro.au
First published on 18th March 2025
Abstract
Green hydrogen production and utilisation technologies employing the polymer electrolyte membrane (PEM) electrolytic cells (both fuel cells and electrolysers) are being deployed to decarbonise the various energy sectors. PEM fuel cells convert the green hydrogen to electrical energy with water as a byproduct, whereas PEM electrolysers produce green hydrogen by splitting the water molecules. However, at present, the PEM-based fuel cells and electrolysers possess some inherent issues stemming from the failures and degradation of the cell components. This review presents the challenges and mitigation strategies for general cell failure and the degradation mechanisms of perfluorinated PEM-based electrolytic cells. The review begins with an overview of the fundamental principles of PEM electrolytic cells, followed by a detailed discussion of fluorinated membranes, including their associated challenges and environmental concerns. The review then examines common cell failures, addressing catalytic system degradation, water management issues, ohmic losses, and other component degradation. A subsequent section focuses on PEM failures, analysing the mechanisms and pathways that lead to defects such as pinholes, hotspots, cracking, thinning, and other structural deteriorations. Building on this analysis, we propose several mitigation strategies to enhance the performance and durability of PEM fuel cell and electrolyser components. We then recommend these strategies to improve overall PEM fuel cell and electrolyser system performance and offer perspectives on future pathways for commercialising these technologies.
1. Introduction
Scientists and engineers, worldwide, are actively pursuing the generation and storage of renewable energy through environmentally benign routes with a view to addressing the depletion of and also the emissions from fossil fuels, which currently dominate power generation. Recognising the desirable attributes, such as environmental friendliness, sustainability, and abundance of renewable energy sources, researchers are focusing more on overcoming challenges related to their erratic nature. The storage of energy from renewable sources is a critical issue due to fluctuations in production that often do not go in hand with demand. One proposed solution is hydrogen production by water electrolysis, and its storage to counter the inherent intermittent nature of renewable sources like wind, wave, solar, etc. Developing efficient and cost-effective polymer electrolyte membranes (PEMs) for water electrolysis technologies1 is crucial for harnessing the high energy density of hydrogen and ensuring reliable utilization of renewable sources to generate electricity to meet demand cycles. Advantages of PEM water electrolysers include enhanced hydrogen-production capacity, purity, and efficiency at higher current densities while operating at low temperatures (<100 °C). For higher-temperature applications in electrolysers, PEM cannot be used; instead, a solid oxide electrolyte is used in the range of 800 to 1000 °C.2 Hydrogen fuel cells can also serve as emission-free energy converters with immense potential, especially in addressing environmental implications, as they can be considered essentially carbon neutral during their operational cycles. Using renewable sources for hydrogen production can also make the entire process emission-free, thus offering a significant opportunity to reduce CO2 emissions in various industrial processes.3General Electric pioneered PEM water electrolysis in the 1960s as a replacement for alkaline electrolysis, with Grubb4 conceptualising a solid polymeric material known as the proton exchange membrane (PEM) in electrolytic cells. The PEM facilitates proton transport, minimizing gas crossover and enabling high-pressure operation. These characteristics also contribute to lower operational costs and overall device expenses. Furthermore, the thin membrane reduces ohmic losses, improves protonic conductivity, and offers higher current density in PEM-mediated water electrolysis.3 In addition, PEM-mediated electrolysis offers an environmentally friendly option for generating hydrogen and integrates seamlessly with renewable sources like wind and solar energy. In addition to the limited exploration in the past century due to low demand for electrolytically produced hydrogen, PEM electrolysis faces numerous unexplored challenges. During the 1980s, there was a rise in interest in producing hydrogen on a large scale through water electrolysis with PEM technology. However, the emphasis of this research was primarily on smaller units producing hydrogen, mainly for generating oxygen in space and underwater environments. The growing demand for sustainable energy has sparked renewed interest in PEM electrolysis in the past century.5 Currently, hydrogen is derived from natural resources such as gas, oil, and coal through processes like steam reforming or gasification, leading to substantial carbon dioxide (CO2) emissions. Water electrolysis can be considered as a convenient and adaptable alternative for hydrogen production to meet energy requirements. The PEM electrolysis technology is generally viable at lower temperatures (100–120 °C). It is also essential for the lifespan of PEM water electrolysers to fall within the 104–105 hour timeframe.6 One key benefit of PEM cells is their ability to operate at relatively lower temperatures, often facilitated by a non-corrosive electrolyte. Furthermore, these cells exhibit a commendable tolerance towards CO2 when utilizing atmospheric air. Some noteworthy advantages include the elimination of constraints associated with handling liquid phases coupled with relatively high voltage, current, and power density. PEM cells also excel in functioning at lower pressures, typically ranging from 1 to 2 bars, while maintaining resilience to pressure variations of the reactant gases. Furthermore, the compact and robust build of these cells, along with a simple mechanical design, add to their appeal, often complemented by stable building materials.
Despite the advantages mentioned above, the current state of cell development faces challenges in achieving a target operational lifetime exceeding 5000 hours (150![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 miles) for automotive and stationary applications for 10
000 miles) for automotive and stationary applications for 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–40
000–40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 hours.7,8 The amount of energy needed to obtain hydrogen from water through electrolysis is four times greater than the energy required to produce it from methane. Hence, hydrogen energy production using water electrolysis is an expensive process.3 Only deionized water can be used to feed the anode, as any impurities in the feed can affect the efficiency of the cell. Deionized water interacting with PEM is also corrosive to metals, which can cause corrosion of stainless-steel parts, releasing unwanted cations into the system and resulting in a loss of efficiency. Titanium metal parts can form an oxide layer on their surface, protecting them from harsh cell operating conditions. Therefore, self-protecting metals like titanium are generally used for cell parts of electrolysers, as they need to operate under harsh conditions. However, this oxide layer's growth on titanium can lead to increased cell resistance, which accelerates at higher temperatures. This, combined with membrane thinning, contributes to aging problems.9,10 All of these factors can contribute to an increase in the production and operation costs of electrolysers (>2000€ per kW) and lower durability.9,11
000 hours.7,8 The amount of energy needed to obtain hydrogen from water through electrolysis is four times greater than the energy required to produce it from methane. Hence, hydrogen energy production using water electrolysis is an expensive process.3 Only deionized water can be used to feed the anode, as any impurities in the feed can affect the efficiency of the cell. Deionized water interacting with PEM is also corrosive to metals, which can cause corrosion of stainless-steel parts, releasing unwanted cations into the system and resulting in a loss of efficiency. Titanium metal parts can form an oxide layer on their surface, protecting them from harsh cell operating conditions. Therefore, self-protecting metals like titanium are generally used for cell parts of electrolysers, as they need to operate under harsh conditions. However, this oxide layer's growth on titanium can lead to increased cell resistance, which accelerates at higher temperatures. This, combined with membrane thinning, contributes to aging problems.9,10 All of these factors can contribute to an increase in the production and operation costs of electrolysers (>2000€ per kW) and lower durability.9,11
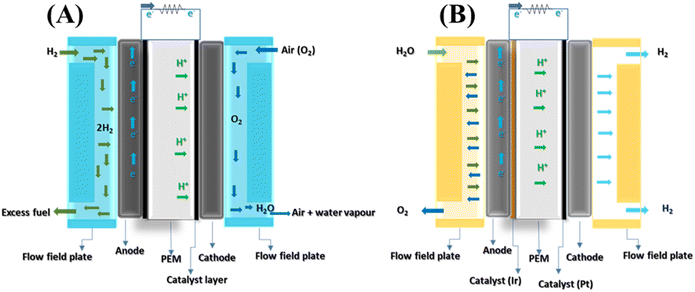
PEM fuel cells (PEMFCs), a crucial technology in this field, are still in their early stages, warranting further development for the successful commercialization of this promising technology.12 A significant obstacle to implementing fuel cells in automobiles is their cost. Currently, the production cost is around USD 45 per kilowatt for volumes under 500 thousand units per year. A crucial prerequisite for commercialising PEMFCs is a reliable hydrogen fuelling station network. Furthermore, for these fuel cells to operate within a temperature range of −40 to 120 °C, they need expensive electrocatalysts, primarily platinum-based. Despite the cost, their desirable characteristics include low operating temperatures, high power density, and ease of scalability, positioning PEMFCs as a viable alternative for internal combustion engine (ICE) automobiles in moving forward.13 Typically, a PEMFC (Fig. 1A) comprises several components: the membrane electrode assembly (MEA) (see Fig. 2 (ref. 14)), which includes a polymer electrolyte membrane (PEM), catalyst layers (CL), gas diffusion layers (GDL) with micro-porous layers (MPL), gas flow channels (GFC), and bipolar plates (BPP). Similarly, an electrolyser (Fig. 1B) also consists of a PEM, catalytic layers, porous transport layer (PTL), gaskets, titanium-based bipolar, and current collector plates.15 The catalyst layers within these cells typically consist of a specialised catalyst layer comprising a catalyst (i.e., Pt/C, Ir/Ru) and an ionomer. The ionomer plays a critical role in binding the catalyst particles together and providing triple-phase boundaries as active sites for the reaction. Consequently, the composition of the ionomer ink is pivotal for managing ohmic losses and optimizing gas transport within the cell.13
 | ||
| Fig. 2 SEM image of a typical membrane electrode assembly. Reproduced with permission from ref. 14, copyright© 2017 ECS – The Electrochemical Society. | ||
Reactions taking place in fuel cells and electrolysers are shown in eqn (1)–(4) below, where the half-cell potentials (E°a) for each reaction are shown below:
PEMFC anode:
| H2 = 2H+ + 2e−, E°a = 0 V | (1) |
PEMFC cathode:16
| 2H+ + ½O2 + 2e− = H2O, E°a = 1.229 V | (2) |
PEMWE anode:17
| H2O = 2H+ + ½O2 + 2e−, E°a = 1.229 V | (3) |
PEMWE cathode:
| H+ + e− = ½H2, E°a = 0 V | (4) |
Obviously, the electrolyte membrane, usually crafted from a highly specialised polymer, plays a crucial role in fuel cells. Generally, it acts as a barrier, preventing gas exchange between the hydrogen feed at the anode and air at the cathode while also serving as an insulator between the electrodes. Some of the important requirements of membranes used in PEM cells are: (i) they should have good mechanical strength and chemical resistance; (ii) they should have high proton conductivity (around 90–100 mS cm−1) at low to medium temperature ranges (less than 100 °C). Generally, electrolytes used in electrolytic cells are typically classified into two types: liquid electrolytes and solid electrolytes. Common liquid electrolytes include alkali-based solutions, molten carbonates, and phosphoric acid. Solid electrolytes, on the other hand, consist of polymer electrolyte membranes (PEMs), anion exchange membranes (AEMs) and solid oxide electrolytes (SOEs). Generally, PEMs offer several advantages over AEMs, SOEs, and liquid electrolytes. They often enable cells to operate efficiently at low temperatures (60–80 °C) while providing durability and requiring less maintenance. Additionally, PEM-based cells can achieve higher current and power densities by offering lower internal resistances compared to liquid and other solid electrolyte systems.18,19 The PEMFC, originating from General Electric's use of an ion-exchange resin as an electrolyte in 1959 for space applications, subsequently has become a key player in portable power sources. The recent adoption of polymer membrane-based electrolytes has bolstered fuel cells and electrolysers, positioning them as leading candidates for automobiles and buildings and replacements for rechargeable batteries.
Generally, the performance factors for PEMFCs include operating temperature, pressure, and gas stream humidity. Wang et al.13 found that increasing operating temperature and pressure, along with proper gas stream humidification, enhances the general performance of PEMFCs. However, inefficiencies, especially at low current densities, were observed at operating temperatures that are higher than the gas flow humidification temperatures. As mentioned earlier, the development of membranes for electrolytic cell applications traces back to 1959 when General Electric (GE) initiated tests with phenolic membranes. These early membranes generally had a short lifespan of up to 1000 hours, had low mechanical strength, and a power density of 0.05–0.1 kW m−2. In the early 1960s, they enhanced the power density of their cells by introducing partially sulfonated polystyrene sulfonic acid membranes, reaching 0.4–0.6 kW m−2, and these were employed application in NASA's Gemini flights. In the late 1960s, GE improved mechanical strengths and membrane lifespans with cross-linked polystyrene-divinylbenzene sulfonic acid membranes, achieving a power density of 0.75–0.8 kW m−2. However, proton conductivities remained sub-optimum. In the 1970s, Du Pont's development of the perfluorosulfonic acid “Nafion®” marked a breakthrough in membrane technology, doubling specific conductivity and extending the membrane's durability by four orders of magnitude (104–105 hours).20 Nafion® became a standard for PEMFC and continues to hold that status. Dow and Asahi Chemical Companies later developed advanced perfluorosulfonic acid (PFSA) membranes having shorter side chains with a higher ratio of ion conducting groups to the main chain.21,22 Details regarding different types of membranes, their relevant properties, and their manufacturers are collated in the table (Table 1) below.
| Sr. No. | Types | Examples | Manufacturers | Advantages |
|---|---|---|---|---|
| a Information not available. | ||||
| 1 | Fluorinated | • Perfluorosulfonic acid-based membranes | • DuPont | High stability, excellent chemical and mechanical strength, and high proton conductivity |
| • Asahi glass | ||||
| • Solvay | ||||
| • Solvay-Solexis | ||||
| • Aciplex-S | ||||
| 2 | Partially fluorinated | • Sulphonated polymer of α,β,β-trifluorostyrene | • Ballard advanced materials | Better mechanical properties, higher stability and relatively high proton conductivity, and cheaper than perfluorinated counterparts |
| • Crosslinked sulfonated polystyrene membranes | • Ionics Inc., Watertown | |||
| 3 | Non-fluorinated | • Sulfonated polyether ether ketone-(SPEEK) | • Produced by sulfonating commercial Victrex® and Gatone® PEEK | Cheaper, suitable for water purification and treatment, and more environmentally friendly |
| • Sulfonated polyarylene | • Asahi glass Company Ltd. and Asahi chemical industry Company Ltd., Japan | |||
| 4 | Acid-based blends | Polyethyleneimine (PEI)/SPEEK blend | —a | Lower vanadium ion permeability, higher water uptake, higher energy efficiency, lower charge capacity loss, good stability, and suitable for vanadium redox flow battery applications |
| 5 | Other types | Composite membranes | Can be prepared by combining Nafion (produced by DuPont, Aciplex, Asahi) with refinforcing fillers | Comparatively cheaper, high operating temperature range, can be recycled, higher water uptake, and more environmentally friendly |
Generally, PFSA membranes can be modified through the incorporation of organic or inorganic additives such as Zr(HPO4)2 nH2O, ZrO2, SiO2, SiO2/TiO2 blends, SiO2/Al2O3 particles, resulting in the formation of composite membranes for better properties.23 Alternative materials are also employed for membranes in addition to fluorinated ones, thus aiming to lower costs compared to exclusive use of fluorinated materials. Some of these partially fluorinated membranes have been found to yield enhanced stability and superior mechanical properties. While partially fluorinated membranes exhibit proton conductivity, their cost is at par with that of fully fluorinated counterparts. One of the widely accepted partially fluorinated membranes is a sulfonated copolymer that incorporates the α,β,β-trifluorostyrene monomer.18 One of the initial commercial membranes, made of polystyrene-sulfonic acid (PSSA), can undergo modification by replacing tertiary hydrogens with methyl groups and fluorine. This modification can result in the creation of sulfonated poly(α,β,β-trifluorostyrene), thus enhancing stability and prolonging the membrane's lifespan. Polymer materials known for good chemical resistance, mechanical properties and thermal stability, such as tetrafluoroethylene, poly(tetrafluoroethylene-co-perfluoropropylvinylether), poly(tetrafluoroethylene-co-hexafluoropropylene), polyethylene, polyvinyl fluoride, polyvinylidene fluoride, poly(vinylidene fluoride-co-hexafluoropropylene), poly(ethylene-alt-tetrafluoroethylene), etc. possess promising structures for grafting sulfonic acid (SA) onto these membranes. Radiation-induced grafting stands out as a prevalent method for preparing such polymers, incorporating SA and modified SA segments. While PSSA-based membrane materials exhibit a significantly higher degree of hydration compared to Nafion membranes, their conductivity is relatively lower.24
Polybenzimidazole, an aromatic polymer, has been studied recently for membrane applications because of its favorable chemical resistance and thermal stability, along with cost-effectiveness compared to fluorinated counterparts. Nevertheless, its proton conductivity remains relatively modest (around 0.02 to 0.06 S cm−1).25,26 Another prospective class of membrane materials, polyarylene ethers, has demonstrated commendable mechanical and chemical attributes. To make these polymers more desirable for membrane applications, they can be functionalized using sulfonic acid groups.27 Polyimide-based membranes possess noticeable thermal stability, albeit their manufacturing process has faced significant challenges associated with casting films from solutions.28 Polyphosphazene-based membranes represent yet another category, displaying reasonable proton conductivity, complemented by favourable thermal and chemical properties. Moreover, these polymers offer facile functionalization capabilities, though they often exhibit poorer mechanical strengths.29 Out of all the types of polymer electrolyte membranes available to date, perfluorinated membranes still stand out as the superior choice. This is primarily due to their exceptional physio-chemical properties, performance, and durability. However, they do have significant drawbacks, which will be discussed in the following section.
2. Fluorinated membranes
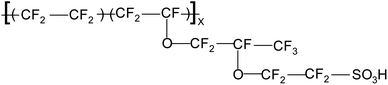
As already stated, perfluorinated membranes exhibit excellent chemical and mechanical properties, high ion conductivity, and prolonged performance. Therefore, they are the most commonly used solid electrolyte in both fuel cells and electrolysers. The carbon–fluorine bond situated in the main-chain backbone not only endows the material with inherent stability but also contributes to hydrophobicity and good thermal and electrical insulation properties.30 However, a notable limitation is their operating temperature, as temperatures exceeding 90 °C can lead to membrane dehydration, resulting in diminished performance. The rather high hydrophobic nature of the fluorinated polytetrafluoroethylene backbone contrasts with the hydrophilic nature of sulfonic acid groups in the sidechains (see Fig. 3).The frequently employed perfluoropolymer membranes include Nafion, produced by DuPont, Aciplex from Asahi Chemical, Flemion by Asahi Glass, and Gore-Select by Gore and Associates. These polymers are characterized by a long-side-chain structure that bears the ionic groups crucial for facilitating proton conductivity.30 This unique structural feature of these polymers allows for efficient proton transport, making them integral components in various applications, particularly in electrolysers, fuel cells, and other such devices where proton exchange is a key factor. In 1964, DuPont identified the desirable properties of Nafion for use as a membrane separator in chlor-alkali cells, producing chlorine and caustic soda. Simultaneously, in the USA, GE was working on PEM fuel cells for the space program, initially using unstable polystyrene-based sulfonic acid polymer membranes. While DuPont was exploring potential applications, GE discovered that Nafion membranes had the necessary oxidative stability and proton conductivity, marking the introduction of Nafion membranes in PEMFCs for space and military applications. Polymeric membranes gained acceptance in chlor-alkali cells due to environmental concerns and energy cost issues. In the 1990s, interest in PEMFC technology grew substantially, prompting DuPont to redirect efforts toward developing Nafion technology for the industry. The initially developed membranes, N-115 and N-117, were suitable for high catalyst loadings and low current density.20,31 Nafion membranes also offer an extended operational lifespan lasting up to 57![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 hours.30 The figure (Fig. 3) below shows the general structure of Nafion membranes.
000 hours.30 The figure (Fig. 3) below shows the general structure of Nafion membranes.
Preparing perfluorinated membranes involves a complicated process of copolymerizing tetrafluoroethylene with fluorocarbon vinyl ether containing a sulfonic acid group. This process, while effective, presents challenges in terms of higher costs, thus limiting its commercialisation. To address these issues, Solvay Solexis developed a new membrane prepared by copolymerising perfluoro-3-oxa-pentene sulfonyl fluoride with tetrafluoroethylene, resulting in the product now known as Aquivion. Generally, Aquivion stands out for its short sulfonic acid sidechains, providing high crystallinity, a greater ion exchange capacity, and enhanced hydration. However, the relatively shorter side-chains lack the ability to form interconnected hydrophilic networks, as seen in long side-chain membranes like Nafion, thus compromising some of the other desirable properties.32
3. Limitations of fluorinated membranes
Nafion, while the dominant product in the market for PEM electrolyser and fuel cell (FC) design and production, has many drawbacks. Firstly, its proton conductivity relies on water-filled channels, making it unsuitable for temperatures significantly below 0 °C or above 100 °C. While stable against peroxide-type degradation, it can decompose when exposed to adventitious cations or peroxide radicals. In addition, contaminating ions can reduce the conductivity of the membrane. The degradation of PEM affects the performance of electrolytic cells. The main reasons are mechanical, chemical, and thermal stresses due to the operating conditions. These stresses can cause cracks, tears, punctures, and pinholes.2,33 Such deformations can lead to short circuits, combustion from gas mixing (H2/O2), and other safety hazards. The release of hydrogen fluoride indicates membrane thinning and pinhole formation, as it is produced during the attack of ˙OH radicals on the main chain. Moreover, Nafion generally exhibits poor mechanical properties and chemical stability, especially at elevated temperatures, often leading to noticeable degradation over multiple working cycles. According to a study conducted by Xiao-Zi Yuan et al.34 on Nafion membranes, it was found that after 800 hours of operation, membranes underwent thinning and formed pinholes. In electrolysers, typically, a thicker PEM is used in comparison to a fuel cell. However, the degradation effect on the membranes is very similar to that found in fuel cells. This leads to higher hydrogen crossover and, eventually, a decline in cell performance.3As we know, the glass transition temperature (Tg) of a PEM is a significant aspect to consider for its end-use applications. Differential Scanning Calorimetric (DSC) studies have shown that Nafion exhibits two glass transition temperatures: a lower glass transition temperature (Tg) around 125 to 130 °C, attributed to the main chain mobility, and a higher Tg ranging from 195 to 240 °C, owing to the motion of the sidechains.35 Water has a plasticizing effect on Nafion, which can slightly reduce the membrane's crystallinity. In a study by Huang et al.,36 it was observed that relative humidity (RH) significantly affected the stress–strain behaviour of MEAs based on Nafion membranes at room temperature (see Fig. 4). As humidity increased, the elastic modulus decreased, indicating an inverse correlation between the two.
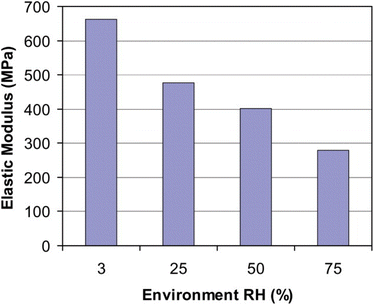 | ||
| Fig. 4 A plot showing the relationship between elastic modulus and relative humidity. Reproduced with permission from ref. 36, copyright© 2006 Wiley Periodicals, Inc. | ||
This observation confirmed that an increase in relative humidity has a plasticizing effect on the PEM, reducing its stiffness.
Additionally, operation at temperatures above the Tg of PEMs can cause a reduction in their dimensional stability. Therefore, generally, temperature and humidity are crucial factors for optimizing the performance of polymeric membranes in electrolyser and fuel cell applications.37,38 Some of the major drawbacks of Nafion membranes are discussed in detail below:
(1) As discussed earlier, one of the most extensively researched materials for PEM electrolyser and fuel-cell membranes is Nafion® which is a copolymer composed of tetrafluoroethylene and perfluoro(4-methyl-3,6-dioxa-7-octene-1-sulfonic acid), developed by DuPont. According to the sources, the cost of Nafion 117 is approximately USD 711 for a piece of ca. 61 × 50 cm2.39,40 This high cost is attributed to the intensive process of fluorinating polyethylene into polytetrafluoroethylene and then functionalizing the resultant product with sulfonic acid groups. Therefore, producing this type of membrane on a commercial scale is quite challenging.
(2) Ionic clusters within fluorinated membranes, composed of sulfonic acid species, undergo dehydration when exposed to temperatures exceeding 80 °C. This characteristic often renders them unsuitable for high-temperature applications. Dehydrating these ionic clusters within the membrane can result in a significant loss of proton conductivity. The sulfonic acid species, crucial components forming the ion clusters in the fluorinated membranes, play a pivotal role in facilitating proton transport. However, as the temperature rises beyond the 80 °C threshold, the loss of water from the clusters disrupts the proton conduction pathways, thus adversely affecting the general performance of the membrane in high-temperature conditions.41 This limitation underscores the necessity for alternative materials or design strategies to address the challenges posed by dehydration in order to enhance the suitability of fluorinated membranes for applications requiring elevated temperatures. The operational capabilities of Nafion membranes are also constrained by their limited performance under specific conditions, primarily stemming from poor ionic conductivities at low humidity and high temperatures. The challenge arises when the humidity levels decrease, impacting the ability of Nafion membranes to facilitate efficient ion transport. At lower humidities, the reduced water contents within the membrane hinder proton mobility, thereby diminishing overall ionic conductivity. Therefore, a combination of elevated temperatures and low humidity aggravates the challenges, as the membrane's ability to retain sufficient water content becomes severely compromised.42
(3) The high methanol permeability of Nafion membranes can pose a significant problem in the context of DMFCs. This trait of Nafion leads to a phenomenon known as ‘methanol crossover’. This occurs when methanol molecules readily pass through the Nafion membrane, migrating from the anode to the cathode of the fuel cell. Methanol crossover is problematic because it results in the mixing of feed and oxidant flows, thus reducing the efficiency of the fuel cell. This effect, in turn, diminishes the overall performance of the fuel cell, leading to lower power output and decreased efficiency. Secondly, the methanol crossover contributes to mechanical instability. The interaction of methanol with the cathode can lead to detrimental side reactions, such as methanol oxidation, to form unwanted by-products. These by-products, in turn, can adversely affect the structural integrity of the fuel cell components, potentially causing material degradation, corrosion, or other related issues.
(4) The fluorine content in materials, particularly the stability of the carbon–fluorine (C–F) bond, contributes to environmental concerns. The C–F bond's remarkable stability makes it resistant to easy decomposition in the environment. Hence, this enhanced stability comes with a downside, as materials containing high levels of fluorine, such as per- and polyfluoroalkyl substances (PFASs), pose a risk of bioaccumulation.43 PFAS is a general term used for materials containing fluorine atoms attached to an alkyl chain. Perfluorinated membrane materials and their degradation products fall under this category.
The high mobility of PFASs in water facilitates their leaching into water systems, causing environmental issues like soil and water contamination. This contamination, in turn, can potentially be a significant threat to both humans and ecosystems (Fig. 5 (ref. 44)). The persistence of PFASs in the environment allows them to accumulate over time, and their presence in water systems can lead to long-term exposure for aquatic organisms and, subsequently, organisms higher in the food chain, including humans.38,45,46
 | ||
| Fig. 5 Toxic effects of PFAS released into the environment from various sources, reproduced with permission from ref. 44, copyright 2019 Springer Nature LTD. | ||
The bioaccumulation of PFASs raises concerns about their potential adverse effects on human health, as these substances can enter the food chain and accumulate in various tissues.47,48 Additionally, the impact on ecosystems is substantial, as PFASs have been associated with various ecological disruptions, affecting aquatic life and potentially causing long-term harm to the balance of ecosystems. Addressing the environmental implications of fluorinated compounds, especially PFASs, necessitates a comprehensive approach, including developing environmentally friendly alternatives and stringent regulations to mitigate the release and accumulation of these substances in the environment. Perfluorocarboxylic acids (PFCAs), which are environmentally persistent and possibly toxic, have become widely distributed globally. Trace amounts, as low as nanograms per milliliter, have even been detected in human blood.49 Toxicology research on laboratory animals has associated PFCAs with tumor formation, hormonal disruptions, developmental issues, and immune system toxicity. In humans, exposure to PFAS compounds has been linked to various health risks, such as thyroid disorders, ulcerative colitis, high cholesterol, liver disease, developmental delays, cancers, and immune suppression. Furthermore, Nafion by-product 2 has been identified in the blood of most study participants, including young children.
Moreover, the water-soluble nature of per- and polyfluoroalkyl substances (PFAS) allows them to traverse longer distances in both freshwater and marine environments and can lead to the contamination of drinking water. In addition, PFAS proves challenging to eliminate fully through water treatment processes, thus contributing to concerns about drinking water safety. Numerous studies have also documented elevated concentrations of PFAS in drinking water near industrial areas, emphasizing the wide-ranging consequences of these substances in water ecosystems.49–52 Consequently, fluorine-containing membranes are being banned. This restricts the use of such membranes in PEM fuel cells and electrolysers, necessitating the development of non-fluorinated, non-toxic materials for membrane preparation.
When applied in marine settings, membrane-based fuel cells can encounter challenges due to harsh conditions impacting their performance. The presence of sodium, potassium, chromium, and magnesium ions in the marine environment poses a problem related to the efficient working of fluorinated membranes. These cations have the ability to replace protons in the sulfonic acid group sites, causing degradation of the electrolyte membrane and reducing its conductivity. In addition to the above-mentioned contaminants, NO2 and SO2 also emerge as major pollutants, with salt being a predominant factor in the marine environment. Mechanical degradation of PEMs plays a significant role in the loss of efficiency in electrolysers by reducing protonic conductivity and causing ohmic losses. The rate of degradation is strongly dependent on the cell's operating conditions.33 Currently used Nafion membranes are believed to lose mechanical strength when they swell in water at higher temperatures. A study showed that polymer membranes cast at higher temperatures (200 °C) are more prone to degradation than those cast at lower temperatures.53 High-temperature membrane casting could also lead to the chemical degradation of the ionomer. On the other hand, it has been observed that the membranes can shrink (thin) when subjected to compression and dehydration under a high vacuum inside the cell.
4. Failures of fuel cells and electrolysers
As mentioned in the previous sections, PEM fuel cells comprise multiple parts like catalysts, membranes, gas diffusion layers (GDLs), bipolar plates, and seals. During the service life, these parts can deteriorate or malfunction, resulting in the breakdown of the entire fuel cell system. Examples of degradation mechanisms encompass catalyst particle growth, preferential dissolution of alloys, corrosion of carbon supports, catalyst poisoning, membrane breakdown, loss of sulfonic acid groups, formation of surface films on bipolar plates, alterations in hydrophilicity, and decomposition of PTFE within catalyst layers and GDLs.54,55 The following section overviews the prevalent and substantial failure issues linked to the currently used PEM-based electrolytic cells. Fig. 6 represents some of the general operating conditions that trigger cell failures.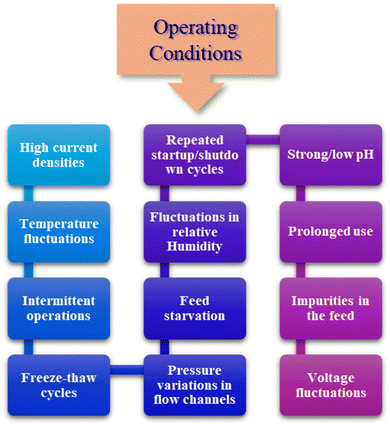 | ||
| Fig. 6 A schematic representation of the general operating conditions that trigger cell failure in PEMFCs and PEMWEs. | ||
4.1 General cell failures
In a typical PEMFC stack, the serial (bipolar) cell arrangement makes the stack susceptible to catastrophic failure if one cell fails. Meeting durability targets for automotive applications proves challenging due to changes in operating conditions, including cyclic power output and environmental factors such as relative humidity and temperature. Current designs are not adequate to demonstrate stable performance beyond a few thousand hours under these demanding conditions. A significant risk for water electrolysers is the mixing of the resultant gases produced during the electrolysis process, namely H2 and O2, which are then stored inside the unit.15 The gradual performance decline observed in electrolytic cells is often linked to electrode degradation, involving issues like platinum catalyst sintering, dissolution, ohmic losses, membrane degradation, and carbon corrosion. In this context, ensuring the durability of catalyst supports during start/stop cycling remains an ongoing challenge.36Electrochemical platinum dissolution is a concern in fuel cells and electrolysers, particularly under high cell voltage or open-circuit voltage (OCV) conditions.57–59 Another important factor affecting the performance of both fuel cells and electrolysers is the migration of the platinum catalyst, as well as platinum agglomeration (see Fig. 7 (ref. 60)). Generally, platinum stability is compromised at potentials beyond 1.188 V in acidic environments. At potentials higher than 0.98 V, oxidation can occur, leading to platinum oxide formation. Anodic and cathodic dissolution is assumed to occur by forming metastable platinum complexes. The presence of chlorides accelerates catalyst dissolution by shifting the equilibrium between platinum oxidation and reduction. In fuel cell mode, higher potentials, typically ranging from 0.9 to 1 V, amplify this effect. Furthermore, platinum ions naturally tend to precipitate on larger particles, significantly reducing the catalyst's active surface area. This reduction in active area not only decreases catalytic efficiency but also increases the cathode potential, thereby leading to higher activation overpotential.
 | ||
| Fig. 7 Platinum catalyst failure types.60 | ||
In water electrolysers, platinum catalysts are mainly used in the cathode, where the potential is usually lower. As a result, platinum catalysts are relatively stable at the cathode in PEMWEs. However, they are prone to detachment from the carbon support due to the low potential. The carbon support can suffer from mechanical degradation after prolonged use, especially under harsh operating conditions in PEMWEs, such as high current density and temperature fluctuations. This degradation can manifest as cracks, structural weakening, or even material loss. Additionally, the presence of metallic impurities in the cathode can inhibit catalytic activity by forming agglomerates that block the active sites.61 On the other hand, iridium is used as the catalyst in the anode. However, the potential at the anode is usually higher. Therefore, iridium can undergo electrochemical dissolution by forming soluble iridium oxides. In addition, higher chances of catalyst failures such as Ostwald ripening, migration, and agglomeration are in the anode. In addition, oxygen bubbles can nucleate in the anode where the oxygen evolution occurs. This can affect the stability of the catalytic layer. The formation and detachment of these bubbles can cause mechanical damage to the catalyst, potentially leading to catalyst detachment.62 Corrosion due to carbon-mediated corrosion can be a significant issue in fuel cells, occurring during a fuel shortage or localised flooding. Generally, the catalytic layer in a fuel cell consists of a catalyst, catalyst support, and a polymer membrane. Carbon-supported platinum nanoparticles are used as catalysts due to their high surface area. However, carbon becomes unstable in PEMFC operating conditions, often leading to performance degradation as Pt detaches or agglomerates, owing to weakened Pt–carbon support interaction. The degradation is primarily caused by carbon's electrochemical oxidation,57,63–65 which is generally accelerated at elevated temperatures and potentials. Although PEMFCs operate at low temperatures, certain conditions during transportation, such as high water content, high oxidation potential, low pH, etc., can accelerate carbon-mediated corrosion. A typical electrochemical oxidation reaction of carbon is shown in eqn (5).
| C + 2H2O = CO2 + 4H+ + 4e− | (5) |
The three main conditions causing carbon-mediated corrosion are high potential from feed starvation during flooding, repeated startup/shutdown cycles, and long-time effects during normal operation. During cyclic operations, it can be induced or exacerbated, leading to insufficient fuel and causing carbon-mediated corrosion to support the current flow. The standard carbon corrosion potential is 0.207 V. When the potential at the anode is below this value, the current is generated by utilising the fuel. When the value is beyond 0.027 V, corrosion of the anode catalyst layer occurs, which is driven by the degradation of the carbon support to facilitate the supply of protons. As a consequence, platinum agglomeration can also happen. A mitigation strategy to prevent this type of corrosion from occurring is operating the cells under fixed flowrates and current densities, which helps avoid these processes and reduces the extent of corrosion.57,58 The platinum catalyst used in the cathode of electrolysers is also sensitive and requires careful handling.33 Any impurities in the catalyst layer can alter its properties and lead to the formation of free radicals that degrade cell components. In a PEMWE, the porous transport layer (PTL) and the bipolar plates perform several critical functions.63 They facilitate the distribution of electrical current and provide mechanical support for the MEA. Potential (voltage) and pH are two critical factors affecting the performance of the bipolar plates and PTL.
In PEMWEs and PEMFCs, a decoupling phenomenon has been observed between the applied current density and the potential at the PTL/CCM interface. The potentials at the anode and cathode remain constant, irrespective of the applied current density, and are independent of the PTL/CCM interface potential, which is considerably higher. Additionally, the pH at the interface is acidic, ranging from 0 to 3.66 Due to the high electrochemical potential and the harsh environment at the anode, the catalyst layer is at high risk of corrosion during prolonged use. This is particularly critical since the anode is where water is oxidised to evolve oxygen. Therefore, titanium is preferred as the material for the PTL and BPP at the anode. However, the passive layer formed on titanium has a high interfacial contact resistance and is prone to corrosion after about 1000 hours of constant operation. To mitigate this, titanium is generally coated with noble metals such as gold, platinum, or iridium, resulting in 50 to 70% of the expense being devoted to making the bipolar plates and PTL robust for operation. Using other affordable metals like steel or copper to manufacture the bipolar plates and PTL parts would result in corrosion under the harsh conditions of the anode. This can lead to the release of unwanted metal ions, which can then attack the ionomers and PEMs, leading to their degradation, similar to the degradation observed in PEMFCs. Thinning of the polymer membrane through Fenton's reaction at the cathode, caused by ferric ion species, is also observed. The water used in a typical PEMWE is highly deionized, with a conductivity of approximately 1.0 μS cm−1. This prevents membrane and ionomer poisoning due to foreign cations.63,66
On the other hand, in a PEMWE, product gases can accumulate inside the cells, leading to bubbling and blockage of the flow channels. The problem occurs when gas generation at the cathode surpasses the flow channels' ability to remove it. Gas removal capacity depends on the water flow rate and the cross-sectional area of the channels. If bubbles persistently block the channels, localised water shortages can develop, leading to uneven current distribution and decreased efficiency. An increase in current densities has been observed to raise the risk of stagnant bubbles forming at the top end of the channels (as can be seen in Fig. 8).62,67
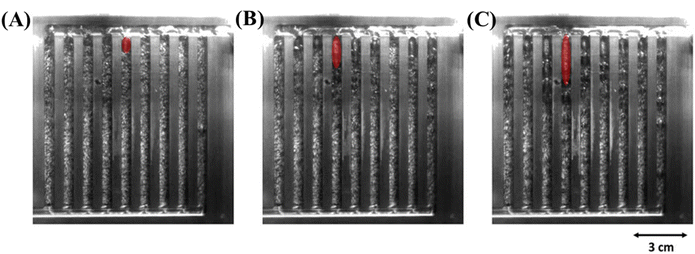 | ||
| Fig. 8 Flow field of a PEMWE at different current densities (A) 0.3 A cm−2, (B) 0.6 A cm−2, and (C) 1.0 A cm−2, showing stagnant bubbles at the top end of the channel.67 | ||
Water starvation is a significant problem in electrolysers. Overall starvation occurs when the total water supplied to the electrolyser is less than the water consumed, whereas local starvation results from a non-uniform supply of water, high current densities, and high pressure.68 Water starvation leads to membrane drying, hotspots, and ohmic losses, as insufficient water flow causes uneven temperature distribution and impairs heat removal, leading to the degradation of the MEA. At high current densities (above 0.5 A cm−2), membrane drying has been observed near the water outlet due to inadequate water flow, resulting in an increase in local ohmic resistance. Therefore, high current densities combined with insufficient water flow can significantly affect the voltage distribution.
Ohmic losses in a PEMWE can result from the ohmic resistances caused by the internal components. However, most losses are attributed to the resistance to proton transport within the membrane. Like a fuel cell, membrane thickness is an important aspect contributing to ohmic losses. Activation losses in an electrolyser depend on the rates of the OER and HER at the electrodes.72 Insufficient feed at the anode can result in mass transport losses (see Fig. 9). This occurs when the reactant water at the anode is consumed quickly, leading to a shortage of reactant at the anode surface. Slow diffusion of product gases can also result in mass transport losses. Electrochemical impedance spectroscopy (EIS) can be employed to detect ohmic losses and resistance in fuel cells and electrolysers.
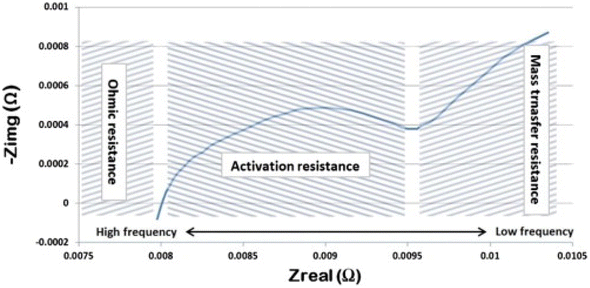 | ||
| Fig. 9 An EIS plot showing the types of internal resistances causing losses in a PEMWE, reproduced with permission from ref. 72, copyright© 2014 Hydrogen Energy Publications, LLC. Published by Elsevier Ltd. | ||
A durability study of MEA73 was performed at 90 °C, ambient pressure, and an RH of 10% at the anode and 30% at the cathode on a PEMFC stack under OCV holding mode, with intervals of 24, 48, and 144 hours, for a total duration of about 250 hours. The cell voltage decreased gradually, first exponentially and then linearly, during OCV holding. After reactivation, the voltage drop was almost fully recovered. It was also found that hydrogen crossover current densities (HCCD) were increased owing to the irreversible membrane degradation, leading to a reduction in OCV. From Fig. 10A below, it can be seen that the HCCD rate steeply increased after the 144 hour interval, continuing until the test was finished. The membrane thickness also decreased from 25.6 microns to a minimum of 13.9 microns.
 | ||
| Fig. 10 (A) Plot showing an increase in hydrogen crossover overtime during the OCV holding tests on PEMFCs, reproduced with permission from ref. 73, copyright 2023 Springer Springer Nature SharedIt content; (B) graph comparing the IVP characteristics of two fuel cell stacks, reproduced with permission from ref. 74, copyright© 2003 Published by Elsevier B.V.; (C) graphs showing a drop in mean cell voltage overtime during the durability tests conducted on PEMFC stacks, reproduced with permission from ref. 75, copyright© 2021 Elsevier Ltd. | ||
Fig. 10B shows the I–V characteristics of a commercial fuel cell stack and a constructed 19-cell ‘CMIT’ stack discussed in a study by Giddey et al.74 As we can see, with an increase in current, the voltage slowly drops for both stacks, possibly because of losses such as ohmic, activation, and mass transport losses, while the power increases until it reaches a peak before eventually decreasing. The commercial stack produced the highest output power of 1.02 kW when running at 90 A and 11.3 V at a temperature of 64 °C. In comparison, the CMIT stack produced 1.01 kW at a lower voltage of 8.1 V at a higher current of 125 A at 60 °C. This suggests that the commercial stack was relatively more efficient than the CMIT stack, and internal resistances within the cells and other components could influence cell performance.
In a 1000 hour durability study of 3-cell PEMFC stacks conducted by Chu et al.,75 it was found that the cell voltage eventually dropped, indicating performance degradation, during two dynamic load cycles with varying operating conditions and load ranges. In the first 400 hours, the performance loss was slower but accelerated after 400 hours. By 1000 hours, the mean cell voltages of the stacks had dropped by 6% (Fig. 10C). After 500 hours, the hydrogen crossover rate was also rapidly increased in the cell stacks due to the failure of the MEA components, which could primarily be attributed to pinholes or cracks formed in the membrane due to overheating. Higher operating temperatures have been found to improve the efficiency of the electrolysis process; however, higher temperatures may reduce the durability of electrolysers.76 Fig. 11A shows the effect of operating temperatures on cell voltage and the resulting improvement in efficiency with an increase in temperature.
 | ||
| Fig. 11 (A) Graph showing the effect of temperature on voltage and current densities in a PEMWE, reproduced with permission from ref. 76, copyright© 2022 Hydrogen Energy Publications LLC. Published by Elsevier Ltd.; (B) graph showing the changes in voltage in 4000 hour durability test of a PEM electrolyser.77 | ||
In the above study (see Fig. 11B), the performance of the MEA was assessed over 4000 hours. The changes in cell voltage were measured at different current densities (0.2 A cm−2, 1.0 A cm−2, and 2.0 A cm−2). Initially, the cell voltage increased over time for all current densities until 1000 hours, after which it slightly decreased and then remained stable until 4000 hours of operation. The authors attributed this initial increase to the degradation of the MEA. However, the results varied depending on the types of catalysts and membranes used. This degradation can be mitigated to some extent by improving contact between the MEA components, which reduces the ohmic resistance within the cell. The choice of materials and robust design of the cell components play a crucial role in minimizing degradation, ensuring long-term efficiency and durability.77
Generally, nitrogen (N2), nitrogen oxides (NOx), and sulfur oxides (SOx) can poison the catalyst and reduce reaction rates in the hydrogen cell. Carbon monoxide (CO) can also strongly bind to the catalyst, inhibiting the hydrogen oxidation reaction and impacting the cell's efficiency. Furthermore, hydrogen sulfide (H2S), sulfur (S), metals, and ammonia (NH3) can deposit and foul the membrane of the hydrogen cell, leading to decreased performance. Additionally, NH3 can etch the membrane, potentially causing damage and reducing the cell's effectiveness. Oils, if present as contaminants, can clog flow channels within the cell, reducing gas transport and hindering overall performance. If these contaminants are not properly managed, they can also cause the degradation of the components of the cell over time, thus leading to decreased efficiency and potentially a shorter lifespan. Maintaining a high-purity hydrogen feed and preventing membrane fouling are crucial steps to optimize the performance and longevity of a hydrogen cell. Hydrogen crossover within PEM cells is another important problem that often accelerates the degradation of both the membrane and catalyst layer, thereby causing the development of flaws such as pinholes and cracks.78 This, in turn, leads to the escalation of local hot spots and thermal degradation. Consequently, there is a loss of mass in both the membrane and catalyst layers, a reduction in activation surface area, catalyst sintering, and the generation of free radicals. These factors collectively diminish performance by lowering the open circuit voltage, voltage efficiency, fuel utilization efficiency, and overall efficiency of the PEMFC. Research indicates that fluctuations in relative humidity percentages can impact hydrogen crossover rates.79 This phenomenon may arise from the maintenance of specific humidity levels within the membrane, which reduces its porosity and consequently lowers the rate of hydrogen crossover.79
4.2 Polymer electrolyte membrane failures
The failure of the membrane is thought to stem from a combination of thermal, chemical, and mechanical factors, often working in tandem, especially under harsh operating conditions.80 Operational parameters, including humidity, temperature, freeze–thaw cycles, intermittent operation, and start-up/shut-down processes, significantly influence the deterioration of the membrane. The abrupt failure of a PEM cell or stack results from significant gas crossover occurring through cracks, pinholes, punctures, and damages in the membrane. Swelling and contracting caused by changes in humidity and temperature also influence the membrane's fatigue lifespan, with greater strain oscillations ultimately resulting in shorter fatigue life. Generally, the membrane's water content decreases as temperature rises, making it more susceptible to cracking. Basically, dry membranes are less mechanically stable, so elevated temperatures can compromise their integrity. Studies also indicate that the chemical structure of PFSA membranes starts to alter at temperatures exceeding 150 °C. However, this is not significant in the case of current fuel cells as they operate at a temperature below 100 °C. In addition, insufficient water feed may lead to the local drying of the membrane at the anode in electrolysers.9,15 Metallic impurities in the feed water also hinder the smooth conductivity of protons, causing higher resistance. Some metal ion impurities can deposit on the catalyst layer, obstructing hydrogen evolution at the cathode. For instance, impurities like Ca2+ can form metallic hydroxides at the cathode site, resulting in additional ohmic losses.Cyclic stresses, in addition, typically result in fatigue-related issues like pinholes and cracks, while continuous stress can induce membrane creep.79 Although membranes can tolerate a certain degree of mechanical stress, such as pinholes and tears, in the long run, this can reduce the lifetime of PEM cells. Fluctuations in temperature and humidity cause stress in the MEA during cell operation, subjecting it to mechanical loading. Fig. 12A and B (ref. 55 and 81) shows the typical factors contributing to the degradation and failure of PEMs under the above-mentioned conditions.
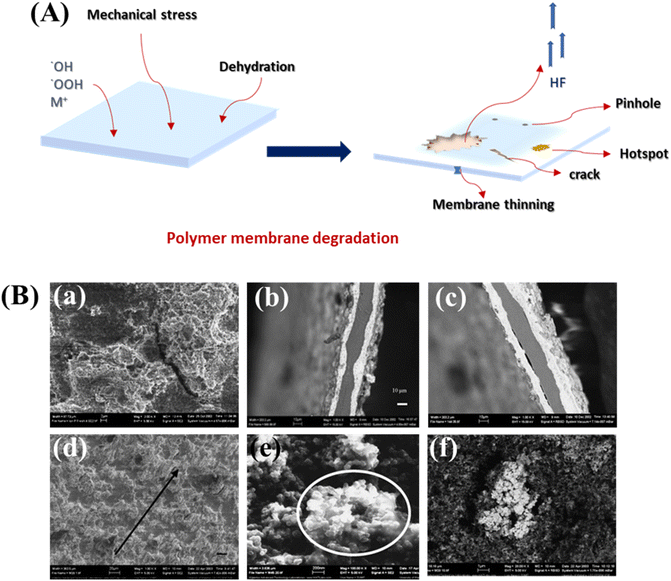 | ||
| Fig. 12 (A) Depiction of common factors contributing to the degradation and failure of polymer electrolyte membranes; and (B) SEM images of different types of membrane failures: (a) cracks (b) variations in thickness, (c) delamination, (d) catalyst orientation, (e) electrolyte clusters, and (f) platinum clusters, reproduced with permission from ref. 55 and 81, copyright© 2019 Elsevier Ltd. | ||
Thinning of the membrane is an additional factor contributing to elevated hydrogen cross-over, resulting in declines in the overall performance. Consequently, the formation of hot spots may occur, and the impact of gas cross-permeation can intensify. Apart from thinning, chemical degradation also causes bubble formation on the surface and pores of the membrane. While chemical degradation alone doesn't generate cracks, it can accelerate crack development through two mechanisms: by merging bubbles on the surface of the membrane and by linking pores within the cross-sectional area.79 Nevertheless, this degradation phenomenon has been noted in electrolysis stacks only after approximately 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 hours of periodic operation at 1 A cm−2 and 80 °C. This implies that it is a relatively prolonged process, although operating at a higher current density could hasten the occurrence.6 Research focusing on employing higher current densities (2 A cm−2 and above) observed increased hydrogen production. However, this was also found to accelerate the formation of hotspots in the case of electrolysers.6 Higher current densities and operating temperatures also led to lower cell efficiency, with a voltage drop as the ohmic resistance and mass-transport resistance increased accordingly, which, in turn, raised the operational costs.10,82–84
000 hours of periodic operation at 1 A cm−2 and 80 °C. This implies that it is a relatively prolonged process, although operating at a higher current density could hasten the occurrence.6 Research focusing on employing higher current densities (2 A cm−2 and above) observed increased hydrogen production. However, this was also found to accelerate the formation of hotspots in the case of electrolysers.6 Higher current densities and operating temperatures also led to lower cell efficiency, with a voltage drop as the ohmic resistance and mass-transport resistance increased accordingly, which, in turn, raised the operational costs.10,82–84
The chemical decomposition of PEMs is generally attributed to factors such as hydrogen peroxide formation, reactant gas crossover, platinum catalyst dissolution, recrystallization, and the presence of cationic contaminants. The onset of localized membrane degradation typically initiates at the cathode side and accelerates in dry and high-temperature conditions. A commonly used marker for identifying the degradation in fluorinated membranes is the presence of hydrogen fluoride and/or other chain fragments in the condensed discharge water.
In PEM fuel cells, the oxygen molecules can diffuse through the polymer membrane, especially if there are defects or if the membrane is not completely impermeable. As these oxygen molecules reach the anode, incomplete reduction can occur during the electrochemical reactions. This incomplete reduction process contributes to the formation of free radical species that are very reactive, such as HO˙ and HO2˙. Incomplete reduction refers to the situation where oxygen molecules are not fully converted to water during the electrochemical reactions at the anode. The incomplete reduction often leads to the formation of active radicals, particularly HO˙ radicals, on the catalyst surface. Overall, the rate of membrane degradation is directly linked to the extent of gas cross-over, where more significant amounts of oxygen reach the anode. This incomplete reduction process is evidenced by detecting hydrogen peroxide (H2O2) in the product water. H2O2 is a by-product of ORR and hence indicates the presence of active radicals in the system. Increased gas cross-over intensifies the incomplete reduction process, resulting in higher concentrations of active radicals and accelerating membrane degradation.85 The standard operating temperature of a fuel cell falls within the range of 60 to 80 °C.12 Nevertheless, ongoing research is focused on developing PEMFCs that can be operated at temperatures surpassing 100 °C. However, operating at higher temperatures comes with certain drawbacks, such as increased component degradation, corrosion, and low humidity, which can lead to membrane degradation.
In fuel cell/electrolysis cell systems, stainless steel is often used for end plates due to its relatively high durability and corrosion resistance. However, over time, metal ions from stainless steel can leach into the system.86,87 The presence of these metal ions, particularly in the form of contaminants, can speed up the degradation of PEM in cells. A proposed pathway for the degradation process involves a Fenton reaction, which is a type of chemical reaction involving the generation of hydroxyl radicals (˙OH) from hydrogen peroxide (H2O2) in the presence of metal ions, typically transition metals like iron (Fe). These highly reactive hydroxyl radicals can initiate chain reactions that lead to the breakdown of the polymer chains in the membrane. The free radicals, particularly hydroxyl radicals, generated through the Fenton reaction are highly reactive species. In fuel cells, these radicals can initiate oxidative reactions that contribute to the deterioration of the polymer structure in the membrane. The eqn (6)–(12) below show the general pathways leading to the formation of free radicals from metal ions, where M is the metal ion.
| M2+ + H2O2 → M3+ + ˙OH + OH− | (6) |
| M3+ + H2O2 → M2+ + ˙OOH + H+ | (7) |
| M3+ + ˙OOH → M2+O2 + H+ | (8) |
| H2O2 + ˙OH → ˙OOH + H2O | (9) |
| M3+ + e− → M2+ | (10) |
| M2+ + ˙OH → M3+ + OH− | (11) |
| M2+ + H+ + ˙OOH → M3+ + H2O2 | (12) |
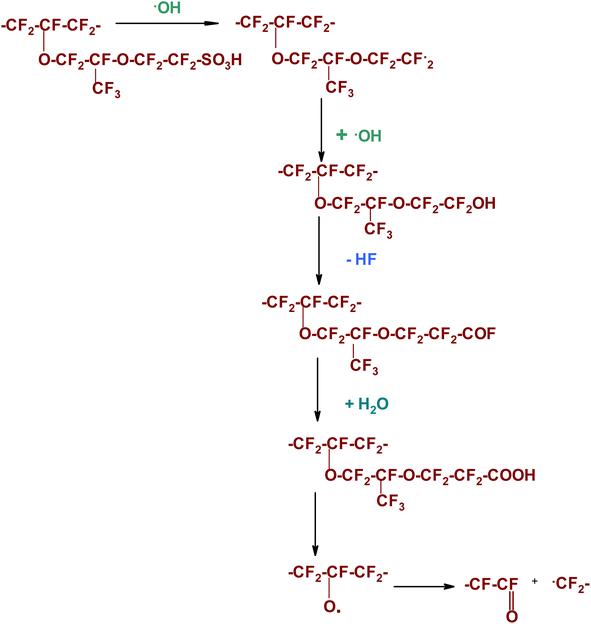
Another pathway for generating free radical species is the interaction between hydrogen and oxygen at the surfaces of a platinum catalyst, resulting in the formation of highly reactive species such as ˙OH, ˙H, and ˙OOH, which can contribute to membrane degradation. This process may occur on either the anode or cathode catalytic sites, depending on the current densities. The degradation of the main chain through the unzipping mechanism is considered one of the most common types of membrane degradation. During the polymerization of perfluorinated membrane materials, the end product acquires carboxylic acid end groups (–COOH) by using persulfate initiators. These end groups are prone to attack by any free radicals in the system. The ˙OH species can undergo a reaction with the –COOH group, resulting in the formation of carbon dioxide and water. This process leaves an active radical site on the main chain (see Scheme 1). The newly formed active chain component can react with additional ˙OH, initiating a series of chain-stripping reactions. These reactions culminate in substantially degrading the main-chain backbone.88,89
 | ||
| Scheme 1 The degradation mechanism in the main chain leads to the unzipping of the polymer molecules. | ||
A predominant degradation pattern is found in the side chains, primarily due to more reactive linkages (see Scheme 2). The hydroxyl radical can attack the C–S bond in the sulfonic acid end-group, leading to the generation of an active radical site in the side chain and the release of molecules such as water and sulfur trioxide. This active radical site formed in the sidechain can undergo further loss of molecules by reacting with more ˙OH, releasing hydrogen fluoride as a by-product. Another pathway involves free radicals attacking the C–F bond, either in the main chain or side chains.58,89
Temperature variations can also affect the degradation rate. When a Nafion membrane is immersed in water exceeding 110 °C, it undergoes a complete loss of mechanical integrity, leading to extensive disintegration. Furthermore, heightened membrane swelling can result in substantial dimensional alterations and the creation of internal constraints, diminishing its mechanical strength. These factors collectively contribute to the membrane's perforation. Research indicates that insufficient water supply to the anode causes localised membrane drying, exacerbating the issue. The challenge intensifies with increasing current density, making it increasingly challenging to provide adequate liquid water to the anode. Consequently, the electrical resistance in this region of the membrane rises, triggering a temperature increase due to the Joule effect.6 Another contributing factor to membrane breach is the mechanical stress caused by relative humidity (RH) cycling. To comprehensively address this issue, it is crucial to investigate the alterations in mechanical properties, particularly strength, arising from chemical aging and RH cycling.
5. Mitigation strategies
As discussed before, the performance of electrolytic cells influenced by factors like design, assembly, operational conditions, material degradation, and impurities. While performance degradation is inevitable as it is a natural consequence during the lifespan of a cell, an understanding of degradation mechanisms will help to minimize the rate, thereby enhancing the reliability, durability, and stability of these devices. Furthermore, ensuring the optimal performance of hydrogen cells requires a comprehensive approach to address potential contaminants. Purifying the hydrogen feedstock to fuel cells is a critical first step, involving advanced techniques such as pressure swing adsorption, membrane separation, or chemical scrubbing to guarantee high-purity hydrogen. In addition, to protect catalysts from poisoning by contaminants like CO, NOx, and SOx, it is essential to develop and employ more resistant catalysts, potentially utilizing alloyed catalysts or appropriate coatings that prevent the adsorption of these harmful substances. Improving membrane technology is equally vital, focusing on resistance to fouling and chemical attack by contaminants such as H2S, NH3, and metals. These may involve the development of new membrane materials or coatings with repellent properties against the contaminants. Regular maintenance and monitoring practices, including scheduled cleaning and replacement of affected parts, are also crucial for combating the impact of contaminants on the hydrogen cell. Table 2 below outlines the common challenges encountered during the operation of PEMFCs and PEMWEs, along with the corresponding mitigation strategies.| Sr no | General challenges | Mitigation strategies |
|---|---|---|
| 1 | Catalyst layer failures | • Avoiding high cell voltage or OCV, as well as frequent load and voltage changes |
| • Particle dissolution, detachment, agglomeration, Ostwald ripening | • Maintaining optimal humidity and temperature suitable for operation, depending on the type of membrane used | |
| • CO poisoning | • Preventing chloride impurities from entering the system | |
| • Optimizing and homogenizing particle size to achieve a uniform catalyst layer distribution | ||
| • Preventing carbon corrosion can mitigate particle detachment | ||
| • Lowering water content in the CL can reduce Pt leaching, prevent carbon corrosion, and also enhance efficiency | ||
| • Modification of the CL morphology by introducing additives like nanotubes, nanofilms, etc., can enhance the structural reinforcement | ||
| • Traditional Pt/C catalysts can be replaced with de-alloyed Pt–Cu core–shell type catalysts, which enhances the durability | ||
| Using carbides, nitrides, or oxides of metals such as titanium, molybdenum, and tungsten as the support may enhance durability and resistance to corrosion | ||
| • Oxidizing CO by passing O2 in the feed, also by introducing H2O2 in the anode gas supply can recover catalyst from CO poisoning | ||
| • A higher cell temperature can reduce CO adsorption on Pt catalysts; however, the upper limit depends on the temperature range suitable for the specific type of PEM used | ||
| 2 | Corrosion of the metallic parts of the cell assembly (PTL, BPPs, carbon support) | • Avoiding high cell voltage or OCV, as well as frequent load and voltage changes |
| • Avoiding thermal and mechanical stresses | ||
| • Using top-grade materials: graphite, alloyed stainless steel, etc. that are less corrosive | ||
| • Platinization of the PTL and nitridation of BPPs ensure the formation of a protective layer on the surface, which protects against corrosive triggers. | ||
| • Adding inert oxides like SnO2, TiO2, Ta2O5, and Nb2O5 to catalyst support enhances its stability in oxidative environments | ||
| • In PEMFCs, hydrogen starvation must be avoided to prevent extremely high cathodic potential, which accelerates the corrosion of metallic components | ||
| 3 | Water flow problems (flooding/starvation/etc.) | • Avoid over-humidifying the membranes and operating at high current densities. |
| • Operating at higher temperatures, suited for the type of PEM used, ensures a higher evaporation rate of liquid water, which can help lower flooding | ||
| • Use of thinner membranes lowers water retention, facilitating faster water transport from CLs to GDLs | ||
| • Ensuring homogenous water flows through the system by arranging the inlet and outlet channels in an interdigitated pattern, where the pathways end at the CL | ||
| • Optimise reactant flow channel designs to avoid obstructions such as sharp angles and longer pathways | ||
| • Anode water removal by forcing liquid water through membrane from cathode to anode reduces cathode flooding. | ||
| • Adding a microporous layer (MPL) to the GDL improves the contact between the CL and GDL, which in turn improves water management by enhancing water removal from the catalyst layer | ||
| • Avoiding operation at high current densities and pressures can reduce water starvation in electrolysers | ||
| 4 | Bubble formation | • Optimizing the ionomer content in the catalyst layer (CL) affects its porous structure, thickness, and distribution of triple-phase sites, thereby enhancing bubble evolution and transport efficiency |
| • Use of superaerophobic CL facilitates formation of smaller bubbles that can be released much easily | ||
| • Using porous PTLs can manage bubble transport more effectively | ||
| • Optimising flow channel designs by adopting serpentine and parallel flow field types, with shorter paths can enhance bubble movement and removal. | ||
| • Application of external physical fields, such as magnetic, ultrasonic, and gravitational fields, has been shown to enhance bubble evolution and transport | ||
| 5 | Degradation of GDLs | • Optimising compression pressure can reduce degradation due to mechanical stress |
| • Coating the GDL with a PTFE layer enhances its mechanical and chemical stability while also making it hydrophobic, reducing excess water buildup | ||
| • Doing corrosion-resistance treatments | ||
| • Minimizing ionomer degradation can prevent the release of fluoride ions, which contribute to issues such as GDL degradation, corrosion, and damage to other components | ||
| 6 | Ohmic losses | • Minimising corrosion of the components can enhance electrical conductivity |
| • Robust interface contacts ensure less contact resistance and strong interfacial bonding of the MEA | ||
| • Enhancing bulk electrical conductivity by employing metallic parts that are highly conductive | ||
| • Adding a microporous layer also enhances proper contact between CLs and GDLs | ||
| • Optimal humidity and temperature specific to the PEM are also important for improving electrical conductivity | ||
| 7 | Degradation of PEM | • Minimising local drying can prevent the formation of hot spots |
| • Ensuring uniform membrane thickness can reduce mechanical failures, thereby controlling hydrogen crossover issues | ||
| • Ensuring high purity of the reactants prevents free radicals and other species from entering the membrane, which can trigger degradation | ||
| • Ensuring uniform temperature and humidity control measures is vital in preventing membrane dehydration, shrinkage and rupture | ||
| • Reducing voltage fluctuations is critical in preventing degradation and formation of pinholes | ||
| • Hydrogen starvation must be avoided in the case of FCs to prevent damage | ||
| • Reducing input feed pressure is critical in some cases of PEMWEs, however, for optimal performance, a certain level of pressure is typically necessary to ensure high gas production rates and effective electrolysis. | ||
| • Optimising ionomer content is crucial in preventing hydrogen crossover and maintaining adequate proton conductivity of the membrane |
Additionally, implementing contaminant detection systems can provide real-time awareness of their presence in the hydrogen feed or within the cell. This also allows for prompt corrective actions to be taken before contaminants can cause significant damage. Design modifications, such as specialized flow channels or filters strategically placed to capture contaminants before they reach sensitive cell areas, can also minimise their impacts. By integrating these measures, the adverse effects of contaminants on electrolytic cell performance can be significantly reduced, ensuring more reliable and efficient operation over time.90 A detailed discussion of the mitigation strategies mentioned in the table above is provided in the following sections.
5.1 Strategies for mitigating general failures in fuel cells and electrolysers
In the context of PEMFCs, it is crucial to maintain an optimal water flow within the system to ensure effective proton conductivity while mitigating ohmic losses. Adequate hydration of the membrane in a fuel cell stands as a fundamental goal for fuel cells utilising PFSA-based membranes. A promising approach to address this challenge involves the utilization of thin membranes with a thickness of fewer than 50 micrometers. Such thin membranes can facilitate a homogeneous water flow throughout the system, thereby optimising proton transport. It is critical to avoid operating at high current densities and abrupt, intermittent operating cycles, as rapid changes in load and voltage can significantly compromise the structural integrity of the MEA.58,61 Another crucial approach involves reducing the impedance within the cell. Reducing the resistance of the conducting elements within the cell system can be achieved through several strategies. This includes enhancing bulk electrical conductivity, minimising corrosion of components, and ensuring robust interface contacts by implementing gas diffusion layers between catalyst layers and bipolar plates. These measures collectively serve to minimise ohmic losses, thereby enhancing overall efficiency. Catalyst layers often utilise perfluoro sulfonic acid (PFSA) ionomers to enhance the triple-phase boundaries (active reaction sites). Applying a thin ionomer layer on the catalyst layer can enhance the proton transport to the PEM. However, this can affect the electronic conductivity and mass transport, especially at lower catalyst loadings and high current densities. This leads to a reduction in O2 concentration near the Pt surface (cathode). To address this, various strategies have been proposed to improve local O2 transport. These include modifying the ionomer backbone to increase free volume, reducing sulfonic acid groups, and restricting their mobility by shortening the side chains. Other approaches involve replacing sulfonic acid groups with less adsorptive alternatives and tailoring these changes to the lower water content in catalyst-layer ionomers compared to bulk membranes.13Furthermore, to limit platinum agglomeration in the cathode, it is beneficial to purge the anode channel with air. This proactively helps to maintain the integrity and performance of the cathode, thus contributing to the prolonged functionality of the fuel cell system. Furthermore, by employing a multifaceted approach involving membrane hydration, component optimization, and operational techniques, fuel cell systems can effectively manage proton conductivity while mitigating undesirable failure conditions, thus maximizing their performance and longevity. Running PEMFCs on hydrocarbon reformate may lead to the exposure of the anode catalyst layer to carbon monoxide (CO). To mitigate this, alloying the platinum catalyst can decrease CO coverage. In addition, maintaining the saturated vapor pressure of the feed during operation can be achieved by thinning the catalyst layer. Furthermore, raising the cell temperature can help in this regard. Introducing oxygen into the hydrogen feed is also another strategy to oxidize CO. Alternatively, modifying the gas diffusion layer with elements such as cobalt, iron, and copper can facilitate CO oxidation. These methods serve to enhance the performance and durability of PEFCs when operating with hydrocarbon reformation, ensuring efficient and reliable energy conversion. Furthermore, optimal catalytic supports can be carefully selected to reduce oxidation rates effectively. One proposed technique to enhance thermal stability involves graphitising carbon supports. Additionally, maintaining fixed current densities and flow rates during operation can help prevent the processes leading to carbon corrosion mentioned earlier. Introducing a microporous layer consisting of densely packed carbon particles held together by PTFE has been found to have several benefits. It reduces overall ohmic loss, mitigates electrode flooding, and enhances the mechanical stability of the MEA. Additionally, PTFE can be incorporated into gas diffusion layers (GDL) to impart hydrophobicity, thereby preventing cell flooding.13 Fig. 13 summarises the important strategies that can be adopted for enhancing cell performance and minimising component failures of PEMFCs and PEMWEs.
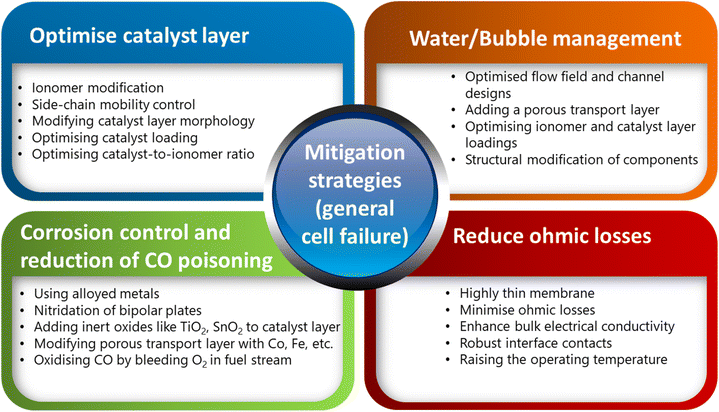 | ||
| Fig. 13 An analysis chart shows some common mitigation strategies for enhancing the overall efficiency of PEMFCs and PEMWEs. | ||
To reduce the risk of flooding in PEM fuel cells, it is beneficial to steer clear of sharp corners in the flow-field path, as this can impede the passage of water. In this context, gas diffusion flow channels (GFCs) play a crucial role in fuel cells by effectively managing reactants and facilitating water removal. Extensive research has been conducted on various GFC designs, including parallel, serpentine, pin-type, and porous media flow fields, with channel dimensions typically ranging from 0.1 to 1 mm. By designing pathways with smooth curves instead of sharp angles, the likelihood of flooding decreases. Introducing shorter pathways can also alleviate pressure drops in the flow of liquid water. This adjustment, in turn, ensures that water flows more efficiently through the system without encountering significant resistance. Among these designs, porous media flow fields have attracted increasing attention and are utilised in some recent hydrogen-operated electric vehicles. These channels enhance heat removal and electric conductance, especially by employing materials such as metal and carbon foams, which are renowned for their high porosity, corrosion resistance, and conductivity.13 Water generated on the cathode side can also play a crucial role in keeping the membrane adequately hydrated. When managed correctly, this water can naturally diffuse and pass through the membrane by convection, thus assisting in maintaining its hydration levels. Therefore, proper redirection of water formed on the cathode side can contribute to some degree to preserving the state of hydration of the membranes within the fuel cell. As mentioned in the previous paragraph, incorporating a microporous layer into the interface of the gas diffusion layer can additionally reduce the likelihood of flooding. Generally, the accumulation of ice in cells can pose a problem, especially when there's an excess of water present. To prevent issues, it is essential to keep the cell's temperature above freezing. One solution is to remove excess water before starting cell operation through gas purging. Generally, optimising the compression pressure during cell assembly can help reduce mechanical stress on GDLs to some extent.69 Furthermore, increasing the hydrophobicity of GDL materials is an effective way to ensure proper water management, which is also critical for preventing the dissolution of GDLs in water. Carbon corrosion in GDLs can be mitigated by applying PTFE coatings and performing corrosion-resistant treatments.
Reducing the corrosion of bipolar plates can often be achieved by employing top-grade materials with excellent corrosion resistance, high electrical conductivity, and strong mechanical properties. Materials such as graphite and stainless steel are viable options. Graphite, being a standard choice, generally offers high conductivity and corrosion resistance, although it is prone to mechanical failures and can be expensive. On the contrary, stainless steel possesses strong corrosion resistance and mechanical properties. However, its electrical conductivity is hindered by oxide layers forming on its surfaces. These characteristics of stainless steel can be adjusted by altering the chromium content in various grades of steel alloys.
PEMWEs typically operate under harsher conditions than PEMFCs, such as higher pressures, current densities, temperatures, and power load cycles. These factors accelerate component degradation. However, selecting materials with high chemical, mechanical, and thermal stability can mitigate this degradation to some extent. SPEEK and PBI membranes, for example, demonstrate excellent mechanical and chemical properties. Additionally, nitriding bipolar plates (BPPs) have been found to minimise corrosion, protecting the gas diffusion layer (GDL) and preventing pinhole formation.91 Adding inert oxides like SnO2, TiO2, Ta2O5, and Nb2O5 to the catalyst support reduces corrosion and lowers costs by decreasing noble metal loading.92 Studies also suggest that increasing catalyst loading can reduce the MEA degradation rate. Modifying catalyst morphology can address other challenges; for instance, platinum nanotubes are less prone to dissolution, and nano-thin films help prevent agglomeration.3
Bubble management in PEMWEs can be enhanced by optimizing the catalyst layer structure, including thickness, porosity, and triple-phase boundary distribution. Controlling ionomer content is crucial for regulating pore size. Structural modifications such as super wetting electrode designs, 3D ordered layers, or magnetized catalysts improve bubble management. Flow field designs, particularly parallel configurations, also aid in the efficient removal of bubbles.62
5.2 Strategies for mitigating membrane degradation in fuel cells and electrolysers
Firstly, mechanical failures of membranes can be significantly reduced by minimising local drying. Carefully designing the flow field structure is also essential in this case. Additionally, reducing voltage fluctuations, such as overshoots and undershoots, can, in turn, minimise membrane degradation and pinhole formations.93 Effective heat transfer and temperature control play a crucial role in preventing membrane damage. A notable issue with fluorinated membranes is that, at temperatures above 100 °C, water evaporation can occur, leading to membrane dehydration. This dehydration can cause various problems, such as cracks, pinholes, hotspots, and degradation of the conductive side chains. These issues can be mitigated to some extent by modifying the polymer structure through the incorporation of fillers such as metal oxides or carbon nanotubes,94,95 using non-fluorinated membranes, or employing polymers with shorter side chains.96Other critical factors must be considered, including reducing the feed input pressure and maintaining uniform moderate temperature and humidity conditions throughout the operation. In operational applications requiring high feed pressure, it is essential to balance power requirements with input pressure. During load demand variations, hydrogen starvation in fuel cells must be avoided to prevent damage to the membrane and associated catalyst layers. Pressure management is equally critical for PEMWEs.97 Temperature control is vital for protecting electrolyte membranes from dehydration, shrinkage, and rupture.98 Therefore, implementing a thermal management subsystem is crucial to ensure uniform temperature distribution throughout the system. Another important aspect is maintaining optimal membrane humidity. In the case of PEMFCs, this can be partially achieved by passing the feed gases through external humidifiers set to the required temperature.97 It is also essential to maintain uniform membrane thickness to effectively manage mechanical failures. Secondly, addressing issues related to corrosion is of paramount importance. The impurities can originate from BPPs, membranes (specifically Nafion®), and sealants, thus highlighting the diverse range of impurity sources and associated potential contaminants. Here, the mitigating steps involve minimizing the corrosion of metallic parts, such as BPP, in the system and preventing the degradation of cell parts like silicone seals. To further enhance the system's longevity, it is also crucial to avoid the intake of metal ion impurities and air pollutants in the cell. It is noted here that air contains contaminants such as N2, NOx, SOx, NH3, and ozone. In addition, reformate hydrogen, another source, includes CO, CO2, H2S, ammonia, and methane. By carefully managing these factors, one can ensure the optimal performance and durability of the membrane system.58 Fig. 14 shows some common strategies that can be used to reduce membrane degradation. Therefore, ensuring the durability of associated components, such as catalyst layers, GDLs, sealants, BPPs, and other components, is crucial for maintaining the integrity of the PEM. The degradation of these components can release free radicals into the system, which may attack the membrane, leading to its degradation and eventual failure. The feed gases or water must also be highly purified to prevent impurities from entering the system. Impurities can cause corrosion and degradation of cell components, and the resulting degradation of products can, in turn, contribute to the deterioration of the membrane assembly.
 | ||
| Fig. 14 An analysis chart shows some common mitigation strategies for minimizing membrane degradation in electrolyser and fuel cells. | ||
Employing proper GDLs can help mitigate pinhole formation in the membrane and associated hydrogen crossover by effectively managing the heat generated by electrolytic reactions within the cells.79 Reducing hydrogen crossover also lowers the degree of chemical degradation of the membranes by decreasing the probability of free radicals, which trigger chemical degradation, being carried through the membranes.99 Ensuring close contact and even assembling of the MEA can significantly reduce these issues. Hydrogen crossover also depends on membrane thickness, surface morphology, and ionomer concentration. Optimizing these factors can again reduce the crossover rate.91
Efforts to improve Nafion's properties generally focus on enhancing mechanical stability, typically through controlling membrane swelling, temperature, and reinforcement with inert matrices. Composite membranes, combining Nafion with inorganic fillers, are also employed to enhance chemical and temperature stabilities. However, due to matrix substitution, these enhancements may reduce proton conductivity and water vapor permeability.
To address Nafion's high methanol crossover, fine inorganic particles can be added to the polymer matrix, or other polymers with different properties can be introduced within the void space. Two approaches currently exist for this modification: semi-interpenetrating polymer networks (semi-IPN) and interpenetrating polymer networks (IPN). However, high methanol permeability remains an issue, especially in proton exchange membranes in direct methanol fuel cells (PEM/DMFCs) that use Nafion membranes. Here, cost is also a significant concern due to the complex manufacturing process of Nafion membranes. Therefore, alternative approaches to enhance performance while reducing costs are actively pursued in the field of hydrogen cell technology.24
A recent study100 examined the challenges of commercialising large-scale high-pressure PEM water electrolysis and highlighted that issues such as gas cross-permeation, membrane degradation, and hydrogen embrittlement of metal components are underexplored. The study found that composite membranes, prepared through various methods, effectively reduce gas permeation. Chemical degradation can be mitigated by introducing free radical scavengers to eliminate free radicals. Additionally, anti-corrosion coatings with high chemical stability and good electrical conductivity can partially inhibit hydrogen embrittlement. Several recent studies have also utilised various modelling techniques to analyse the challenges of commercializing fuel cells and electrolysers, as well as to predict methods for mitigating these challenges.97,101–104
A recent review105 of fuel cell systems explored the challenges of implementing this technology in electric vehicles, identifying hydrogen storage as a significant obstacle. The review proposed that fuel cell/electrolyser hybrid technology could offer a potential solution. Additionally, storing hydrogen in the form of hydrogen-based compounds, such as methanol, was highlighted as a way to simplify storage. The review also discussed strategies to enhance fuel cell performance, including increasing cell temperature, raising reagent pressure, using highly conductive electrodes, employing thinner membranes, and optimizing humidity levels.105–107
6. Conclusions and future perspectives
Hydrogen has emerged as a beacon of promise in the realm of alternative fuels, driven by its unique characteristic of producing water as the sole by-product when oxidised to produce power. This feature positions hydrogen as a standout candidate in the pursuit of carbon-neutral energy sources, as its use does not contribute to greenhouse gas emissions or environmental pollution. Embracing hydrogen holds the potential to revolutionize energy across multiple sectors, paving the way for a cleaner and more sustainable future. The versatility of hydrogen extends beyond its role as a fuel to incorporate applications in fuel cells for electricity generation. Its abundant potential to be produced using electrolysers powered by natural sources, such as solar and wind, further strengthens its sustainability credentials. However, fully realising hydrogen's potential faces challenges related to commercialising polymer electrolytic fuel cells and hydrogen-based technologies.The durability of PEMFCs and PEMWEs is a crucial factor hindering the commercialisation of automotive and stationary applications, along with concerns regarding cost and hydrogen storage. Substantial improvements are needed to make electrolytic cells more durable for automobile applications. For example, PEMFC systems must be upgraded to increase operating time to 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 hours in the coming years.108 Therefore, commercialisation of hydrogen-based cells is still a major challenge owing to the issue of failure and degradation associated with various components within the cell system. The primary accelerators of PEM degradation include elevated partial pressure of reactants, reduced RH, increased temperature, and heightened voltage. Consequently, addressing these factors is crucial in mitigating degradation and enhancing the longevity of fuel cell systems. In PEMFC stacks, the serial cell arrangement makes them vulnerable to catastrophic failure if a single cell fails, and durability is difficult to achieve due to varying operating conditions. Similarly, in water electrolysers, performance declines over time due to electrode degradation, including catalyst sintering, membrane degradation, and ohmic losses, with the risk of gas mixing posing additional safety concerns. Contaminants like nitrogen, sulfur oxides, carbon monoxide, and ammonia can poison the catalyst and foul the membrane in hydrogen cells, leading to reduced performance and efficiency. Additionally, hydrogen crossover within PEM cells accelerates degradation, causing issues like membrane and catalyst layer damage, reduced efficiency, and increased risk of thermal degradation. Maintaining high-purity feed and controlling humidity levels are crucial to optimizing performance and longevity.
000 hours in the coming years.108 Therefore, commercialisation of hydrogen-based cells is still a major challenge owing to the issue of failure and degradation associated with various components within the cell system. The primary accelerators of PEM degradation include elevated partial pressure of reactants, reduced RH, increased temperature, and heightened voltage. Consequently, addressing these factors is crucial in mitigating degradation and enhancing the longevity of fuel cell systems. In PEMFC stacks, the serial cell arrangement makes them vulnerable to catastrophic failure if a single cell fails, and durability is difficult to achieve due to varying operating conditions. Similarly, in water electrolysers, performance declines over time due to electrode degradation, including catalyst sintering, membrane degradation, and ohmic losses, with the risk of gas mixing posing additional safety concerns. Contaminants like nitrogen, sulfur oxides, carbon monoxide, and ammonia can poison the catalyst and foul the membrane in hydrogen cells, leading to reduced performance and efficiency. Additionally, hydrogen crossover within PEM cells accelerates degradation, causing issues like membrane and catalyst layer damage, reduced efficiency, and increased risk of thermal degradation. Maintaining high-purity feed and controlling humidity levels are crucial to optimizing performance and longevity.
The selection of materials for the polymer electrolyte membrane, a critical component in PEM cells, holds immense importance in addressing these challenges. Currently, Nafion stands out as the predominant choice due to its outstanding conductivity and favourable chemical and mechanical properties. However, despite its favourable characteristics, widespread adoption of Nafion faces obstacles due to its high production costs and environmental implications. The stability of the carbon–fluorine (C–F) bond in fluorine-containing materials, such as PFASs, which form the basic structure of Nafion and other perfluorinated membranes, makes them resistant to decomposition in the environment, leading to significant environmental concerns. PFASs are water-soluble and can easily contaminate land and water sources, causing risks to human health and ecosystems due to their persistence and potential for bioaccumulation. The presence of PFASs in water systems can cause long-term exposure for aquatic life and humans, leading to ecological disruptions and health risks. Additionally, PFASs are difficult to remove through water treatment processes, contributing to the contamination of drinking water, particularly near industrial areas. Addressing these issues requires developing environmentally friendly alternatives and implementing strict regulations to mitigate the impact of PFASs. Perfluorinated polymeric membranes, which are currently used in PEM cells, face two primary challenges. Firstly, they struggle to maintain high protonic conductivity when water content is low, particularly under conditions of high temperature and low humidity. Secondly, ensuring durability under these conditions remains a significant obstacle, as existing sulfonic acid-based materials exhibit low conductivity under low hydration conditions.109
To enhance electrolytic cell performance, it is crucial to develop novel polymer-based membranes that operate effectively at higher temperatures, require less hydration, and are made from environmentally friendly materials. Improving proton conductivity at lower hydration levels and elevated temperatures can be achieved by incorporating highly acidic functional groups or designing new polymer backbones. Polymers similar to Nafion, but without halogenated components, are being explored for their potential in fuel cells and electrolysers, focusing on reducing costs and environmental impact. Customising membranes for specific applications, such as varying degrees of hydration tolerance or resistance to contaminants, can further optimise performance for different types of fuel cells and electrolysers. In addition, developing membranes that work synergistically with other cell components, such as electrodes and catalysts, can improve overall system efficiency. Inorganic/organic hybrid materials such as polymer/clay nanocomposite hybrids are one of the promising candidates due to their excellent mechanical and thermal stability, along with low gas cross-permeation effects. Reinforcing polymers with fibres or nanoparticles can enhance their mechanical strength and durability, helping membranes withstand operation stresses, such as swelling and shrinking, due to hydration cycles. Reducing gas crossover, particularly of hydrogen and oxygen, is critical for both safety and efficiency, with nanocomposite membranes incorporating impermeable fillers like clays or graphene to achieve this without compromising proton conductivity. Chemical stability is also essential, as the membrane must resist degradation under oxidative and acidic conditions, extending its lifespan and maintaining efficiency over time. Cost-effective materials are vital for large-scale deployment, potentially through the use of cheaper raw materials or simplified fabrication processes. Using non-toxic, halogen-free materials can reduce the environmental footprint and make membranes safer for disposal and recycling. A proposed model connects PEM electrolysers and fuel cells into a single integrated system. In this setup, hydrogen produced by the electrolyser from feed water is supplied to the fuel cell. The water produced by the fuel cell during operation is then purified and cycled back into the electrolyser. This arrangement aims to create a self-sustaining power generator. However, further research is needed to confirm the feasibility of this system.
To address these challenges, there is a pressing need to develop novel proton-conducting membranes/components and structures capable of meeting the demands of higher-temperature systems. Researchers and industry stakeholders are actively exploring other similar or advanced materials to replace Nafion but with reduced costs and environmental footprint. This ongoing research and development effort is critical in advancing the commercial sustainability of hydrogen-based fuel cells and electrolysers, ultimately contributing to a cleaner and more sustainable energy future.
Abbreviations
| BPP | Bipolar plates |
| CL | Catalyst layers |
| DMFC | Direct methanol fuel cell |
| DSC | Differential scanning calorimetric |
| GDL | Gas diffusion layers |
| GE | General electric |
| GFC | Gas flow channels |
| HER | Hydrogen evolution reaction |
| HDV | Heavy-duty vehicle |
| ICE | Internal combustion engine |
| IPN | Interpenetrating polymer networks |
| MEA | Membrane electrode assembly |
| MPL | Micro-porous layers |
| NOx | Nitrogen oxides |
| OCV | Open-circuit voltage |
| OER | Oxygen evolution reaction |
| ORR | Oxygen reduction reaction |
| PEI | Polyethyleneimine |
| PEM | Proton exchange membranes |
| PEMFCs | PEM fuel cells |
| PEMWEs | PEM water electrolysers |
| PFAS | Polyfluoroalkyl substances |
| PFCA | Perfluorocarboxylic acids |
| PFSA | Perfluorosulfonic acid |
| PSSA | Polystyrene-sulfonic acid |
| PTFE | Polytetrafluoroethylene |
| PTL | Porous transport layer |
| RH | Relative humidity |
| SA | Sulfonic acid |
| SOx | Sulfur oxides |
| SPEEK | Sulfonated polyether ether ketone |
| Tg | Glass transition temperature |
Data availability
Data availability is not applicable to this article as no new data were created or analyzed in this study.Author contributions
Malavika Arun: data collection, data curation, writing-original draft; Sarbjit Giddey: funding acquisition, supervision and conceptualisation, review, and editing; Paul Joseph: supervision, review, and editing; Dattatray S. Dhawale: funding acquisition, project administration, supervision, conceptualisation, data curation, review, and editing.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors gratefully acknowledge the funding support from the Commonwealth Scientific and Industrial Research Organisation (CSIRO) Hydrogen Energy Systems Future Science Platform (HES FSP), Hydrogen Mission, and Energy Technologies BU. We are also thankful for funding from CSIRO and Victoria University, Melbourne, Australia, towards MA's post-doctoral research fellowship.References
- P. Millet, R. Ngameni, S. Grigoriev, N. Mbemba, F. Brisset, A. Ranjbari and C. Etiévant, PEM water electrolyzers: from electrocatalysis to stack development, Int. J. Hydrogen Energy, 2010, 35, 5043–5052 CAS.
- M. Irshad, K. Siraj, R. Raza, A. Ali, P. Tiwari, B. Zhu, A. Rafique, A. Ali, M. Kaleem Ullah and A. Usman, A brief description of high temperature solid oxide fuel cell's operation, materials, design, fabrication technologies and performance, Appl. Sci., 2016, 6, 75 CrossRef.
- F. N. Khatib, T. Wilberforce, O. Ijaodola, E. Ogungbemi, Z. El-Hassan, A. Durrant, J. Thompson and A. G. Olabi, Material degradation of components in polymer electrolyte membrane (PEM) electrolytic cell and mitigation mechanisms: a review, Renew. Sustain. Energy Rev., 2019, 111, 1–14 CrossRef CAS.
- W. Grubb, Batteries with solid ion exchange electrolytes: I. secondary cells employing metal electrodes, J. Electrochem. Soc., 1959, 106, 275 CrossRef CAS.
- M. Carmo, D. L. Fritz, J. Mergel and D. Stolten, A comprehensive review on PEM water electrolysis, Int. J. Hydrogen Energy, 2013, 38, 4901–4934 CrossRef CAS.
- P. Millet, A. Ranjbari, F. De Guglielmo, S. A. Grigoriev and F. Auprêtre, Cell failure mechanisms in PEM water electrolyzers, Int. J. Hydrogen Energy, 2012, 37, 17478–17487 CrossRef CAS.
- R. Borup, J. Meyers, B. Pivovar, Y. S. Kim, R. Mukundan, N. Garland, D. Myers, M. Wilson, F. Garzon and D. Wood, Scientific aspects of polymer electrolyte fuel cell durability and degradation, Chem. Rev., 2007, 107, 3904–3951 CrossRef CAS PubMed.
- Y. Wang, K. S. Chen, J. Mishler, S. C. Cho and X. C. Adroher, A review of polymer electrolyte membrane fuel cells: technology, applications, and needs on fundamental research, Appl. Energy, 2011, 88, 981–1007 CrossRef CAS.
- F. Fouda-Onana, M. Chandesris, V. Médeau, S. Chelghoum, D. Thoby and N. Guillet, Investigation on the degradation of MEAs for PEM water electrolysers part I: effects of testing conditions on MEA performances and membrane properties, Int. J. Hydrogen Energy, 2016, 41, 16627–16636 CrossRef CAS.
- C. Rakousky, G. P. Keeley, K. Wippermann, M. Carmo and D. Stolten, The stability challenge on the pathway to high-current-density polymer electrolyte membrane water electrolyzers, Electrochim. Acta, 2018, 278, 324–331 CAS.
- F. Massari, M. Wenske, R. Kaeppner, I. Aso, R. Rasoini and F. Gamallo, Alkaline 2.0-novel and advanced electrolyzers for a sustainable integration of renewable energy and implementation of a decarbonized private and public transport in Europe, 2014 Search PubMed.
- M. P. Rodgers, L. J. Bonville, H. R. Kunz, D. K. Slattery and J. M. Fenton, Fuel cell perfluorinated sulfonic acid membrane degradation correlating accelerated stress testing and lifetime, Chem. Rev., 2012, 112, 6075–6103 CAS.
- Y. Wang, H. Yuan, A. Martinez, P. Hong, H. Xu and F. R. Bockmiller, Polymer electrolyte membrane fuel cell and hydrogen station networks for automobiles: status, technology, and perspectives, Adv. Appl. Energy, 2021, 2, 100011 CAS.
- X. Yin, L. Lin, H. T. Chung, S. K. Babu, U. Martinez, G. M. Purdy and P. Zelenay, Effects of MEA fabrication and ionomer composition on fuel cell performance of PGM-free ORR catalyst, ECS Trans., 2017, 77, 1273 CAS.
- Y. Chen, C. Liu, J. Xu, C. Xia, P. Wang, B. Y. Xia, Y. Yan and X. Wang, Key components and design strategy for a proton exchange membrane water electrolyzer, Small Structures, 2023, 4, 2200130 CrossRef CAS.
- L. Carrette, K. A. Friedrich and U. Stimming, Fuel cells: principles, types, fuels, and applications, ChemPhysChem, 2000, 1, 162–193 CrossRef CAS PubMed.
- H. Gunaseelan, A. Munde, R. Patel and B. Sathe, Metal-organic framework derived carbon-based electrocatalysis for hydrogen evolution reactions: a review, Mater. Today Sustain., 2023, 22, 100371 Search PubMed.
- E. Ogungbemi, O. Ijaodola, F. N. Khatib, T. Wilberforce, Z. El Hassan, J. Thompson, M. Ramadan and A. G. Olabi, Fuel cell membranes–pros and cons, Energy, 2019, 172, 155–172 CAS.
- M. Steilen and L. Jörissen, Hydrogen conversion into electricity and thermal energy by fuel cells: use of H2-systems and batteries, Electrochemical energy storage for renewable sources and grid balancing, Elsevier, 2015, pp. 143–158 Search PubMed.
- S. Banerjee and D. E. Curtin, Nafion® perfluorinated membranes in fuel cells, J. Fluor. Chem., 2004, 125, 1211–1216 CrossRef CAS.
- B. Smitha, S. Sridhar and A. Khan, Solid polymer electrolyte membranes for fuel cell applications—a review, JJ. Membr. Sci., 2005, 259, 10–26 CrossRef CAS.
- S. J. Peighambardoust, S. Rowshanzamir and M. Amjadi, Review of the proton exchange membranes for fuel cell applications, Int. J. Hydrogen Energy, 2010, 35, 9349–9384 CrossRef CAS.
- E. Y. Safronova, A. A. Lysova, D. Y. Voropaeva and A. B. Yaroslavtsev, Approaches to the Modification of Perfluorosulfonic Acid Membranes, Membranes, 2023, 13, 721 CrossRef CAS PubMed.
- A. Kraytsberg and Y. Ein-Eli, Review of advanced materials for proton exchange membrane fuel cells, Energy Fuels, 2014, 28, 7303–7330 CrossRef CAS.
- Q. Li, C. Pan, J. O. Jensen, P. Noyé and N. J. Bjerrum, Cross-linked polybenzimidazole membranes for fuel cells, Chem. Mater., 2007, 19, 350–352 CrossRef CAS.
- J. S. Yang, L. N. Cleemann, T. Steenberg, C. Terkelsen, Q. Li, J. O. Jensen, H. Hjuler, N. J. Bjerrum and R. He, High molecular weight polybenzimidazole membranes for high temperature PEMFC, Fuel Cells, 2014, 14, 7–15 CAS.
- M. Xu, H. Xue, Q. Wang and L. Jia, Sulfonated poly (arylene ether) s based proton exchange membranes for fuel cells, Int. J. Hydrogen Energy, 2021, 46, 31727–31753 CAS.
- N. Sazali, M. A. Mohamed and W. N. W. Salleh, Membranes for hydrogen separation: a significant review, Int. J. Adv. Des. Manuf. Technol., 2020, 107, 1859–1881 CrossRef.
- R. Wycisk and P. N. Pintauro, Polyphosphazene membranes for fuel cells, Fuel Cells, 2008, II, 157–183 Search PubMed.
- V. Arcella, A. Ghielmi and G. Tommasi, High performance perfluoropolymer films and membranes, Ann. N. Y. Acad. Sci., 2003, 984, 226–244 CrossRef CAS PubMed.
- M. Doyle and G. Rajendran, Perfluorinated membranes, Handbook of fuel cells, ( 2010) Search PubMed.
- O. N. Primachenko, E. A. Marinenko, A. S. Odinokov, S. V. Kononova, Y. V. Kulvelis and V. T. Lebedev, State of the art and prospects in the development of proton-conducting perfluorinated membranes with short side chains: a review, Polym. Adv. Technol., 2021, 32, 1386–1408 CrossRef CAS.
- P. Millet, Degradation processes and failure mechanisms in PEM water electrolyzers, PEM Electrolysis for Hydrogen Production: Principles and Applications, CRC Press-Taylor & Francis Group, New York, 2016, p. 23 Search PubMed.
- X.-Z. Yuan, S. Zhang, S. Ban, C. Huang, H. Wang, V. Singara, M. Fowler, M. Schulze, A. Haug and K. A. Friedrich, Degradation of a PEM fuel cell stack with Nafion® membranes of different thicknesses. Part II: ex situ diagnosis, J. Power Sources, 2012, 205, 324–334 CrossRef CAS.
- H.-Y. Jung and J. W. Kim, Role of the glass transition temperature of Nafion 117 membrane in the preparation of the membrane electrode assembly in a direct methanol fuel cell (DMFC), Int. J. Hydrogen Energy, 2012, 37, 12580–12585 CrossRef CAS.
- X. Huang, R. Solasi, Y. Zou, M. Feshler, K. Reifsnider, D. Condit, S. Burlatsky and T. Madden, Mechanical endurance of polymer electrolyte membrane and PEM fuel cell durability, J. Polym. Sci., Part B: Polym. Phys., 2006, 44, 2346–2357 CrossRef CAS.
- J. Benziger, A. Bocarsly, M. J. Cheah, P. Majsztrik, B. Satterfield and Q. Zhao, Mechanical and transport properties of nafion: effects of temperature and water activity, Fuel Cells Hydrog. Storage, 2011, 85–113 CAS.
- M. Di Virgilio, A. Basso Peressut, V. Arosio, A. Arrigoni, S. Latorrata and G. Dotelli, Functional and environmental performances of novel electrolytic membranes for PEM fuel cells: a lab-scale case study, Clean Technol., 2023, 5, 74–93 Search PubMed.
- B. D. James, F. Lomax and C. Thomas, Manufacturing Cost of Stationary Polymer Electrolyte Membrane (PEM) Fuel Cell Systems, Final Report, Prepared for the US Department of Energy, National Renewable Energy Laboratory, 1999 Search PubMed.
- Y. Prykhodko, K. Fatyeyeva, L. Hespel and S. Marais, Progress in hybrid composite Nafion®-based membranes for proton exchange fuel cell application, Chem. Eng. J., 2021, 409, 127329 CAS.
- M. K. Mistry, N. R. Choudhury, N. K. Dutta, R. Knott, Z. Shi and S. Holdcroft, Novel organic–inorganic hybrids with increased water retention for elevated temperature proton exchange membrane application, Chem. Mater., 2008, 20, 6857–6870 CAS.
- X. Sun, S. C. Simonsen, T. Norby and A. Chatzitakis, Composite membranes for high temperature PEM fuel cells and electrolysers: a critical review, Membranes, 2019, 9, 83 CAS.
- Z. Wang, J. C. DeWitt, C. P. Higgins and I. T. Cousins, A Never-Ending Story of Per-And Polyfluoroalkyl Substances (PFASs)?, Environ. Sci. Technol., 2017, 51, 2508–2518 CAS.
- E. M. Sunderland, X. C. Hu, C. Dassuncao, A. K. Tokranov, C. C. Wagner and J. G. Allen, A review of the pathways of human exposure to poly-and perfluoroalkyl substances (PFASs) and present understanding of health effects, J. Expo. Sci. Environ. Epidemiol., 2019, 29, 131–147 CAS.
- H. Holmquist, P. Fantke, I. T. Cousins, M. Owsianiak, I. Liagkouridis and G. M. Peters, An (eco) toxicity life cycle impact assessment framework for per-and polyfluoroalkyl substances, J. Expo. Sci. Environ. Epidemiol., 2020, 54, 6224–6234 CAS.
- M. S. Shimizu, R. S. Garcia, G. B. Avery, R. J. Kieber, S. A. Skrabal and R. N. Mead, Distribution of legacy and emerging per-and polyfluoroalkyl substances in riverine and coastal sediments of Southeastern North Carolina, USA, Environ. Sci.: Processes Impacts, 2022, 24, 2119–2128 CAS.
- A. O. De Silva, J. M. Armitage, T. A. Bruton, C. Dassuncao, W. Heiger-Bernays, X. C. Hu, A. Kärrman, B. Kelly, C. Ng and A. Robuck, PFAS exposure pathways for humans and wildlife: a synthesis of current knowledge and key gaps in understanding, Environ. Toxicol. Chem., 2021, 40, 631–657 CAS.
- Y. Lu, R. Guan, N. Zhu, J. Hao, H. Peng, A. He, C. Zhao, Y. Wang and G. Jiang, A critical review on the bioaccumulation, transportation, and elimination of per-and polyfluoroalkyl substances in human beings, Crit. Rev. Environ. Sci. Technol., 2024, 54, 95–116 CAS.
- N. Kotlarz, J. McCord, D. Collier, C. S. Lea, M. Strynar, A. B. Lindstrom, A. A. Wilkie, J. Y. Islam, K. Matney and P. Tarte, Measurement of novel, drinking water-associated PFAS in blood from adults and children in Wilmington, North Carolina, Environ. Health Perspect., 2020, 128, 077005 CAS.
- E. A. Emmett, F. S. Shofer, H. Zhang, D. Freeman, C. Desai and L. M. Shaw, Community exposure to perfluorooctanoate: relationships between serum concentrations and exposure sources, J. Occup. Environ. Med., 2006, 48, 759 CAS.
- J. M. Graber, C. Alexander, R. J. Laumbach, K. Black, P. O. Strickland, P. G. Georgopoulos, E. G. Marshall, D. G. Shendell, D. Alderson and Z. Mi, Per and polyfluoroalkyl substances (PFAS) blood levels after contamination of a community water supply and comparison with 2013–2014 NHANES, JJ. Expo. Sci. Environ. Epidemiol., 2019, 29, 172–182 CAS.
- X. C. Hu, D. Q. Andrews, A. B. Lindstrom, T. A. Bruton, L. A. Schaider, P. Grandjean, R. Lohmann, C. C. Carignan, A. Blum and S. A. Balan, Detection of poly-and perfluoroalkyl substances (PFASs) in US drinking water linked to industrial sites, military fire training areas, and wastewater treatment plants, Environ. Sci. Technol. Lett., 2016, 3, 344–350 CAS.
- S. Siracusano, N. Van Dijk, R. Backhouse, L. Merlo, V. Baglio and A. Aricò, Degradation issues of PEM electrolysis MEAs, Renew. Energy, 2018, 123, 52–57 CAS.
- V. Anghel, Prediction failure for pem fuel cells, Int. J. Adv. Eng. Technol., 2012, 4, 1 Search PubMed.
- D. Qiu, L. Peng, X. Lai, M. Ni and W. Lehnert, Mechanical failure and mitigation strategies for the membrane in a proton exchange membrane fuel cell, Renew. Sustain. Energy Rev., 2019, 113, 109289 CAS.
- M. Xie, T. Chu, T. Wang, K. Wan, D. Yang, B. Li, P. Ming and C. Zhang, Preparation, performance and challenges of catalyst layer for proton exchange membrane fuel cell, Membranes, 2021, 11, 879 CAS.
- P. Rama, R. Chen and J. Andrews, A review of performance degradation and failure modes for hydrogen-fuelled polymer electrolyte fuel cells, Proc. Inst. Mech. Eng. A J. Power Energy, 2008, 222, 421–441 CrossRef CAS.
- E. Wallnöfer-Ogris, F. Poimer, R. Köll, M.-G. Macherhammer and A. Trattner, Main degradation mechanisms of polymer electrolyte membrane fuel cell stacks–mechanisms, influencing factors, consequences, and mitigation strategies, Int. J. Hydrogen Energy, 2023, 50, 1159–1182 CrossRef.
- S. Zhang, X.-Z. Yuan, J. N. C. Hin, H. Wang, K. A. Friedrich and M. Schulze, A review of platinum-based catalyst layer degradation in proton exchange membrane fuel cells, J. Power Sources, 2009, 194, 588–600 CrossRef CAS.
- J. C. Meier, C. Galeano, I. Katsounaros, J. Witte, H. J. Bongard, A. A. Topalov, C. Baldizzone, S. Mezzavilla, F. Schüth and K. J. Mayrhofer, Design criteria for stable Pt/C fuel cell catalysts, Beilstein J. Nanotechnol., 2014, 5, 44–67 CrossRef PubMed.
- E. Wallnöfer-Ogris, I. Grimmer, M. Ranz, M. Höglinger, S. Kartusch, J. Rauh, M.-G. Macherhammer, B. Grabner and A. Trattner, A review on understanding and identifying degradation mechanisms in PEM water electrolysis cells: insights for stack application, development, and research, Int. J. Hydrogen Energy, 2024, 65, 381–397 Search PubMed.
- S. Yuan, C. Zhao, X. Cai, L. An, S. Shen, X. Yan and J. Zhang, Bubble evolution and transport in PEM water electrolysis: mechanism, impact, and management, Prog. Energy Combust. Sci., 2023, 96, 101075 Search PubMed.
- H. Becker, E. J. Dickinson, X. Lu, U. Bexell, S. Proch, C. Moffatt, M. Stenström, G. Smith and G. Hinds, Assessing potential profiles in water electrolysers to minimise titanium use, Energy Environ. Sci., 2022, 15, 2508–2518 CAS.
- J. Zhao, Z. Tu and S. H. Chan, Carbon corrosion mechanism and mitigation strategies in a proton exchange membrane fuel cell (PEMFC): a review, J. Power Sources, 2021, 488, 229434 CAS.
- V. Mehta and J. S. Cooper, Review and analysis of PEM fuel cell design and manufacturing, J. Power Sources, 2003, 114, 32–53 CAS.
- M. Prestat, Corrosion of structural components of proton exchange membrane water electrolyzer anodes: a review, J. Power Sources, 2023, 556, 232469 CAS.
- M. Maier, Q. Meyer, J. Majasan, R. E. Owen, J. B. Robinson, J. Dodwell, Y. Wu, L. Castanheira, G. Hinds and P. R. Shearing, Diagnosing stagnant gas bubbles in a polymer electrolyte membrane water electrolyser using acoustic emission, Front. Energy Res., 2020, 8, 582919 Search PubMed.
- S. Sun, Y. Xiao, D. Liang, Z. Shao, H. Yu, M. Hou and B. Yi, Behaviors of a proton exchange membrane electrolyzer under water starvation, RSC Adv., 2015, 5, 14506–14513 CAS.
- J. Park, H. Oh, T. Ha, Y. I. Lee and K. Min, A review of the gas diffusion layer in proton exchange membrane fuel cells: durability and degradation, Appl. Energy, 2015, 155, 866–880 CAS.
- Y. Pan, H. Wang and N. P. Brandon, Gas diffusion layer degradation in proton exchange membrane fuel cells: mechanisms, characterization techniques and modelling approaches, J. Power Sources, 2021, 513, 230560 CAS.
- T. Mennola, M. Mikkola, M. Noponen, T. Hottinen and P. Lund, Measurement of ohmic voltage losses in individual cells of a PEMFC stack, J. Power Sources, 2002, 112, 261–272 CAS.
- J. Van der Merwe, K. Uren, G. Van Schoor and D. Bessarabov, Characterisation tools development for PEM electrolysers, Int. J. Hydrogen Energy, 2014, 39, 14212–14221 CAS.
- D. Yoo, B. Hwang, S. Oh and K. Park, Acceleration of electrolyte membrane degradation by frequent activation in PEMFC electrochemical durability evaluation, Korean J. Chem. Eng., 2023, 40, 2004–2009 CAS.
- S. Giddey, F. Ciacchi and S. Badwal, Design, assembly and operation of polymer electrolyte membrane fuel cell stacks to 1 kWe capacity, J. Power Sources, 2004, 125, 155–165 CAS.
- T. Chu, M. Xie, Y. Yu, B. Wang, D. Yang, B. Li, P. Ming and C. Zhang, Experimental study of the influence of dynamic load cycle and operating parameters on the durability of PEMFC, Energy, 2022, 239, 122356 CrossRef CAS.
- A. Bazarah, E. H. Majlan, T. Husaini, A. Zainoodin, I. Alshami, J. Goh and M. S. Masdar, Factors influencing the performance and durability of polymer electrolyte membrane water electrolyzer: a review, Int. J. Hydrogen Energy, 2022, 47, 35976–35989 CrossRef CAS.
- M. Möckl, M. F. Ernst, M. Kornherr, F. Allebrod, M. Bernt, J. Byrknes, C. Eickes, C. Gebauer, A. Moskovtseva and H. A. Gasteiger, Durability Testing of Low-Iridium PEM Water Electrolysis Membrane Electrode Assemblies, J. Electrochem. Soc., 2022, 169, 064505 CrossRef.
- P. Trinke, B. Bensmann and R. Hanke-Rauschenbach, Current density effect on hydrogen permeation in PEM water electrolyzers, Int. J. Hydrogen Energy, 2017, 42, 14355–14366 CrossRef CAS.
- Q. Tang, B. Li, D. Yang, P. Ming, C. Zhang and Y. Wang, Review of hydrogen crossover through the polymer electrolyte membrane, Int. J. Hydrogen Energy, 2021, 46, 22040–22061 CrossRef CAS.
- S. A. Grigoriev, K. Dzhus, D. G. Bessarabov and P. Millet, Failure of PEM water electrolysis cells: case study involving anode dissolution and membrane thinning, Int. J. Hydrogen Energy, 2014, 39, 20440–20446 CrossRef CAS.
- S. Kundu, M. Fowler, L. Simon and S. Grot, Morphological features (defects) in fuel cell membrane electrode assemblies, J. Power Sources, 2006, 157, 650–656 CrossRef CAS.
- A. Gago, S. Ansar, B. Saruhan, U. Schulz, P. Lettenmeier, N. A. Cañas, P. Gazdzicki, T. Morawietz, R. Hiesgen and J. Arnold, Protective coatings on stainless steel bipolar plates for proton exchange membrane (PEM) electrolysers, J. Power Sources, 2016, 307, 815–825 CrossRef CAS.
- C. Rozain, E. Mayousse, N. Guillet and P. Millet, Influence of iridium oxide loadings on the performance of PEM water electrolysis cells: part II–advanced oxygen electrodes, Appl. Catal. B Environ., 2016, 182, 123–131 CrossRef CAS.
- M. Debe, S. Hendricks, G. Vernstrom, M. Meyers, M. Brostrom, M. Stephens, Q. Chan, J. Willey, M. Hamden and C. Mittelsteadt, Initial performance and durability of ultra-low loaded NSTF electrodes for PEM electrolyzers, J. Electrochem. Soc., 2012, 159, K165 CrossRef CAS.
- V. O. Mittal, H. R. Kunz and J. M. Fenton, Membrane degradation mechanisms in PEMFCs, J. Electrochem. Soc., 2007, 154, B652 CrossRef CAS.
- P. Ren, P. Pei, Y. Li, Z. Wu, D. Chen and S. Huang, Degradation mechanisms of proton exchange membrane fuel cell under typical automotive operating conditions, Prog. Energy Combust. Sci., 2020, 80, 100859 CrossRef.
- N. Zamel and X. Li, Effect of contaminants on polymer electrolyte membrane fuel cells, Prog. Energy Combust. Sci., 2011, 37, 292–329 CrossRef CAS.
- D. E. Curtin, R. D. Lousenberg, T. J. Henry, P. C. Tangeman and M. E. Tisack, Advanced materials for improved PEMFC performance and life, J. Power Sources, 2004, 131, 41–48 CrossRef CAS.
- L. Ghassemzadeh, K.-D. Kreuer, J. Maier and K. Müller, Chemical degradation of Nafion membranes under mimic fuel cell conditions as investigated by solid-state NMR spectroscopy, J. Phys. Chem. C, 2010, 114, 14635–14645 CrossRef CAS.
- X. Cheng, Z. Shi, N. Glass, L. Zhang, J. Zhang, D. Song, Z.-S. Liu, H. Wang and J. Shen, A review of PEM hydrogen fuel cell contamination: impacts, mechanisms, and mitigation, J. Power Sources, 2007, 165, 739–756 CrossRef CAS.
- M. N. I. Salehmin, T. Husaini, J. Goh and A. B. Sulong, High-pressure PEM water electrolyser: a review on challenges and mitigation strategies towards green and low-cost hydrogen production, Energy Convers. Manage., 2022, 268, 115985 CrossRef CAS.
- Q. Feng, G. Liu, B. Wei, Z. Zhang, H. Li and H. Wang, A review of proton exchange membrane water electrolysis on degradation mechanisms and mitigation strategies, J. Power Sources, 2017, 366, 33–55 CrossRef CAS.
- A. K. Taylor, C. Smith and K. C. Neyerlin, Mitigation and diagnosis of pin-hole formation in polymer electrolyte membrane fuel cells, J. Power Sources, 2023, 571, 232971 CrossRef CAS.
- W.-F. Chen, J.-S. Wu and P.-L. Kuo, Poly (oxyalkylene) diamine-functionalized carbon nanotube/perfluorosulfonated polymer composites: synthesis, water state, and conductivity, Chem. Mater., 2008, 20, 5756–5767 CrossRef CAS.
- R. Kannan, B. A. Kakade and V. K. Pillai, Polymer electrolyte fuel cells using Nafion-based composite membranes with functionalized carbon nanotubes, Angew. Chem., Int. Ed., 2008, 47, 2653–2656 CrossRef CAS PubMed.
- K. D. Kreuer, M. Schuster, B. Obliers, O. Diat, U. Traub, A. Fuchs, U. Klock, S. J. Paddison and J. Maier, Short-side-chain proton conducting perfluorosulfonic acid ionomers: Why they perform better in PEM fuel cells, J. Power Sources, 2008, 178, 499–509 CrossRef CAS.
- J. Mao, Z. Li, J. Xuan, X. Du, M. Ni and L. Xing, A review of control strategies for proton exchange membrane (PEM) fuel cells and water electrolyser: from automation to autonomy, Energy AI, 2024, 100406 CrossRef.
- T. Lochner, R. M. Kluge, J. Fichtner, H. A. El-Sayed, B. Garlyyev and A. S. Bandarenka, Temperature effects in polymer electrolyte membrane fuel cells, ChemElectroChem, 2020, 7, 3545–3568 CrossRef CAS.
- N. T. Truc, S. Ito and K. Fushinobu, Numerical and experimental investigation on the reactant gas crossover in a PEM fuel cell, Int. J. Heat Mass Transfer, 2018, 127, 447–456 CAS.
- S. Bin, Z. Chen, Y. Zhu, Y. Zhang, Y. Xia, S. Gong, F. Zhang, L. Shi, X. Duan and Z. Sun, High-pressure proton exchange membrane water electrolysis: current status and challenges in hydrogen production, Int. J. Hydrogen Energy, 2024, 67, 390–405 CrossRef CAS.
- K. Kabouchi and M. K. Ettouhami, CFD Modelling and Simulation of the Thermal Management in a Polymer Electrolyte Membrane (PEM) Fuel Cell Stack, J. Adv. Res. Numer. Heat Transf., 2024, 27, 1–13 Search PubMed.
- P. Sharma, M. Cirrincione, A. Mohammadi, G. Cirrincione and R. R. Kumar, An overview of artificial intelligence-based techniques for PEMFC system diagnosis, IEEE Access, 2024, 12, 165708–165735 Search PubMed.
- M. J. Izadi, P. Hassani, M. Raeesi and P. Ahmadi, A novel WaveNet-GRU deep learning model for PEM fuel cells degradation prediction based on transfer learning, Energy, 2024, 293, 130602 Search PubMed.
- N. Norazahar, F. Khan, N. Rahmani and A. Ahmad, Degradation modelling and reliability analysis of PEM electrolyzer, Int. J. Hydrogen Energy, 2024, 50, 842–856 CAS.
- E. Savran, M. BÜYÜK and F. Karpat, Yakıt Hücreli Elektrikli Araçların Zorlukları ve Son Gelişmeleri üzerine Derleme, Osmaniye Korkut Ata Üniversitesi, J. Indian Inst. Sci., 2024, 7, 424–439 Search PubMed.
- A. L. Dicks and D. A. Rand, Fuel Cell Systems Explained, John Wiley & Sons, 2018 Search PubMed.
- A. Alaswad, A. Omran, J. R. Sodre, T. Wilberforce, G. Pignatelli, M. Dassisti, A. Baroutaji and A. G. Olabi, Technical and commercial challenges of proton-exchange membrane (PEM) fuel cells, Energies, 2020, 14, 144 Search PubMed.
- S. Dirkes, J. Leidig, P. Fisch and S. Pischinger, Prescriptive lifetime management for PEM fuel cell systems in transportation applications, part II: on-board operando feature extraction, condition assessment and lifetime prediction, Energy Convers. Manage., 2023, 283, 116943 Search PubMed.
- M. A. Hickner, H. Ghassemi, Y. S. Kim, B. R. Einsla and J. E. McGrath, Alternative polymer systems for proton exchange membranes (PEMs), Chem. Rev., 2004, 104, 4587–4612 CAS.
| This journal is © The Royal Society of Chemistry 2025 |