 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Optimization strategies for flexible aqueous rechargeable sodium-ion batteries (ARSIBs)
Hehe Ren
,
Xinzhan Du
,
Jing Liang
* and
Wei Wu
 *
*
Laboratory of Printable Functional Materials and Printed Electronics, School of Physics and Technology, Wuhan University, Wuhan 430072, PR China. E-mail: jingliang@whu.edu.cn; weiwu@whu.edu.cn; Fax: +86-27-68778491; Tel: +86-27-68778491
First published on 19th March 2025
Abstract
Flexible aqueous rechargeable sodium-ion batteries (ARSIBs) have emerged as promising energy storage systems for flexible and wearable electronics due to their safety, eco-friendliness, flexibility, and low cost, especially for large-scale and high-throughput storage needs. However, the design of flexible ARSIBs with high performance and excellent mechanical performance still faces challenges including the structural stability of electrodes, narrow electrochemical stability window of electrolytes, various side reactions, and structural design. It is urgent to design and develop ARSIBs with superior electrochemical performance, excellent mechanical performance and broader application scenarios. While there have been numerous reviews on sodium-ion batteries, there is a scarcity of exclusive reviews focusing on flexible ARSIBs. This review systematically introduces the advancements in electrode materials, electrolytes, and their optimization strategies for flexible ARSIBs. In addition, the recent advancements of batteries are discussed in terms of design strategies, multifunctional applications, and damage resistance. To manufacture flexible ARSIBs with coordinated high energy density and multi-functionality, not only the active materials and electrolytes should be optimized, but also the structural design should be improved. This review aims to evaluate the challenges and future prospects, and offer valuable references for designing advanced and high-performance flexible ARSIBs.
1. Introduction
In view of the success of flexible and wearable electronics, urgently developing an energy storage system with safety and flexibility has become a top priority. Since their commercialization in 1991, lithium-ion batteries (LIBs) have been widely used in portable electronics (such as watches, mobile phones, cameras, and computers), as well as automotive applications.1–5 Although conventional LIBs offer high energy density, their rigid nature, safety issues, limited lithium resources, and high costs remain unavoidable, hindering their further development in the fields of wearable electronics and foldable flexible electronics. Due to the advantages of eco-friendliness, flexibility and abundant sodium resources, flexible aqueous rechargeable sodium-ion batteries (ARSIBs) have greater market competitiveness in the development of portable flexible electronics.6–11During the charging process of flexible ARSIBs, Na+ migrates from the cathode to the anode via the electrolyte, while electrons flow from the cathode to the anode through the external circuit, often accompanied by redox reactions (Fig. 1). Therefore, the development of active materials and electrolytes has received increasing attention in recent years. However, unlike non-aqueous systems, aqueous batteries encounter special challenges, including the limited electrochemical stability window (ESW) of water (1.23 V) and irreversible side reactions involving the hydrogen evolution reaction (HER), oxygen evolution reaction (OER), and proton co-insertion. The above problems not only lead to low coulomb efficiency (CE), poor cycling performance, and low energy density, but also cause battery bloating/expansion. Therefore, designing and preparing suitable active materials and electrolytes are essential for high-performance flexible ARSIBs.
The Na/S battery with metallic sodium as anode and elemental sulfur as cathode is the earliest sodium battery in the world.12 Subsequently, people began to explore the electrochemical behavior of Na+ in aqueous electrolytes, as well as the structural design of flexible aqueous batteries to obtain safe and environmentally friendly flexible ARSIBs.13–17 Fig. 2 shows the evolution of flexible ARSIBs from 2017 to 2024.18–24 The electrochemical performance and applications of flexible ARSIBs including fiber-based, paper-based, metal current collector-based, and self-supporting ARSIBs were further explored.25–27 The above studies have demonstrated the potential of flexible ARSIBs as promising energy storage systems in wearable and implantable electronics fields.
 | ||
| Fig. 2 The major development of flexible ARSIBs with different structural designs in recent years.18–24 | ||
This review offers an overview of the most recent research progress in flexible ARSIBs, including battery constituent components, structural design strategies, and practical applications. Firstly, we introduce the developments of active materials in ARSIBs, and summarize the optimization and improvement strategies, aiming at enhancing the electrochemical performance of active materials. Secondly, we systematically discuss diluted/concentrated electrolytes, low freezing point electrolytes, and hydrogel electrolytes in ARSIBs. Our findings suggest that utilizing optimized electrolytes could potentially alleviate the limitations of certain electrode materials, thereby enhancing their electrochemical performance. And then, according to the structural design, we elucidate advanced configurations for flexible ARSIBs, such as fiber-based, paper-based, metal current collector-based, and self-supporting ARSIBs. Then the adaptability of flexible ARSIBs in practical applications and their stability under extreme conditions are discussed. Finally, we briefly discuss the future prospects and challenges for flexible ARSIBs. To manufacture flexible ARSIBs with coordinated energy density and functionality, it is crucial to optimize active materials and electrolytes, and improve structural design strategies. The purpose of this review is not only to summarize the development and applications of flexible ARSIBs, but also to provide comprehensive information on flexible ARSIBs.
2. Cathode materials and optimization strategies
Compared with conventional LIBs, ARSIBs hold great potential as next-generation energy storage devices due to their abundant sodium resources and aqueous electrolytes, which meet the requirements of safety, low cost, and environmental friendliness. Currently, the active materials used for the cathodes of ARSIBs primarily include Mn-based oxides, polyanionic compounds, Prussian blue analogues (PBAs), metal organic frameworks (MOFs), and organic polymer materials. Fig. 3 provides a performance comparison of these cathode materials, including energy density, cycle performance, conductivity, structural stability, cost effectiveness, and environmental friendliness. In the following sections, we will specifically discuss the performance of cathode materials and optimization strategies, providing valuable insights for the development of high-performance ARSIBs.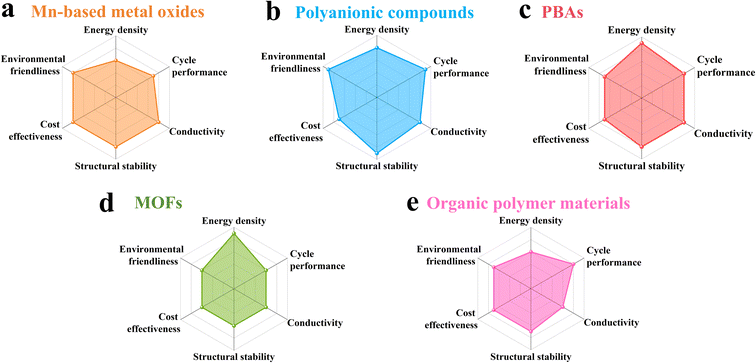 | ||
| Fig. 3 Performance comparison of (a) Mn-based oxides, (b) polyanionic compounds, (c) PBAs, (d) MOFs, and (e) organic polymer materials. | ||
2.1. Cathode materials
MnO2 exhibits a representative pseudocapacitance behavior in the neutral electrolyte.31 Moreover, the insertion of Na+ into MnO2 will induce an irreversible phase, which limits the wide applications of MnO2 in ARSIBs.32 In recent years, NaxMnO2 compounds have been considered promising cathode materials in ARSIBs, which have recently attracted major attention. A variety of NaxMnO2 sodium compounds were synthesized with different sodium to manganese ratios, such as Na0.27MnO2, Na0.40MnO2, and Na0.44MnO2. It has been demonstrated that Na0.44MnO2 undergoes a biphasic reaction involving Na+ intercalation, different from the capacitive behavior of MnO2 in aqueous electrolytes (Fig. 4a).33 After charging/discharging for 1000 cycles, the Na0.44MnO2 cathode still exhibits excellent electrochemical performance, and the capacity of Na0.44MnO2 can reach 35 mA h g−1 (0.5 A g−1). Subsequently, a sodium rich disordered birnessite Na0.27MnO2 exhibits excellent discharge capacity (138 mA h g−1), high energy efficiency, and appealing cycle performance, as well as nearly 100% CE after 5000 cycles (Fig. 4b).34 In summary, various NaxMnO2 compounds are developed as potential cathodes in ARSIBs, and their crystal structures and properties depend on the ratio of sodium. Nevertheless, these materials present significant volume shrinkage/expansion throughout the charging/discharging process, resulting in structural collapse and rapid capacity reduction.
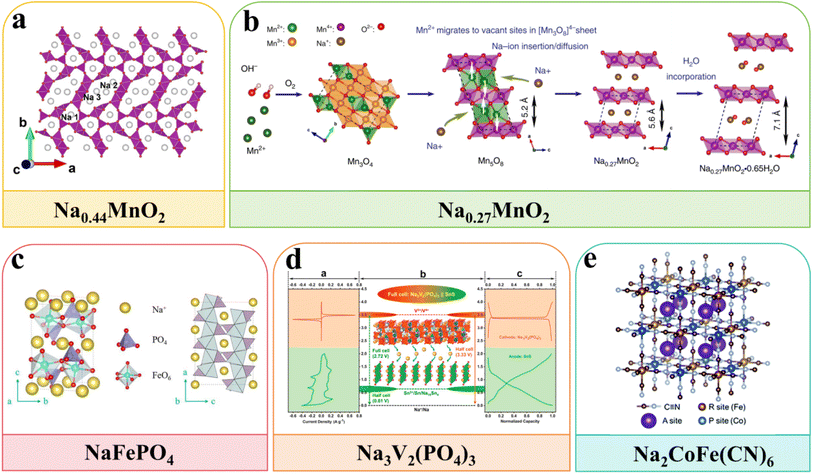 | ||
| Fig. 4 Schematic illustration of the crystal structure of (a) Na0.44MnO2,33 reproduced from ref. 33 with permission from Elsevier, copyright 2019. (b) Na0.27MnO2,34 reproduced from ref. 34 with permission from Springer Nature, copyright 2019. (c) NaFePO4,36 reproduced from ref. 36 with permission from the American Chemical Society, copyright 2020. (d) Na3V2(PO)4,37 reproduced from ref. 37 with permission from Elsevier, copyright 2019 and (e) Na2CoFe(CN)6.46 | ||
Olivine-type LiFePO4 is widely used in LIBs due to its stable structure and appealing energy density. The sodium-rich polyanionic compound NaFePO4 is extensively investigated as an active material for ARSIBs, presenting a similar structure to LiFePO4. As shown in Fig. 4c, the open framework structure of NaFePO4 is formed by the sharing of a side and corners between the PO43− tetrahedron and the FeO6 octahedron.36 NaFePO4 provides high theoretical capacity (154 mA h g−1) and a wide ESW of 2.8 V, due to the existence of the Fe3+/Fe2+ redox couple. As shown in Fig. 4d, the framework structure of NVP is made up of sharing corners between three PO4 tetrahedra and a VO6 octahedron, which creates a large interstitial space and a three-dimensional (3D) network of interconnected channels to facilitate the insertion/extraction behaviors of Na+. The NVP presents high theoretical capacity and appealing stability, due to the multivalent redox reaction of V2+/V5+ redox couples and the strong V–P bonds. The Na3V2(PO4)3/C (NVP/C) with 3D framework structure is prepared by electrospinning technology, which presents phenomenal discharge capacity (101 mA h g−1), outstanding electrochemical performance and great stability.37 However, polyanionic compounds still face the following challenges: (1) the inherent low conductivity, (2) the instability of electrode materials in aqueous electrolytes. These problems lead to poor cycle performance and limit the practical applications of polyanionic compounds.
Prussian blue materials containing only Fe metal ions undergo single-point redox reactions.42 In recent years, the stability of the PBA structure has been improved by doping other transition metal ions. In particular, NiFe,43 CuFe,44 FeFe,45 CoFe,46 and MnFe47 can perform double-site redox reactions, which shows excellent electrochemical performance. The crystal structure of PBAs influences their redox potential, and the different positions of Na+ in various crystal structures leads to varying electron polarizations. For example, Na2CoFe(CN)6 (CoHCF) serves as a promising cathode for ARSIBs, which was prepared using a citric acid-assisted co-precipitation method.46 The addition of Co2+ is conducive to control crystallization, thus enhancing the structural stability of CoHCF throughout the cycle process (Fig. 4e). After charging/discharging 100 times at 2C, the discharge capacity of CoHCF still remains at 100% of the initial capacity. However, there are limited reports regarding PBAs as cathode materials for ARSIBs, due to the inherent instability of PBAs in aqueous electrolytes.
As shown in Fig. 5a, the Sn/SnO@C nano-composites are prepared by the reduction of Sn-MOF with H2, in which SnO nanorods and Sn nanospheres are embedded in the hexagonal carbon framework.49 As the anode material for SIBs, Sn/SnO@C exhibits a high capacity of 130.1 mA h g−1 (2000 mA g−1), excellent rate performance, and appealing cyclability (Fig. 5b). In Fig. 5c, the nitrogen-carbon coated FeSe2 (FeSe2@NC) with spindle nanostructure is fabricated by polymerization, carbonization and selenization of MIL-BBA.50 The nitrogen-containing carbon layer effectively improves electronic conductivity, provides more active sites, and alleviates volume expansion caused by the Na+ diffusion process. FeSe2@NC exhibits excellent cycling stability with the capacity of 269.8 mA h g−1 after charging/discharging 500 times. As shown in Fig. 5d, the sulfur-doped porous carbon (S/C) is fabricated by carbonization and vulcanization of MOF-5.51 The ordered framework carbon shows a large specific surface area and provides more active sites, making it an excellent host for sulfur. The unique structure endows the S/C anode with outstanding cycle stability, and the discharge capacity is 268.8 mA h g−1 after 100 cycles at 0.1 A g−1. The active materials prepared by carbonization, selenization and vulcanization of MOFs possess phenomenal Na+ storage capacities. However, the chemical stability of MOFs in aqueous electrolytes is limited, and there are few literature reports on MOFs as electrode materials for ARSIBs.
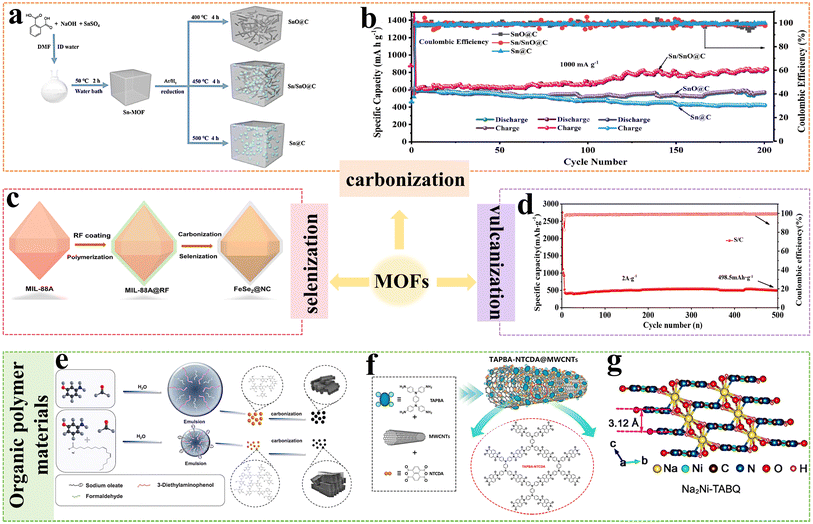 | ||
| Fig. 5 (a) Schematic illustration of the fabrication of the samples, (b) cycle performance.49 Reproduced from ref. 49 with permission from Elsevier, copyright 2020. (c) The synthetic process of FeSe2@NC.50 Reproduced from ref. 50 with permission from Elsevier, copyright 2023. (d) Cycle performance of S/C.51 Reproduced from ref. 51 with permission from Elsevier, copyright 2024. (e) Schematic diagram of the PFMS preparation process.52 Reproduced from ref. 52 with permission from Elsevier, copyright 2024. (f) Synthetic process of TAPBA-NTCDA@MWCNTs.53 Reproduced from ref. 53 with permission from Elsevier, copyright 2023. (g) Crystal structures of Na2Ni–TABQ.54 Reproduced from ref. 54 with permission from John Wiley and Sons, copyright 2020. | ||
The HC microspheres are obtained by the carbonization of phenol–formaldehyde resin microspheres (PFMS); the graphitization of the materials increases the layer spacing, and facilitates electron and ion transfer (Fig. 5e). The HC microspheres exhibit a high capacity of 96.9 mA h g−1 even at a high current density of 5.0 A g−1.52 As shown in Fig. 5f, the conjugated microporous polyimide (TAPBA-NTCDA) is grown on multi-walled carbon nanotubes (MWCNTs) to form TAPBA-NTCDA@MWCNTs composites.53 The combination of MWCNTs can enhance the conductivity, increase the active site, and improve the stability of materials. As a cathode material for SIBs, the TAPBA-NTCDA@MWCNTs composites provide a high capacity of 98.4 mA h g−1 (0.1 A g−1) and outstanding cycle performance, with a capacity of 51.4 mA h g−1 being maintained after over 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles (2 A g−1).
000 cycles (2 A g−1).
The preparation of organometallic polymers can also effectively solve the problems of low conductivity and poor structural stability of organic polymer materials. Ni-coordinated tetramino-benzoquinone (Ni–TABQ) organometallic polymers are synthesized by d–π hybridization.54 In Fig. 5g, the Ni–N bonds and hydrogen bonds (HBs) in the polymer endow it with a strong two-dimensional (2D) layered structure, which can facilitate electron conduction and Na+ diffusion. Moreover, the Ni–TABQ shows outstanding rate performance; the discharge capacity is 469.5 mA h g−1 at 100 mA g−1 and 345.4 mA h g−1 at 8 A g−1. Although polymerization is an effective way to prevent the dissolution of organic electrode materials, excessive polymerization will limit the exposure of active sites, thus reducing capacity utilization. Therefore, the control of polymerization to fully release the capacity of materials remains a significant challenge.
2.2. Optimization strategies
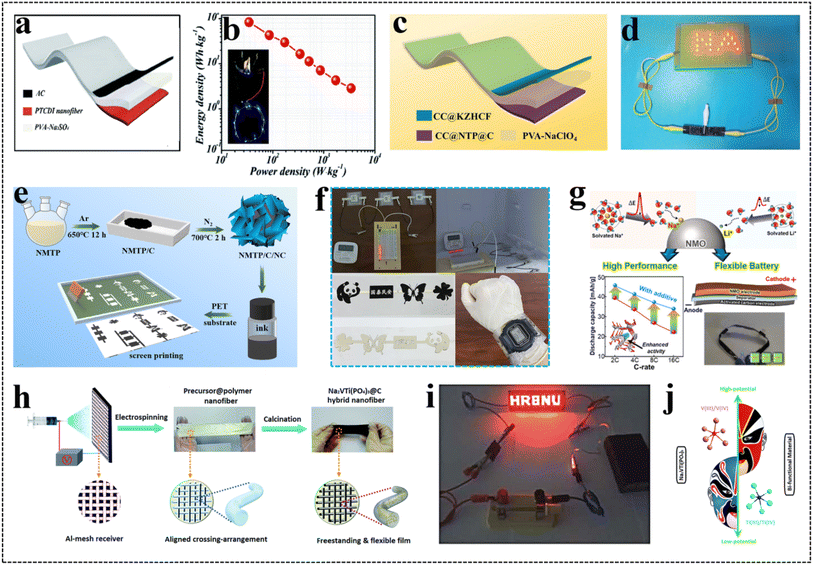
In general, since the radius of Na+ is larger than that of Li+, the stable active materials with microporous structure need to be selected in ARSIBs, which highlights the significance of selecting electrode materials for achieving high-performance ARSIBs. To obtain the high-performance active materials with appealing stability, further optimization is necessary. Design and optimization strategies, such as morphological engineering, defect engineering, and composite modification, can maximize the performance of active materials. The inherent advantages of materials with optimization strategies can be combined to develop active materials with high energy density and excellent cycling performance, making them very promising candidates for energy storage systems.As promising 1D nanostructured materials, the special structure of nanowire materials provides abundant active sites for Na+ storage and transfer. Dense nanowire electrodes possess larger surface areas, offering more effective transfer pathways for Na+ and electrons, thereby maximizing the application of active materials and enhancing electrochemical performance. For example, the carbon-coated Na3V2(PO4)3 (NVP@C) is fabricated using the electrospinning technique, which is uniformly arranged and densely packed intersecting nanowires (Fig. 6a). In 19 m (mol kg−1) NaClO4–NaOTF electrolyte, the NVP@C cathode provides a wide ESW (3.45 V) and excellent discharge capacity (112.2 mA h g−1), closely approaching its theoretical specific capacity. The NVP//NVP symmetric ARSIBs (Fig. 6b) present excellent energy density (70 W h kg−1) and appealing cycle life, and even after charging/discharging 500 times, the capacity of the batteries can reach 75.6% of their initial capacity, and the CE is 100% (Fig. 6c).55
 | ||
| Fig. 6 (a) Morphology of NVP@C nanowires, (b) schematic illustration and (c) cycle performance of the full battery.55 Reproduced from ref. 55 with permission from John Wiley and Sons, copyright 2021. (d) SEM images of NVP nano-flowers, (e) the symmetric battery NVP‖NaClO4‖NVP, (f) schematic illustration of the batteries at −40 °C.56 Reproduced from ref. 56 with permission from Elsevier, copyright 2021. (g) Schematic diagram of the aqueous full-cell, (h) morphological characterization of N24 samples, (i) rate capability and (j) cycle performance of NaVOPO4 materials.57 Reproduced from ref. 57 with permission from the American Chemical Society, copyright 2022. (k) The structure and (l) cycle performance of Na2FePO4F microparticles.58 Reproduced from ref. 58 with permission from John Wiley and Sons, copyright 2018. | ||
The nanosheet and nano-flower materials are promising 2D nanostructured materials, which provide numerous advantages of increased surface area and affluent Na+ sites. In Fig. 6d, NVP nano-flowers with large surface area, prepared by high temperature annealing, consist of many ultrathin nanosheets. The symmetric aqueous NVP‖NaClO4‖NVP micro-batteries exhibit a 2.3 V wide ESW, excellent volume capacity, and outstanding energy density (77 mW h cm−3), surpassing most reported aqueous sodium-ion micro-batteries (Fig. 6e). Notably, even at −40 °C, the CE of this symmetric micro-battery remains as high as 99% (Fig. 6f).56 As shown in Fig. 6g, the NaVOPO4 materials with various morphologies were fabricated using the hydrothermal approach by adjusting the V/Na ratios in the raw materials. These morphologies encompass microparticles (N6) at a size of 50 μm and ellipsoidal microparticles (N12) at a size of 30 μm × 15 μm, and flower-like micro-sized materials (N24) with 20 μm size.57 Interestingly, the internally ordered structure of the flower-like N24 provides abundant Na+ sites, providing buffer space for the volume expansion of the materials throughout the transfer process of Na+ (Fig. 6h). In Fig. 6i, the discharge capacities of N6 and N12 at 0.2C are 13.7 and 46.8 mA g−1, respectively, while N24 shows the most excellent discharge capacity (67.1 mA h g−1). Furthermore, the capacity retention of N24 still remains at 83.8% after charging/discharging 200 times (Fig. 6j), demonstrating the phenomenal rate performance and appealing cycle performance. The nano-flower materials composed of ultrathin nanosheets offer a larger specific surface area, while their unique nanostructure shortens the diffusion distance of Na+, facilitating efficient electron transfer. Consequently, this enables the active materials to engage in electrochemical reactions with optimal efficiency, showing outstanding electrochemical performance.
The 3D framework structure and 3D ion transport channels of the 3D nanostructured materials are crucial in offering more Na+ sites, which facilitated the insertion/extraction behaviors of Na+, and enhanced the efficiency of electrochemical reactions. The porous nanomaterials with 3D framework structure offer rapid transfer pathways for Na+ and electrons, benefiting the diffusion of electrolyte, thereby enhancing interface reaction effects. As shown in Fig. 6k, Na2FePO4F microparticles were synthesized using the solvothermal method, exhibiting a morphology characteristic of stacked block-like structures with rough porous surfaces. The porous structure increases the interface contact area and alleviates the material volume expansion during the charging/discharging process. The 3D ion transport channel is beneficial to the insertion/extraction process of Na+, which further improves its electrochemical activity and cycle stability. In 17 m (mol kg−1) NaClO4 electrolyte, the capacity of Na2FePO4F is 84 mA h g−1 (1 mA cm−2), and the capacity retention and CE are up to 100% after 100 cycles (Fig. 6l).58
The rational design of the nanostructure endows materials with unique surface morphologies, which is conducive to improving the structural stability and obtaining high-performance active materials. The development of multifunctional nanomaterials has made significant contributions to the widespread applications of high-performance ARSIBs in energy storage systems.
In recent years, tunnel-structured Na0.4MnO2 (NMO) materials have been presented as cathodes with promising electrochemical performance. Subsequently, Ca2+ was doped to develop a novel cathode material, Ca0.07Na0.26MnO2 (CNMO), for ARSIBs,62 and CNMO showed enhanced discharge capacity and appealing cycle performance compared to NMO. As shown in Fig. 7a, the doped Ca2+ at the Na(1) sites can reduce the manganese ions at the adjacent manganese sites, and the doped Ca2+ at the Na(2) and Na(3) sites in the manganese oxide skeleton can form vacancy defects, thus improving the diffusion kinetics of Na+. The CNMO displays higher capacity enhancement compared with NMO, and the capacity of CNMO increased 43% at 50C. In addition, active carbon//CNMO (AC//CNMO) full batteries deliver phenomenal rate performance and superior cycle performance, and the capacity retention can reach 98.8% even after charging/discharging 1000 times at 50C (Fig. 7b). The above suggest that the doping of Ca2+ leads to vacancy defects, increasing active sites and facilitating the transfer process of Na+, which improves the electrochemical performance.
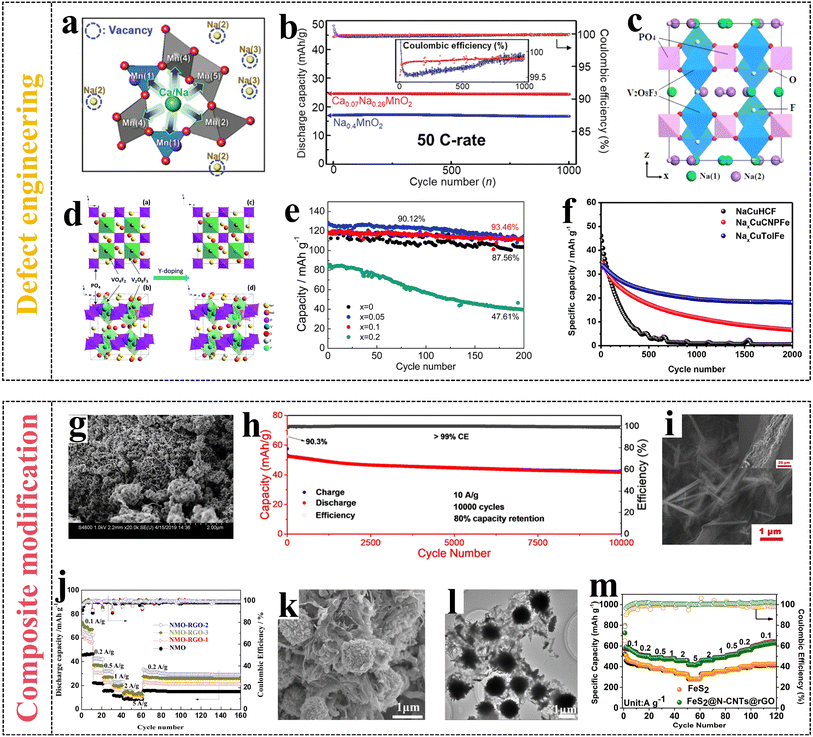 | ||
| Fig. 7 (a) CNMO with local structure around Na(1), (b) cycle performance of CNMO.62 Reproduced from ref. 62 with permission from John Wiley and Sons, copyright 2020. (c) Crystal structure of NVPF.74 Reproduced from ref. 74 with permission from the American Chemical Society, copyright 2014. (d) Schematic illustration of the NVPF crystal structure before and after Y-doping, (e) cycle performance of NVYPF/C.73 (f) Capacity retention of NaCuHCF, NaxCuCNPFe and NaxCuTolFe.75 Reproduced from ref. 75 with permission from Elsevier, copyright 2022. (g) SEM image of NiHCF@CNTs, (h) cycle performance.77 Reproduced from ref. 77 with permission from the American Chemical Society, copyright 2020. (i) Morphology of NM/rGO, (j) cycle performance.78 Reproduced from ref. 78 with permission from Elsevier, copyright 2018. (k) SEM images and (l) TEM images of the FeS2@NCNTs@rGO, (m) rate capability.79 Reproduced from ref. 79 with permission from Elsevier, copyright 2021. | ||
NVP has the advantages of high operating voltage, 3D framework structure, and appealing stability, making it a potential cathode material for ARSIBs.63–66 However, there are challenges in improving the rate performance and cycling stability, which need to be solved for wide application compatibility. Introducing transition metal ions (Cr3+, Ti3+, Mg2+, Zr3+),67–70 nonmetallic ions such as F− (ref. 71) and rare earths ions (Gd3+ and Y3+)72,73 into NVP materials can increase defect sites, leading to enhanced capacity, appealing cycle performance, and excellent energy density. The doping of F− can not only introduce extrinsic defects, but also significantly increase the working voltage, further enhancing structural stability, because of its strong inductive effect and high electronegativity. Na3V2(PO4)2F3 (NVPF) presents outstanding reversible theoretical capacity (128 mA h g−1) and a wide ESW (3.8 V), making it a suitable cathode. As shown in Fig. 7c, the crystal structure of NVPF is made up of [V2O8F3] octahedra and [PO4] tetrahedra, where two [V2O8F3] octahedra are connected by a F along the c-axis. Stable phosphate–metal bonds can develop on cathodes in fluorophosphate and phosphate materials, lowering the possibility of oxygen release.74 Due to the existence of PO43− polyanions, the material volume expansion can be considerably reduced throughout the charging/discharging process of Na+.
The Na3V1.9Y0.1(PO4)3F3/C (NVYPF/C) materials are prepared by adding an appropriate amount of rare earth element yttrium (Y) to NVPF, where some V sites are replaced by Y, which can increase the active sites and enhance the performance of the materials (Fig. 7d). The V3+ sites are replaced by Y3+ with a larger radius in the NVPF structure, leading to vacancy defects, achieving rapid Na+ transfer, and both Y–O and V–O bonds can improve the conductivity and stability of materials. Furthermore, the inert Y3+ substitutes the V3+ sites in the NVPF, which can buffer material volume expansion throughout the transfer process of Na+, thus potentially improving the structural stability. As shown in Fig. 7e, NVYPF/C exhibits excellent discharge capacity (121.3 mA h g−1) and appealing cycle performance, and the capacity still remains at 93.5% of the initial capacity after charging/discharging 200 times.73
The organic ligand CN6− in NaCu[Fe(CN)6] (NaCuHCF) is replaced by other organic ligands, which change the framework structure of PBAs and form internal vacancies, thereby enhancing the cycle performance of materials. The capacity retentions of NaxCu[Fe(CN)5(C6H4N2)] (NaxCuCNPFe) and NaxCu[Fe(CN)5(CH3C6H4NH2)] (NaxCuTolFe) cathodes are all 50% in 1 M Na2SO4 electrolytes, far higher than that of NaCuHCF (0%) after charging/discharging 2000 times (Fig. 7f).75 These results demonstrate that ligand substitution strategies can effectively optimize the internal structure of materials to enhance structural stability, and increase the active sites for the transfer of Na+. Simultaneously, both doping and substitution methods give rise to additional ions or ligands occupying a small amount of sodium sites. Excessive occupancy would have a counterproductive effect, leading to reduced capacity and decreased cycling performance.
The porous structure and excellent conductivity of carbon materials play a crucial role in shortening ion transport pathways, reducing the interface resistance and polarization, and making insertion/extraction of Na+ convenient. As a substrate or surface coating of active materials, the addition of carbon materials is conducive to prevent material aggregation, ensuring better contact between materials and electrolytes. The commonly used carbon materials are carbon nanofibers (CNFs), CNTs, and rGO. CNTs with unique tubular structure exhibit great conductivity, expansive surface area, and appealing mechanical performance. Nevertheless, using CNTs alone as electrode materials for SIBs makes it difficult to meet practical applications. Therefore, combining CNTs with other active materials is indispensable to enhancing electrochemical performance. Nickel hexacyanoferrate with CNT (NiHCF@CNTs) nano-materials are synthesized using a typical coprecipitation method.77 In Fig. 7g, CNTs are intertwined with NiHCF nanoparticles to form a 3D interconnection network, which helps to enhance the interaction kinetics and conductivity of electrode materials. After 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles, the NiHCF@CNTs cathode shows high energy density and appealing cycle performance, and capacity retention is still at 80% at 10 A g−1 (Fig. 7h).
000 cycles, the NiHCF@CNTs cathode shows high energy density and appealing cycle performance, and capacity retention is still at 80% at 10 A g−1 (Fig. 7h).
The composite material Na4Mn9O18/reduced graphene oxide (NM/rGO) exhibits enhanced electrochemical performance, because of the excellent in-plane conductivity, extensive surface area, and high mechanical durability of graphene.78 Having NM/rGO films without current collectors, conductive additives and binders as the cathodes of ARSIBs streamlines the synthesis process and reduces the cost of the battery. As shown in Fig. 7i, the needle-like NM is densely fixed onto the wrinkled graphene sheet to form the NM/rGO composite material with 3D channels, which provides a fast electron conduction path, enhances the structural stability, and reduces mechanical stress caused by the transfer of Na+. As shown in Fig. 7j, the NM/rGO offers great rate performance and appealing cycle performance, and its specific capacity is about 83 mA h g−1, marking an approximately 78% increase compared to NM.
FeS2 was combined with N-doped CNTs (NCNTs) and rGO using the hydrothermal method to prepare FeS2@NCNTs@rGO composites with microsphere structure.79 The urchin-like FeS2 and conductive NCNTs within the layered rGO form a stable network structure (Fig. 7k and l), which helps alleviate the stress and strain caused by Na+ transfer. The unique structure of FeS2@NCNTs@rGO enables effective electron charge transfer and reduces the transfer pathway of Na+, significantly enhancing the conductivity and structural stability of the composite materials. The incorporation of NCNTs and rGO enlarges the specific surface area, buffers the volume expansion of composite materials throughout the cycling process and reduces the dissolution of the electrode materials caused by related strains, thereby leading to outstanding electrochemical performance. As shown in Fig. 7m, compared with FeS2, the FeS2@NCNTs@rGO composite materials exhibit excellent specific capacity, phenomenal rate capability, and appealing cycle performance, after charging/discharging 750 times at 2 A g−1. In the FeS2@NCNTs@rGO composite materials, the bent NCNTs form a cross-linked network structure, and FeS2 is wrapped together with NCNTs and rGO to form a 3D structure, which provides an additional electron pathway, accelerates the transfer of Na+, and effectively enhances the performance of active materials, thereby facilitating the attainment of high-performance SIBs.
Overall, the composite of two or more materials can synergize their respective advantages, significantly enhancing the conductivity and designing active materials with excellent energy density and phenomenal cycle performance. Nevertheless, the bonding mechanisms between multiple materials (physical or chemical bonding) require further investigation.
3. Anode materials
In recent years, many studies have shown that carbon materials, polysulfides, and poly-anionic compounds are the common anode materials for ARSIBs.80 Compared with the cathodes, the anodes with low potential and poor stability need extensive attention, and the further development of anodes is significant for enhancing the electrochemical performance of ARSIBs. The battery construction and reaction mechanism of SIBs are analogical to those of LIBs, and Li can serve as the anodes for LIBs, but Na is unsuitable for SIBs. The reasons are as follows: (1) Na with a low melting point (97.7 °C) presents safety issues, (2) Na exhibits strong chemical reactivity with water-based electrolytes, (3) Na will form dendrites during the charging and discharging process, causing short circuits. It is an effective strategy to use non-metal anodes instead of Na anodes in ARSIBs, but the use of non-metal anodes will result in a decrease in energy density. According to previous reports, high-performance sodium-rich active materials, “water-in-salt” (WIS) electrolytes and the sodium compensation method can replenish the Na+ lost during the charging/discharging processes, effectively improving the energy density.81–83 In ARSIBs, Na undergoes intense reactions with the aqueous electrolyte, and there are problems of dissolution and structural collapse of some active materials in the aqueous electrolyte, hindering the development of anode materials. Therefore, it is essential to optimize active materials for enhancing the performance of ARSIBs.3.1. Carbon materials
Based on differences in microstructure and the degree of graphitization, carbon-based electrode materials can be classified as graphite, soft carbon, or HC. Compared with Li+, Na+ with a larger radius and mass are difficult to be quickly inserted/extracted in graphite electrodes.84,85 Both HC (such as biochar, metal organic framework-based carbon, and other porous AC materials) and soft carbon (such as CNTs) have been effectively used as anode materials for SIBs.86–89 HC is formed by highly disordered stacking of graphite-like crystallites and graphene-like sheets, and there is a strong force between the carbon layers, giving it a tight layered structure. HC has advantages of porous structure and high conductivity, which provide excellent performance. Nevertheless, the problems of low CE and poor cycling stability need to be resolved in the practical applications of carbon materials for ARSIBs.In ARSIBs, carbon materials are mainly used as substrates to composite with other active materials as anode materials, which can enhance the conductivity and increase Na+ active sites. As shown in Fig. 8a, the MWCNT is used as the core to fabricate MWCNT@polyimide core–shell nanowires with a polypyrrole coating (MWCNT@polyimide/polypyrrole) as the anode of ARSIBs.90 The existence of MWCNT would be beneficial for accelerating the Na+ transfer speed throughout the charging/discharging process, and making up for the seriously poor conductivity of polyimide. Moreover, the polypyrrole improves the conductivity of the material, prevents degradation of electrode material structure, and improves the cycling stability of the materials. After 100 charging/discharging cycles, the MWCNT@polyimide/polypyrrole nanowire presents a capacity retention of 77.8%, and the electrode material without expansion and structural damage shows appealing electrochemical performance (Fig. 8b).
 | ||
| Fig. 8 (a) The synthetic process of MWCNT@polyimide/polypyrrole, (b) cycle performance.90 Reproduced from ref. 90 with permission from Elsevier, copyright 2021. (c) Aqueous Na/S battery S@C//Na0.44MnO2.22 Reproduced from ref. 22 with permission from Elsevier, copyright 2022. (d) Schematic representation of the S@Co-IL//Na0.44MnO2 full battery, (e) cycle performance.91 Reproduced from ref. 91 with permission from Elsevier, copyright 2022. (f) Na+ storage mechanism, (g) rate capability, and (h) cycle performance of NTP@C.98 Reproduced from ref. 98 with permission from Elsevier, copyright 2023. | ||
3.2. Polysulfides
Polysulfides are at risk of dissolving in aqueous electrolytes, ultimately causing irrecoverable H2S gas, irreversible material structure damage and high interfacial resistance, eventually resulting in short cycle life of the battery. As the dissolution occurs at the electrode–electrolyte interface, the energy density and cycling stability of the batteries principally rely on the characteristics of electrolytes and active materials. Carbon materials with stable structure provide large surface area and abundant anchoring sites, making them suitable hosts for sulfides and effectively alleviating the issues of polysulfide dissolution. Tubular mesoporous nitrogen-doped carbon (MNC-600) materials exhibit high surface area and great conductivity, providing abundant anchoring sites and improving the redox kinetics. Sulfur is embedded in the MNC-600 to prepare S@MNC-600 (S@C) as the anode of ARSIBs (Fig. 8c). As a suitable host for S, NMC-600 improves the conductivity and structural stability of the S@C materials. S@C still maintains 78.2% of the initial capacity, and the CE is about 98.2% after charging/discharging 300 times. The high-performance ARSIBs are assembled with the S@C anode, Na0.44MnO2 cathode and 17 m NaClO4 electrolyte, and the energy density of S@C//Na0.44MnO2 can reach 110.6 W h kg−1.22Fig. 8d shows that 70% elemental sulfur, CoS2, and 1-butyl-3-methylimidazolium-o,o-bis(2-ethylhexyl)dithiophosphate ionic liquid (BMIm-DDTP-IL) are present in the anode (S@Co-IL) for aqueous rechargeable Na/S batteries.91 After charging/discharging 100 times, the S@Co-IL anode shows appealing cycle performance, and the capacity retention and CE remain at 98% and 100%, respectively. Moreover, the synergistic effect of BMIm-DDTP-IL and CoS2 can enhance conductivity and efficiently inhibit the structural collapse of polysulfides. As shown in Fig. 8e, the S@Co-IL//Na0.44MnO2 full battery exhibits outstanding discharge capacity of 778 mA h g−1 (based on the mass of S) at 1C and excellent cycle performance, with 91.8% retention of its initial capacity, and the CE is 100% after charging/discharging 400 times at 2C.
3.3. Polyanionic compounds
NASICON-type polyanionic compounds have garnered widespread attention owing to their high structural stability, large ion channels, and abundant active sites.92–95 Among them, NaTi2(PO4)3 (NTP), Na3Fe2(PO4)3 (NFP), Na3MnTi(PO4)3 (NMTP), and Na2VTi(PO4)3 (NVTP) Na-rich polyanion compounds are regarded as the most promising anode materials for high-performance ARSIBs, due to their structural advantages.The conductivity of materials can be enhanced and the electron transport path can be shortened by applying a carbon layer to the surface of NTP. The conductive carbon layers on the materials increase the Na+ attachment sites, facilitating insertion/extraction behaviors of Na+, and alleviating volume expansion throughout the charging/discharging process. The porous NTP micron materials coated with a nano-carbon layer (NTP@C) exhibit a unique single crystal structure, which promotes rapid transport of electrons and Na+ (Fig. 8f).98 Furthermore, the carbon layer enhances the conductivity of NTP and serves as a protective interface, shields the electrode material from dissolution, and enhances its stability. In three electrolytes (1 M Na2SO4 electrolyte, 1 M N2-purged Na2SO4 electrolyte, and 1 M O2-dissolved Na2SO4 electrolyte), the NTP@C anodes provide both excellent capacity and phenomenal CE (none: 87.3 mA h g−1, 81.4%; N2: 101.4 mA h g−1, 90.8%; O2: 79.2 mA h g−1, 73.6%) (Fig. 8g and h). It is well known that precious metals show excellent conductivity, and the carbon layer containing precious metals can also provide enhanced conductivity. The NTP@C/Ag materials are prepared as the anode materials for ARSIBs by adding Ag to the NTP carbon layers.99 Compared with NTP materials, NTP@C/Ag materials obviously show higher conductivity, better structural stability and more excellent electrochemical performance. Even after charging/discharging 400 times at 5C, the capacity retention of materials capacity is still at 82.7% (Fig. 9a). Therefore, coating a carbon layer on the active materials is an effective method to improve the conductivity and enhance the structural stability of materials.
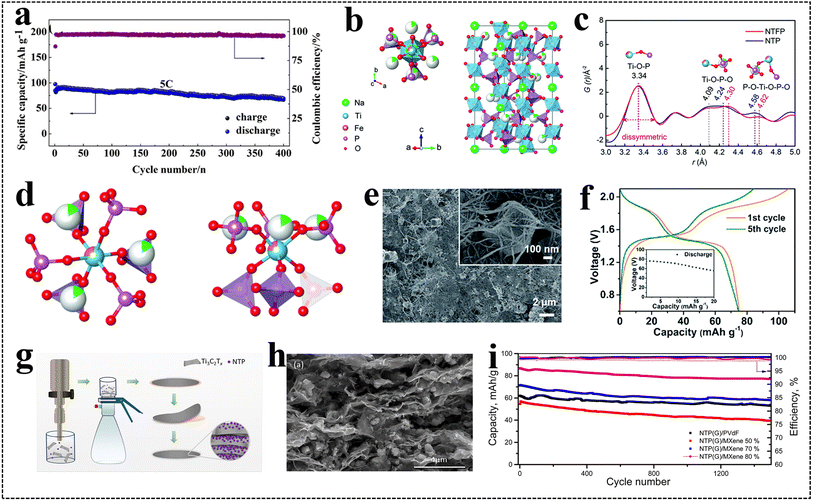 | ||
| Fig. 9 (a) Cycle performance of NTP@C/Ag.99 Reproduced from ref. 99 with permission from Elsevier, copyright 2018. (b) Schematic illustration of the crystal structure of NTFP, (c) representative PDF data of NTFP. (d) Ti/Fe-centered local structures of NTFP.100 (e) SEM images of NTP-MWCNT, (f) cycle performance of the NVPF-SWCNT//NTP-MWCNT full battery.102 (g) Preparation procedure, (h) SEM images, and (i) long-term cycling performance of NTP/MXene.103 Reproduced from ref. 103 with permission from Elsevier, copyright 2021. | ||
As is well known, the hydrogen evolution potential is −0.817 V (vs. Ag/AgCl). However, as the anode of ARSIBs, the Ti4+/Ti3+ redox potential in NTP is −0.807 V (vs. Ag/AgCl), and it faces a great risk of HER in aqueous electrolytes, which hinders the wide applications of NTP anodes in ARSIBs, so it is necessary to find an effective improvement strategy. Heteroatom doping can solve this problem, which can introduce vacancy defects to establish additional sodium storage sites, alleviate the electrostatic repulsion and stress among layers, help to overcome the migration resistance and diffusion obstacles. Na1.5Ti1.5Fe0.5(PO4)3 (NTFP) was prepared by doping Fe3+ into NTP, in which the combination of Fe3+/Fe2+ and Ti3+/Ti4+ redox pairs increased the working potential to −0.721 V, availably preventing overlap with the potential of water decomposition (Fig. 9b).100 The different bond lengths of Fe–O and Ti–O result in different bond angles of Ti–O–P and Fe–O–P, changing the symmetry of the O–P–O bonds and decreasing the average distance between O–P–O, resulting in a slight asymmetric feature in NTFP, according to the pair distribution function (PDF) analysis (Fig. 9c and d). Moreover, the NTFP anode presents high discharge capacity (122.1 mA h g−1), and appealing rate performance in 5 M NaClO4 electrolyte. Subsequently, Na1+xAlxTi2−x(PO4)3/C (x = 0, 0.05, 0.10, 0.20) were prepared by doping Al3+ on NTP, which establishes vacancy defects and introduces extra active sites.101 Although the doping of Al3+ has no evident impact on the structure of the materials, it accelerates the migration of Na+, improves reaction kinetics, and enhances the electrochemical performance. Heteroatom doping can introduce defects for active materials, increase the active sites of Na+, and enhance the structural stability, making it a promising anode for ARSIBs.
Combining two or more electrode materials to prepare composite materials can leverage the strengths of each material and enhance the electrochemical performance. The MWCNT with pore structure possess enhanced conductivity, high specific surface area, and appealing mechanical performance. As anode materials for ARSIBs, NTP-MWCNT composite materials are beneficial for enhancing conductivity, increasing specific surface area, and facilitating the transfer process of Na+, thereby improving the electrochemical performance of the composite materials (Fig. 9e).102 In Fig. 9f, the NVPF-SWCNT//NTP-MWCNT full batteries exhibit a discharge capacity of 75.2 mA h g−1, a high CE (99%), and an excellent energy density (150 W h kg−1). In addition, MXene presents excellent mechanical performance and high conductivity, and the existence of O, OH, and F enables MXene to form HBs with other active materials. As shown in Fig. 9g and h, the combination of NTP nanoparticles and MXenes forms the composite materials NTP/MXenes,103 where the introduction of external particles between MXene sheets effectively prevents material accumulation. As shown in Fig. 9i, the NTP/MXene composites deliver outstanding cycle performance and excellent capacity retention (88%) even after charging/discharging 1500 times (20C). The fabrication of composite materials can combine the advantages of various materials, enhance the structural stability, and increase the Na+ active sites, which enables the composite materials to provide high energy density and excellent cycle performance.
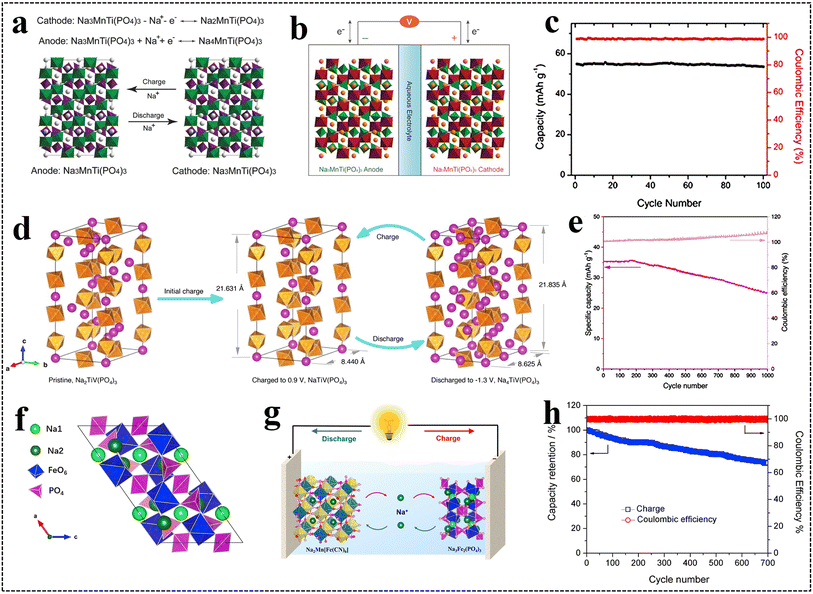 | ||
| Fig. 10 (a) Mechanism diagram and (b) schematic illustration of the symmetric ARSIBs, (c) cycle performance.108 Reproduced from ref. 108 with permission from John Wiley and Sons, copyright 2016. (d) Charge/discharge reaction mechanism of NVTP, (e) cycle performance of the symmetrical ARSIBs.95 Reproduced from ref. 95 with permission from John Wiley and Sons, copyright 2024. (f) The crystal structure of the NFP anode, (g) schematic illustration of ARSIBs, (h) cycle performance at 5C.109 Reproduced from ref. 109 with permission from Elsevier, copyright 2019. | ||
The NISICON-structured NVTP consists of VO6/TiO6 octahedra and PO4 tetrahedra to form a 3D framework. As shown in Fig. 10d, during the charging/discharging process, Na+ was extracted from NVTP to form NaVTi(PO4)3 intermediates through the V4+/V3+ redox couple, while Na+ transfers to another electrode to form Na4VTi(PO4)3 with the redox reactions with the Ti4+/Ti3+ redox couple. In addition, the symmetric ARSIBs exhibit excellent rate performance, high energy density of 55.9 W h kg−1, and appealing cycle performance (Fig. 10e). After charging/discharging 1000 times at 5 A g−1, the capacity retention of this battery is still at 60%, and the CE is closed to 100%.95
Currently, carbon materials, polysulfides, and polyanionic compounds are the most common anode materials for ARSIBs. Optimization strategies for these materials include carbon coating, heteroatom doping, and preparation of composite materials to alleviate material volume expansion, reduce charge diffusion distance, and increase conductivity, which can provide excellent cycling stability and rapid kinetics.
4. Recent development of electrolytes
The electrolyte is an important component of the battery, which is the intermediary for ion transfer between the anode and cathode and has a significant impact on the electrochemical performance of the battery. Outstanding electrolytes should possess excellent stability, superior conductivity, and a wide ESW. Different from traditional organic electrolytes, aqueous electrolytes exhibit high conductivity, safety, and eco-friendliness. Aqueous electrolytes can be divided into liquid electrolytes and hydrogel electrolytes according to the physical properties of the electrolyte; an aqueous electrolyte solution is often influenced by the inherent properties of electrolyte components (salts concentrations, solubility, and freezing point).4.1. Solvation behavior in electrolytes
The solvation behavior determines the composition, thickness, and structure of the solid electrolyte interphase (SEI), which indirectly affects the electrochemical performance of batteries. The solvation structure refers to a relatively stable sheath structure formed by the interactions among the cations, anions, solvent molecules and additives molecules. It has been reported that solvated ions typically possess two solvation layers, a tight first solvation layer and a relatively incompact second solvation layer.110 The strong electrostatic forces and coordination bonds between solvent molecules and cations lead to a balance of attraction and repulsion, forming the tight first solvation layer. The second solvation layer is slightly incompact, including solvent molecules influenced by remote dipole interactions, solvent molecules attracted by the cation, free solvent molecules, and free anions.111–113 During the charging/discharging process, cations undergo an insertion/extraction process within the active materials. Before cations can be inserted into the electrode materials through the SEI layer, desolvation should occur, including the removal of solvent molecules and anions. The desolvation process of cations significantly affects the discharge capacity, ESW, CE, and cycle performance of batteries.As an excellent solvent, water molecules exhibit high polarity and a high dielectric constant, where the lone pairs on the oxygen atom can coordinate with cations, which increases the oxidation potential of the water, thus improving the stability. These characteristics grant water molecules a strong solvation capability for most salts. There are many more water molecules than salt in a diluted sodium salt solution with a concentration of less than 5 M, which usually results in two solvation layers surrounding the Na+. When the salt concentration exceeds 9 M, there are hardly sufficient water molecules to generate the first solvation layer, and the WIS electrolytes can be regarded as dissolved salt. For example, the 9.26 m NaOTF electrolyte can expand the ESW to 2.5 V in ARSIBs.114 The concentrated NaOTF electrolyte prevents the formation of the first solvation layer around Na+, and only an incompact second solvation layer is formed, which facilitates the desolvation behavior of Na+ throughout the charging/discharging process. The concentrated electrolyte (9.26 m NaOTF) contains much more salt than free water, which effectively inhibits the HER/OER. As for 17 m NaClO4 + 2 m NaOTF electrolyte (19 m electrolyte), the solvation structure of Na+ is formed of the ClO4−, OTF−, and water molecules, and an SEI composed of NaF, Na2O, and NaOH is formed on the anode.55 The NVP//NVP symmetric batteries with the 19 m electrolyte present a wide ESW of 1.75 V, excellent energy density (70 W h kg−1), and outstanding electrochemical performance.
4.2. Liquid electrolyte
 | ||
| Fig. 11 (a) The discharging process for M5HCF. (b) CE of the samples.116 (c) The insertion behavior of Mg2+/Na+ in electrode materials. (d) Rate capability of the PNTAQ//MnO2 battery.127 Reproduced from ref. 127 with permission from John Wiley and Sons, copyright 2022. (e) Na/Zn hybrid ion batteries.130 Reproduced from ref. 130 with permission from Elsevier, copyright 2021. (f) Rate capability of NVTP@C//NVTP@C.133 Reproduced from ref. 133 with permission from the American Chemical Society, copyright 2021. (g) The voltage curves at 25 mA g−1, (h) cycle performance in 17 m NaClO4.75 Reproduced from ref. 75 with permission from Elsevier, copyright 2022. (i) Reaction mechanism of the alkaline aqueous battery, (j) cycle life of the battery.137 Reproduced from ref. 137 with permission from Springer Nature, copyright 2024. | ||
The electrochemical performance of ARSIBs can be effectively enhanced by mixing two or more diluted electrolytes to prepare hybrid-ion electrolytes. Among them, aqueous Na/Mg, Na/Li, and Na/Zn hybrid ion batteries collectively show phenomenal electrochemical performance.127–130 An aqueous Na/Mg hybrid ion battery is assembled with 1,4,5,8-naphthalenetetracarboxylic anhydride anthraquinone (PNTAQ) polymer material, MnO2, and 1 M MgCl2 + 0.5 M NaCl hybrid electrolyte. During the charging/discharging process, the insertion/extraction of Na+ and Mg2+ occurs in the active material (Fig. 11c), thereby improving the discharge capacity and cycle performance of the battery, and the capacity of the PNTAQ//MnO2 battery is only reduced by 12.8% (1000 mA g−1) after 1000 cycles (Fig. 11d).127 Zn(CH3COO)2 was added to the CH3COONa electrolyte solution to prepare the Na/Zn hybrid electrolyte, which can expand the ESW to 1.5 V.130 The presence of Na+ promotes the rapid deposition of Zn2+ in the electrolyte on the Zn anode, which can accelerate the occurrence of redox reactions, and endow Zn//NMTP batteries with appealing cycling stability and excellent discharge capacity (55.6 mA h g−1) (Fig. 11e). Despite the many advantages of diluted electrolytes, they still face the problem of narrow ESW, resulting in side reactions such as the HER/OER, proton co-intercalation and active material dissolution, limiting their practical applications in energy storage systems.
In previous reports, 17 m NaClO4 WIS electrolytes have been widely used in high-voltage ARSIBs, in which the amount of free water is less than that of salt, and can expand the ESW to 3 V.134–136 Furthermore, the 17 m NaClO4 electrolytes can greatly inhibit water decomposition, reduce the side reactions of the HER/OER, and avert the structural collapse of active materials. As shown in Fig. 11g, the capacities of NaCuHCF, NaxCuCNPFe, and NaxCuTolFe are 60, 41 and 36 mA h g−1 at 25 mA g−1 in 1 M Na2SO4 electrolyte, respectively. After cycling 2000 times in 1 M Na2SO4 dilute electrolyte, the capacity of NaxCuCNPFe and NaxCuTolFe decreased by half at 1 A g−1, and the capacity of NaCuHCF decreased rapidly to 0%. Nevertheless, after 2000 cycles in 17 m NaClO4 electrolyte, the capacities of NaCuHCF, NaxCuCNPFe, and NaxCuTolFe are rarely reduced (Fig. 11h).75 The above reason is that there is less free water in the concentrated electrolyte than in the dilute electrolyte, which effectively avoids side reactions during the reactions.
With the further development of concentrated electrolytes, in addition to conventional neutral electrolytes, 17 m NaClO4 alkaline electrolytes also exhibit promising applications for ARSIBs. As shown in Fig. 11i, the rich OH− in alkaline electrolyte creates an alkaline environment and inhibits the HER on the NTP anode. A layer of nickel/carbon nanoparticle (Ni/C) is coated on the Na2MnFe(CN)6 to form a H3O+-rich condition near the Na2MnFe(CN)6/Ni/C (NMF) cathode, which significantly inhibits the OER and causes the deterioration of electrode structure. Compared to ARSIBs with neutral electrolytes, NTP//NMF batteries with 17 m NaClO4 alkaline electrolytes (include 1 M NaOH) present outstanding cycle performance over 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles even at 10C (Fig. 11j).137
000 cycles even at 10C (Fig. 11j).137
In summary, the concentrated electrolytes possess many advantages in expanding the ESW and inhibiting side reactions, which make it possible to develop the high-performance aqueous batteries at low cost, with safety and excellent cycling stability. However, some inevitable problems (such as high viscosity) still need to be resolved.
In recent years, the solvation effect has been used to prevent the aqueous electrolytes from freezing, the addition of additives in aqueous electrolyte can form new HBs, expanding the distance and destroying the connection between water molecules.138 Typically, some organic solutions as co-solvents are added into the electrolytes to obtain low freezing point electrolytes, and the ESW can be significantly broadened. According to previous reports, dimethyl sulfoxide (DMSO),139 acetonitrile (ACN),140 methanol,141 formamide (FA)142 and ethylene glycol (EG)143 have been explored as co-solvents for low-freezing-point electrolytes. The proportion of water in the diluted electrolyte is larger than that of salt, and it is easy to crystallize at low temperature. DMSO can be mixed with water in any ratio and used as a receptor for HBs. An appropriate amount of DMSO was added to 2 M NaClO4 solution as a co-solvent to get the mixed electrolyte with a freezing point of −50 °C (2 M-0.3 electrolyte).139 At low temperature, the addition of DMSO destroys the original intermolecular HBs and forms a new HB network, thus avoiding the rapid crystallization and precipitation of the aqueous electrolyte (Fig. 12a). Moreover, the NTP//AC full battery shows great electrochemical performance in 2 M-0.3 electrolyte, and even at −50 °C, the battery capacity remains at 60% of the initial capacity at 25 °C (Fig. 12b).
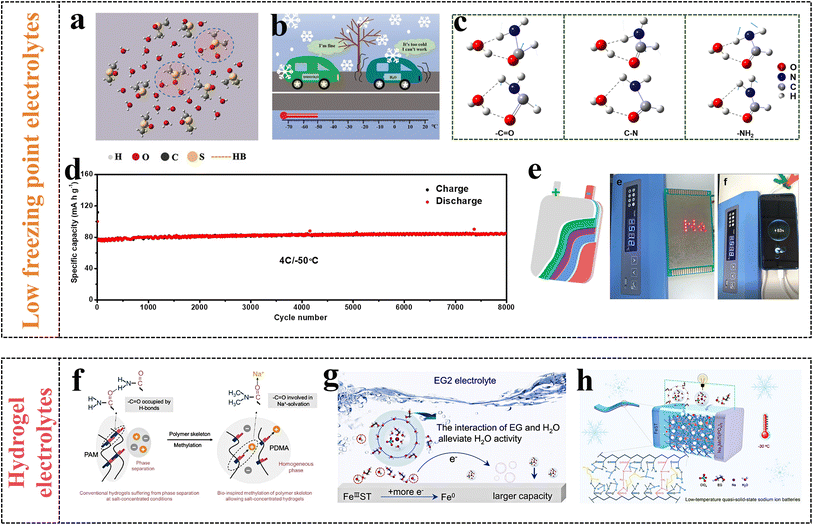 | ||
| Fig. 12 (a) The result of MD simulations, (b) the effect of electrolyte additive DMSO.139 Reproduced from ref. 139 with permission from John Wiley and Sons, copyright 2019. (c) Different functional groups among H2O–FA clusters, (d) cycle performance, (e) the pouch cell can light LEDs and power a mobile phone at −50 °C.142 Reproduced from ref. 142 with permission from Elsevier, copyright 2022. (f) Schematic illustration of the polymer structure.148 Reproduced from ref. 148 with permission from John Wiley and Sons, copyright 2023. (g) Schematic diagram of the function mechanism of electrolyte additive EG, (h) electrochemical performance and mechanisms of the pouch cell at −30 °C.149 Reproduced from ref. 149 with permission from Elsevier, copyright 2024. | ||
At room temperature, WIS electrolytes resemble near-saturated salt solutions. However, at low temperatures, the solubility of the salt decreases, causing salt to crystallize and precipitate on the electrodes, thus reducing the energy density and cycle performance of ARSIBs. A mixed electrolyte was prepared by adding ACN as additive into 17 m NaClO4 electrolyte, and shows a low freezing point. The Na+ detaches from the cation–anion aggregates and coordinates with the ACN molecules, lowing the interactions among ions, thus preventing the precipitation of NaClO4. The device works well even at −50 °C,140 demonstrating excellent electrochemical performance and appealing stability. When FA is used as a co-solvent, FA molecules coordinate with water molecules to form new HBs, breaking direct connections between water molecules and preventing them from forming long-range ordered structures below 0 °C (Fig. 12c). Furthermore, the ARSIBs assembled with AC, organic polymer, and 17 m NaClO4–FA electrolyte exhibit outstanding electrochemical performance and appealing cycling stability.142 The specific capacity of the battery hardly drops at −50 °C after charging/discharging for 8000 cycles (Fig. 12d), and the battery can light up an LED and power a smartphone at −50 °C (Fig. 12e), demonstrating great application potential in ARSIBs.
A suitable additive for aqueous electrolytes should satisfy the needs of high polarity, high boiling points, and thermal stability, which should not only be miscible with water but also dissolve inorganic salts. The development of low-freezing-point aqueous electrolytes offers a valuable strategy for the advancement of high-performance ARSIBs. However, the dosage and reaction mechanisms of additives need to be further explored.
4.3. Hydrogel electrolytes
Hydrogel electrolytes are hydrophilic polymers composed of cross-linked polymer chains. Owing to their light weight, high safety and environmental friendliness, hydrogel electrolytes are promising in flexible electronics. Although liquid electrolytes have high conductivity and fast ion diffusion kinetics, they can reduce the flexibility of the devices. Under extreme conditions (such as bending, piercing, and cutting), flexible devices with liquid electrolytes are more vulnerable to external damage, resulting in electrolyte leakage and device failure. In contrast, the devices with hydrogel electrolytes not only possess excellent mechanical performance, but also eliminate the risk of electrolyte leakage, imparting better resistance to external damage.Hydrogel electrolytes based on proton-conducting polymer matrices, such as polyacrylic acid, polyvinyl alcohol, polyacrylamide, and sodium alginate, are widely used in aqueous metal-ion (Li+, Na+, K+, Zn2+) batteries.144–147 The NVP//NTP full battery with poly(N,N-dimethylacrylamide) (PDMA) hydrogel electrolyte exhibits excellent cycle performance, and its capacity retention is still at 82.8% after cycling 580 times at 2C (Fig. 12f).148 As shown in Fig. 12g, an anti-freezing EG–PAM–NaClO4 hydrogel electrolyte is prepared by using EG, polyacrylamide (PAM), and NaClO4 concentrated electrolyte, and the addition of EG additives to the electrolyte can reduce HBs among solvent molecules, and form new HBs between EG and solvent molecules.149 The interaction between EG and water combines with the synergistic effect of cross-linked polyacrylamide networks, effectively inhibiting water solidification in hydrogel electrolytes, maintaining superior water retention and showing significant mechanical extensibility at −40 °C. In addition, the anti-freezing hydrogel electrolytes, iron-incorporated sodium titanate (FeST) anode, and NMTP cathode are used to fabricate the flexible ARSIBs FeST//NMTP (Fig. 12h). These flexible batteries present high energy density (43.6 W h kg−1), even at −30 °C, and the batteries can work well and a capacity retention of 64% can be achieved. Additionally, this flexible ARSIBs exhibit robust mechanical durability under bending and compression states, which can effectively power electronics at −30 °C. Although designing ultra-high conductivity hydrogel electrolytes needs further exploration, hydrogel electrolytes are still significant in the development of portable and flexible energy storage devices due to their high safety and excellent mechanical performance.
In summary, electrolytes are significant in the advancement of high-performance ARSIBs. Diluted electrolytes possess the advantages of excellent conductivity and low cost, but the narrow ESW (1.23 V) limits their wide applications. Concentrated electrolytes can expand the ESW to 3 V, as well as reducing the side reactions of the HER/OER, structural collapse of active materials and proton co-intercalation, thereby improving the energy density and cycling stability of the batteries. Nevertheless, there is also the problem of high viscosity of the electrolyte, make it easily crystallize at low temperature. Adding organic co-solvents as additives is a effective method of obtaining ARSIBs with low freezing point. By adjusting the solvation structure, the HBs between water molecules are broken, and new HBs between the co-solvent and water are formed, thereby endowing the electrolyte with anti-freezing performance and enhancing the energy density of the batteries. However, the dosage of additives and the reaction mechanism still need to be further explored. In addition, hydrogel electrolytes exhibit light weight, high safety and eco-friendliness, and the flexible ARSIBs with hydrogel electrolytes can achieve excellent electrochemical performance while maintaining high flexibility, which are promising for the advancement of flexible and wearable electronics. Nevertheless, it is essential to further explore high conductivity hydrogel electrolytes and more information about characteristics of the multiple electrolytes is shown in Table 1.
| Advantages | Disadvantages | Common electrolyte (applicable temperature) | Ref. | |
|---|---|---|---|---|
| Diluted electrolytes | High conductivity, low cost, high safety | Narrow voltage window, HER/OER side reaction | 1 M Na2SO4 (25 °C) | 116 |
| 1 M NaNO3 (25 °C) | 118 | |||
| 0.5 M CH3COONa/Zn(CH3COO)2 (25 °C) | 130 | |||
| Concentrated electrolytes | Wide voltage window, high safety, superior stability | Low-cost effectiveness, high viscosity | 9.26 m NaOTF (25 °C) | 114 |
| 17 m NaClO4 + 1 M NaOH (25 °C) | 137 | |||
| 35 m NaFSI (25 °C) | 132 | |||
| Low freezing point electrolytes | Low freezing point, superior stability | Low-cost effectiveness | 2 M NaClO4/DMSO (−50–25 °C) | 139 |
| 17 m NaClO4/ACN (−50–25 °C) | 140 | |||
| 17 m NaClO4/FA (−50–25 °C) | 142 | |||
| Hydrogel electrolytes | Low freezing point, superior stability | Low-cost effectiveness | PDMA hydrogel electrolyte (25 °C) | 148 |
| EG–PAM–NaClO4 hydrogel electrolyte (−30–25 °C) | 149 |
5. Flexible ARSIBs' structural design
The advancement of flexible, wearable, and even implantable electronics has resulted in the success of corresponding energy storage systems. Traditional ARSIBs are bulky, heavy, rigid, failing to satisfy the conditions of flexibility, light weight, and great biocompatibility for wearable electronics. In recent years, the studies on flexible aqueous LIBs, Zn-ion batteries, and Al-ion batteries have been extensive, and those batteries have already been applied in wearable electronics, but there have been few studies on flexible ARSIBs.150–152 The flexible ARSIBs possess abundant Na resources, biocompatibility, safety, and appealing mechanical performance, in accord with the requirements of flexible, wearable and implantable electronics. In addition to electrode materials and electrolytes, the structural design of batteries is also crucial to their performance and applications. To this end, the flexible ARSIBs are categorized based on their structural design into fiber-based, paper-based, metal current collector-based, and self-supporting batteries, with a detailed analysis and summary.5.1. Fiber-based flexible ARSIBs
Different from conventional planar flexible energy storage devices, flexible fiber-based energy storage devices without current collectors and conductive additives not only exhibit high energy density and excellent cycle performance, but also satisfy the needs of flexibility and light weight for wearable electronics. The manufacture of fiber-based flexible electrodes is the priority of flexible fiber-based energy storage devices. Metal wires, CNFs, polymer fibers, and CNTs have been used to prepare flexible fiber-based batteries and capacitors.153–155 For example, CNTs have high flexibility, tensile strength, and high conductivity, which show promise for application as current collectors for flexible fiber-based electrodes. In Fig. 13a, the CNTs were coated with NaTi2(PO4)3@C (NTPO@C) and Na0.44MnO2 active materials, which were twisted into fibrous electrodes, thus fabricating the NTPO@C//Na0.44MnO2 fiber-based flexible ARSIBs.18 As shown in Fig. 13b, the prepared NTPO@C//Na0.44MnO2 fiber-based flexible ARSIBs show appealing cycle performance in 1 M NaSO4 electrolytes, and the capacity retention of the battery can reach 76% after 100 cycles. Moreover, even after bending at 180° for 100 cycles, the capacity of the battery hardly decreased, and the cycle performance of the battery barely changed (Fig. 13c), indicating that the fiber-based ARSIBs possess excellent electrochemical performance and phenomenal mechanical performance. To further explore the application range of fibrous ARSIBs the electrochemical performance of the batteries was tested in saline and cell-culture medium, and the discharge capacities of these batteries still remained at 37 mA h g−1 and 32 mA h g−1, respectively, demonstrating great biocompatibility and appealing stability. Fiber-based flexible ARSIBs with high energy density, outstanding mechanical performance, appealing stability and great biocompatibility are promising in practical applications of wearable and implantable electronics. | ||
| Fig. 13 (a) Schematic diagram of the fibrous battery, (b) cycle performance, (c) the voltage curves of the batteries.18 Reproduced from ref. 18 with permission from Elsevier, copyright 2017. (d) Schematic representation of paper-based flexible ARSIBs, (e) the state of the paper-based flexible battery at various angles, the capacities of the battery (f) under bending states and (g) after punching holes.22 Reproduced from ref. 22 with permission from Elsevier, copyright 2022. (h) Schematic representation of fully-printed ARSIBs, (i) and (j) the electrochemical performance of the battery under bending states.24 Reproduced from ref. 24 with permission from John Wiley and Sons, copyright 2024. | ||
5.2. Paper-based flexible ARSIBs
Paper-based flexible energy storage devices can be employed in wearable electronics due to the abundant resources, high flexibility, and biocompatibility.26,156 The fibers in paper possess strong intermolecular and intramolecular HBs, endowing paper-based electrodes with appealing mechanical performance. The large specific surface area and porous structure enable the paper to absorb more electrolytes through capillary action, allowing for a greater number of extensive interactions between the electrolytes and active materials. Paper-based flexible ARSIBs serve as an appealing energy system that holds promise in the development of wearable electronics. Using paper as a flexible substrate for ARSIBs typically involves incorporating conductive carbon materials to improve their conductivity, such as CNTs, CNFs, and graphene. For example, S@C, Na0.44MnO2, Nafion membrane, graphite paper current collector, and 17 m NaClO4 electrolyte were used to assemble the paper-based flexible ARSIBs S@C//Na0.44MnO2 (Fig. 13d).22 The flexible battery delivers excellent capacity (348 mA h g−1), phenomenal rate performance and appealing Na+ storage performance. Furthermore, under different bending angles, the capacity of the battery decays very little (Fig. 13e and f), and even after punching holes on the battery, the electrochemical performance is rarely affected (Fig. 13g), proving excellent mechanical performance and appealing stability. These paper-based electrodes with ultra-small resistance and volume hold promise for achieving both high power density and energy density simultaneously.5.3. Flexible ARSIBs with metal collectors
Owing to the superior conductivity and high flexibility, metal foils (copper, aluminum, and titanium) used as conductive current collectors for electrodes have gained widespread utilization in flexible energy storage devices.157–159 As is well known, silver foil, despite its excellent conductivity, suffers from poor mechanical performance, rendering it unsuitable as a current collector for flexible devices. Interestingly, the silver current collector is prepared by printing silver paste on a polyethylene terephthalate substrate via the screen-printing technique, which presents high conductivity and excellent mechanical performance. As shown in Fig. 13h, the first fully printed flexible ARSIBs were obtained by the screen-printing technique, including the printed Na3V2(PO4)2F3/C (NVPF/C) cathode, Na3MnTi(PO4)3/C with 2% nitrogenous carbon (NMTP/C/NC) anodes, and printed electrolytes.24 The metal collectors with the advantages of high conductivity and outstanding mechanical performance endow the batteries with excellent energy density (40.1 W h kg−1), high flexibility and stability. Furthermore, the capacity retention of this flexible battery still remains at 81% even after bending at 180° for 200 cycles (Fig. 13i and j). This fully-printed battery enables miniaturization, integration, and patterning, showing outstanding mechanical performance and excellent electrochemical performance. The structural design and manufacturing technique of the fully-printed batteries can offer a new perspective for the advancement of flexible and wearable electronics.5.4. Self-supporting flexible ARSIBs
Flexible electrodes are typically composed of active materials, current collectors, binders, and conductive carbon materials. However, the weight and volume of the electrodes are increased as a result of the addition of current collectors and binders, thereby reducing the mass specific capacity of the batteries. The self-supporting flexible electrodes are free of current collectors, binders and conductive carbon materials, exhibiting excellent energy density and appealing flexibility.160,161 Generally, active materials are attached to carbon cloth or conductive carbon material arrays to prepare conductive films as self-supporting flexible electrodes.The hollow-structure NaTi2(PO4)3 nanoparticles (HNTP) were embedded in porous N-doped carbon nanofiber (PNC) arrays by electrospinning to prepare HNTP@PNC flexible anodes. Moreover, potassium zinc hexacyanoferrate (KZHCF) materials are loaded on CNT films to obtain CNTF@KZHCF flexible cathodes (Fig. 14a).21 The HNTP@PNC//CNTF@KZHCF battery exhibits appealing cycle performance, the discharge capacity still remains at 89.6% of its initial capacity, and the CE can reach 100% (0.48 A cm−3) after 200 cycles (Fig. 14b). Moreover, the capacity of the flexible battery decreases little at 45°, 90°, 135°, and 180° bending states; even upon bending at 90° for 500 cycles, the capacity remains at 89.8% of the initial capacity (Fig. 14c). The self-supporting flexible ARSIBs show excellent mechanical performance and phenomenal stability, offering a development direction for flexible electronics.
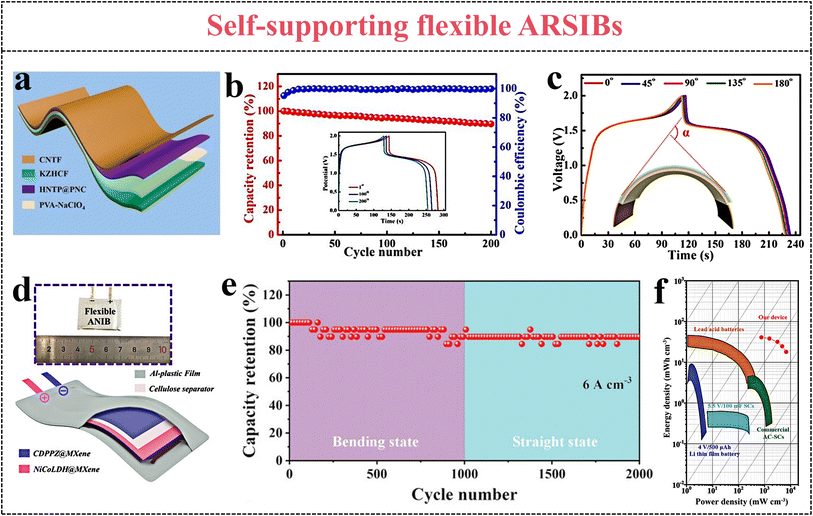 | ||
| Fig. 14 (a) Schematic illustration of self-supporting flexible ARSIBs, (b) cycle performance, (c) the voltage curves of the HNTP@PNC//CNTF@KZHCF battery under different bending angles.21 Reproduced from ref. 21 with permission from Elsevier, copyright 2021. (d) The soft-package flexible ARSIBs, (e) cycle performance, (f) comparison between the energy density of various energy storage devices.23 Reproduced from ref. 23 with permission from John Wiley and Sons, copyright 2023. | ||
As a 2D transition metal carbide and nitride, MXene provides abundant covalent bonds and ionic bonds, leading to excellent conductivity and high hydrophilicity, which is promising as a flexible electrode material. A carboxyl-substituted dipyridophenazine (CDPPZ) suspension was fully mixed with MXene solution to fabricate porous, conductive and flexible MXene@CDPPZ composite films, as a flexible self-supporting anode. The NiCoLDH@MXene composite films were prepared as a flexible self-supporting cathode by the same method. As shown in Fig. 14d, the anodes and cathodes and 10 M NaOH electrolytes were vacuum-encapsulated in aluminum–plastic film to form soft-package flexible ARSIBs.23 The discharge capacity of the battery decreases little under different bending states (0–120°), demonstrating phenomenal electrochemical performance, and appealing flexibility (Fig. 14e). Furthermore, this battery can power an electronic clock whether at flat or bending states, and its performance even surpasses that of commercial lithium iron phosphate and lithium-ion batteries (Fig. 14f).
According to the structural designs, functional flexible ARSIBs including fiber-based, paper-based, metal current collector-based, and self-supporting ARSIBs are developed to meet the requirements of flexible electronics. Table 2 summarizes the reported flexible ARSIBs with the different structural designs of flexible current collectors. Flexible ARSIBs with the advantages of safety, low cost and flexibility are essential in the advancement of wearable electronics. In addition, Fig. 15 summarizes the challenges and key strategies with respect to the cathode, anode, electrolyte, and structural design, providing valuable information for the development of high-performance flexible ARSIBs. In summary, it is significant to optimize active materials and electrolytes, and improve structural design for achieving a balance between energy density and functionality in ARSIBs.
| Battery | System | Working window | Capacity (capacity/current density) | Cycle performance (cycles/capacity retention) | Mechanical performance (bending angle/cycles/capacity retention) | Ref. |
|---|---|---|---|---|---|---|
| NTPO@C//Na0.44MnO2 | Fiber-based flexible ARSIBs | 0–1.6 V | 43 mA h g−1/0.1 A g−1 | 100/76% | 180°/100/88% | 18 |
| S@C//Na0.44MnO2 | Paper-based flexible ARSIBs | 0–1.6 V | 94.3 mA h g−1/0.5C | 300/82% | 60°/100/65% | 22 |
| NMTP/C/NC//NVPF/C | Flexible ARSIBs with a metal collector | 0–1 V | 40.1 mA h g−1/0.1C | 100/78% | 180°/200/81% | 24 |
| HNTP@PNC//CNTF@KZHCF | Self-supporting flexible ARSIBs | 0.6–1.9 V | 24.5 mA h cm−3/0.04 A cm−3 | 200/89.6% | 90°/500/89.8% | 21 |
| MXene@CDPPZ//NiCoLDH@MXene | Self-supporting flexible ARSIBs | 0–1.6 V | 51.2 mA h cm−3/6 A cm−3 | 2000/91% | 120°/1000/91% | 23 |
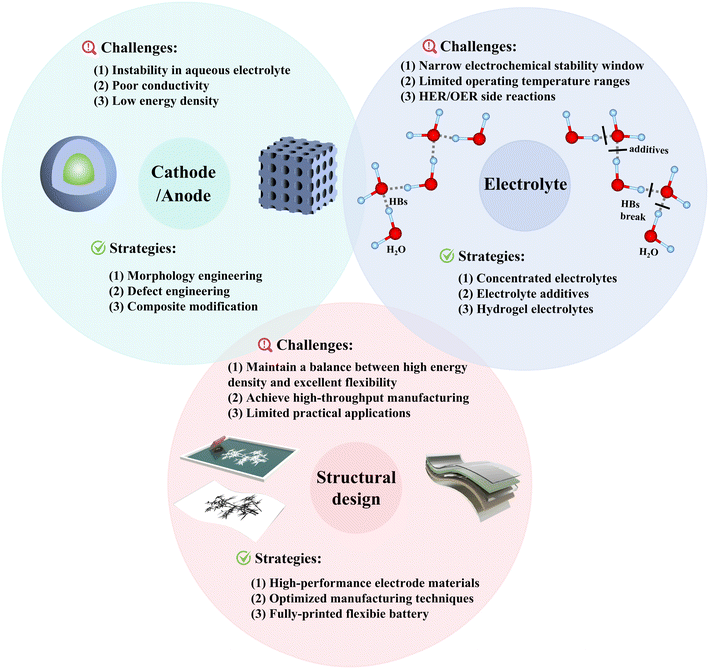 | ||
| Fig. 15 The challenges and strategies with respect to the cathode, anode, electrolyte, and structural design of flexible ARSIBs. | ||
6. Applications of flexible ARSIBs
6.1. Flexible energy systems for portable electronics
The flexibility, lightweight, and biocompatibility characteristics are identified as essential design criteria for flexible energy systems in wearable electronics, ensuring the safety and comfort of these devices during wearing. Compared to conventional LIBs and SIBs, flexible ARSIBs can be widely used in wearable electronic devices, because of their low cost, safety, eco-friendliness, flexibility, and biocompatibility (Table 3). As shown in Fig. 16a, perylene tetracarboxylic diimide (PTCDI) nanofibers are constructed onto flexible indium tin oxide films, thereby fabricating a binder-free PTCDI-nano-organic anode. The aqueous flexible sodium ion storage devices (AFSISD) with a sandwich structure are assembled by combining the PTCDI-nano-organic anode with an activated carbon cathode and polyvinyl alcohol–Na2SO4 (PVA–Na2SO4) gel–electrolyte. This battery provides an excellent gravimetric energy density of 83.8 W h kg−1 and a power density of 3.4 kW kg−1, and it can light up a ring LED strip even in the bent state (Fig. 16b).20 Utilizing a zinc nanosheet as both the zinc source and reducing agent, a micro-cube-like K2Zn3(Fe(CN)6)2·9H2O is grown on carbon cloth (CC) to fabricate the flexible cathode CC@KZHCF, and the flexible anode CC@NTP@C is fabricated by growing NTP onto CC. As shown in Fig. 16c, the flexible ARSIBs with a high output voltage platform are fabricated by the CC@KZHCF cathode and CC@NTP@C anode, which exhibit excellent energy density and attractive mechanical performance. In addition, two batteries connected in series can power 18 LEDs (Fig. 16d).19 As shown in Fig. 16e, the fully-printed ARSIBs NMTP/NC//NVPF/C are fabricated using the screen printing technique, exhibiting excellent electrochemical performance and remarkable mechanical performance. Batteries with different patterns can be integrated in series and parallel to output the required voltage and capacity, which can light up 10 LEDs and serve as an energy bracelet to power an electronic watch (Fig. 16f).24| Battery | Advantages | Challenges |
|---|---|---|
| Flexible ARSIBs | High flexibility | Low energy density |
| Low cost | ||
| Safety | HER/OER side reactions | |
| Environmental friendliness | ||
| Aqueous SIBs | Low cost | Low energy density |
| Safety | Poor flexibility | |
| Environmental friendliness | HER/OER side reactions | |
| SIBs | Low cost | Unsafe |
| Long cycle life | Poor flexibility | |
| Aqueous LIBs | Safety | Low cost-effectiveness |
| Low energy density | ||
| Environmental friendliness | HER/OER side reactions | |
| Poor flexibility | ||
| LIBs | High energy density | Low cost-effectiveness |
| Long cycle life | Unsafe | |
| Mature manufacturing technology | Poor flexibility |
 | ||
| Fig. 16 (a) Schematic illustration and (b) electrochemical performance of AFSISD.20 (c) Schematic illustration of the flexible ARSIBs, (d) two batteries connected in series can power 18 LEDs.19 Reproduced from ref. 19 with permission from John Wiley and Sons, copyright 2019. (e) The preparation process of fully printed flexible electrodes, (f) the batteries can light LEDs and power an electronic watch.24 Reproduced from ref. 24 with permission from John Wiley and Sons, copyright 2024. (g) Schematic illustration of the structure of the triple-stacked flexible battery.128 Reproduced from ref. 128 with permission from the American Chemical Society, copyright 2020. (h) The synthesis of the NVTP/C hybrid nanofibers film, (i) the cross-linked nanofiber film can be used as a connector component in LED circuits, (j) schematic illustration of flexible symmetric ARSIBs.162 | ||
Other cations (such as Li+ and Zn2+) were introduced into the Na+ electrolyte to prepare flexible aqueous hybrid-ion batteries, which present excellent electrochemical performance. For example, the addition of LiNO3 to a Na2SO4 electrolyte enables the co-intercalation of Li+ and Na+, enhancing the performance of the sodium manganese oxide cathode. A triple-stacked flexible aqueous sodium/lithium hybrid-ion battery, comprising activated carbon and Na0.39MnO2, was integrated into an energy band that can power LEDs whether in a flat or bent state (Fig. 16g).128 As shown in Fig. 16h, the NVTP/C hybrid nanofibers were prepared by an electrospinning method, which are well-aligned along two intersecting directions, forming flexible films with 3D network structure. The films with cross-linked architecture exhibit appealing flexibility, excellent stability, and high conductivity, which can serve as a connector component in LED circuits (Fig. 16i). Additionally, flexible self-supported symmetric ARSIBs NVTP/C//NVTP/C without binders, conductive additives, and current collectors present excellent electrochemical performance and high flexibility (Fig. 16j).162
Although flexible ARSIBs have been widely used in certain small, portable wearable electronics, their electrochemical performance still requires further enhancement while maintaining excellent flexibility. Further development is required to advance the applications of these flexible ARSIBs, particularly in integrating these batteries with small energy conversion devices to form integrated electronic systems. For example, combining flexible ARSIBs with flexible sensors, solar cells, wind power generators, and triboelectric nanogenerators can create a wearable integrated system.
6.2. Stability under extreme conditions
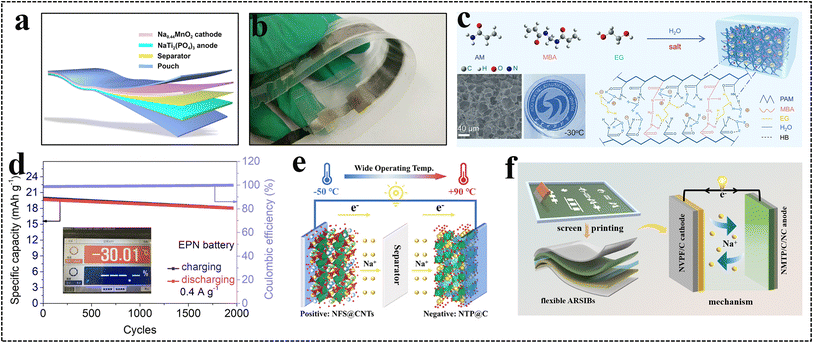 | ||
| Fig. 17 (a) Schematic illustration of the flexible ARSIBs, (b) the photo of flexible strip-shaped ARSIBs.18 Reproduced from ref. 18 with permission from Elsevier, copyright 2017. (c) The synthesis and mechanism of EPN hydrogel electrolyte, (d) electrochemical performance of the battery at −30 °C.149 Reproduced from ref. 149 with permission from Elsevier, copyright 2024. (e) Schematic illustration of the NTP//NFS battery.163 Reproduced from ref. 163 with permission from John Wiley and Sons, copyright 2024. (f) The structure and mechanism of fully-printed flexible ARSIBs.24 Reproduced from ref. 24 with permission from John Wiley and Sons, copyright 2024. | ||
High temperatures can exacerbate the instability of electrode materials and electrolytes. In aqueous electrolytes, solvents evaporate easily under high-temperature conditions, resulting in poor energy density and short cycle life. The development of stable electrode materials and appropriate electrolyte additives is crucial for achieving SIBs with low/high temperature tolerance. As shown in Fig. 17e, the high-performance SIBs NTP//Na2.4Fe1.8(SO4)3 (NTP//NFS) are fabricated with the stable Na2.4Fe1.8(SO4)3 (NFS) cathode, NTP anode, and ester-based electrolyte. These batteries demonstrate an ultra-long cycle life, and the capacity retention can remain at 70.7% after charging/discharging 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 times at 10C. Due to the high voltage platform and excellent stability of NTP and NFS in ester-based electrolytes, the full battery can work safely between −50 °C and 90 °C.163 The stable electrode materials and appropriate electrolyte additives play a significant role in the design of wide-temperature ARSIBs.
000 times at 10C. Due to the high voltage platform and excellent stability of NTP and NFS in ester-based electrolytes, the full battery can work safely between −50 °C and 90 °C.163 The stable electrode materials and appropriate electrolyte additives play a significant role in the design of wide-temperature ARSIBs.
7. Conclusions and outlook
Flexible ARSIBs have great potential for application in portable and wearable electronics due to their low cost, superior mechanical properties, environmental friendliness, and excellent electrochemical performance. The active materials, electrolytes, and structural design are of great significance for the development of flexible ARSIBs with both high energy density and multi-functionality, which have attracted extensive attention. This review introduces and discusses the latest developments in active materials and electrolytes in ARSIBs. The performance of active materials can be enhanced through optimization strategies such as morphology engineering, defect engineering, and composite modification, thereby obtaining high-performance ARSIBs. Furthermore, it is crucial to develop stable active materials with high performance and eco-friendliness, as well as low-cost aqueous electrolytes with a wide ESW and high stability. However, more research is still needed to fully understand the reaction mechanism of ARSIBs and to elucidate their performance enhancement mechanism. In addition, the recent progress of flexible ARSIBs based on fibers, paper, metal current collectors and self-supporting electrodes is also reviewed. These studies offer insightful analysis and helpful recommendations for the success of flexible and wearable electronics. More importantly, the practical applications of ARSIBs still need to be further explored.(1) As an essential component of the battery, the development of high-performance active materials is essential to enhance ARSIB performance. So far, the most promising active materials in ARSIBs are metal oxides, polyanionic compounds, and PBAs, MOFs, and organic polymer materials. However, the instability, poor conductivity and low energy density of active materials in aqueous solution severely limit the advancement of high-performance ARSIBs. Optimization and improvement strategies such as morphology engineering, defect engineering, and composite modification are expected to enhance the performance of active materials. Morphology engineering aims at improving performance by altering the morphologies of the materials, which requires precise control over the structure and surface modification. The purpose of defect engineering is to optimize electrode materials by introducing defects on the surface and inside of the materials, but it is necessary to avoid excessive defects that could destroy the stability of the material and lead to structural collapse during the charging/discharging process. The fabrication of composite materials can improve the electrochemical performance of electrode materials, but it still faces challenges in the complex preparation process and the unclear interactions between materials. Hence, future research on innovative active materials and selecting appropriate optimization strategies is needed, according to specific requirements and practical applications for promoting the development of ARSIBs.
(2) Electrolytes are also an important component of the batteries, so it is a valuable research direction to develop suitable aqueous electrolytes for ARSIBs. So far, dilute electrolytes, concentrated electrolytes, and hydrogel electrolytes have been widely reported. However, they still face the challenges of narrow ESW, limited operating temperature ranges, and side reactions (HER/OER, proton co-intercalation, and active material structure collapse). Moreover, the organic additives used to optimize the aqueous electrolytes still involve the risk of combustion, and the reaction mechanism needs to be further explored. Therefore, further exploration of novel electrolytes and optimization of reported electrolytes are necessary to obtain the high-performance ARSIBs.
(3) The success of high-performance flexible ARSIBs provides significant implications for their commercial viability. To attain this objective, there are multiple aspects that should be considered, such as optimizing electrode materials, developing aqueous electrolytes with a wide ESW, and exploring new design strategies. In addition, the comprehensive understanding of their energy storage mechanisms is necessary to fully utilize the potential of active materials. However, the current research on the reaction mechanism of ARSIBs is not clear enough. Additional computational simulations and more in situ characterization techniques are needed to investigate the reaction mechanism of ARSIBs and enhance their electrochemical performance.
(4) To achieve environmental sustainability goals, the recycling technology of active materials needs further improvement, and the development of biodegradable and eco-friendly materials as next-generation electrode materials is promising. Additionally, the application of flexible ARSIBs requires further expansion. On the one hand, these batteries can be integrated with small energy conversion devices (such as flexible sensors, solar cells, wind power generators, and triboelectric nanogenerators) to create wearable integrated electronic systems. On the other hand, further research into battery assembly and improvement strategies is needed to address the extreme conditions that flexible ARSIBs may encounter, such as high temperatures, high pressures, high salinity, low temperatures, and vacuum environments.
(5) Despite the great progress of aqueous metal ion batteries, the energy density is still lower than that of conventional organic batteries. Due to the advantages of safety, eco-friendliness and low cost, it is significant to explore new manufacturing techniques to prepare flexible ARSIBs with excellent flexibility and high performance. In recent years, screen-printing techniques have shown many advantages in the preparation of multifunctional flexible energy storage devices, including fully-printed flexible aqueous metal ion batteries and supercapacitors. The advantages of miniaturization, high flexibility, the tunability of shapes and sizes, and integration make them promising in portable, wearable, and flexible electronics.
Data availability
No primary research results, software or code have been included and no new data were generated or analysed as part of this review.Author contributions
Hehe Ren: writing-original draft, writing-review & editing, visualization, software, investigation, formal analysis. Xinzhan Du: conceptualization, validation. Jing Liang: writing-review & editing, methodology, funding acquisition, data curation. Wei Wu: writing-review & editing, supervision, resources, project administration, funding acquisition.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (NSFC, Grant No. 52373252), the Fundamental Research, Key Research and Development Project of Hubei Province (2022BCA040), and China Postdoctoral Science Foundation (2022TQ0239, 2022M722465).References
- M. Li, J. Lu, Z. Chen and K. Amine, Adv. Mater., 2018, 30, 1800561 CrossRef PubMed.
- J. B. Goodenough, Nat. Electron., 2018, 1, 204 CrossRef.
- P. Poizot, S. Laruelle, S. Grugeon, L. Dupont and J. M. Tarascon, Nature, 2000, 407, 496–499 CrossRef CAS PubMed.
- Y. Chen, T. Wang, H. Tian, D. Su, Q. Zhang and G. Wang, Adv. Mater., 2021, 33, 2003666 CAS.
- P. K. Nayak, L. Yang, W. Brehm and P. Adelhelm, Angew. Chem., Int. Ed., 2017, 57, 102–120 Search PubMed.
- Y. Wang, D. Liu, M. Sun and J. Liu, Mater. Chem. Front., 2021, 5, 7384 CAS.
- H. Zhang, X. Tan, H. Li, S. Passerini and W. Huang, Energy Environ. Sci., 2021, 14, 5788–5800 RSC.
- M. Liu, H. Ao, Y. Jin, Z. Hou, X. Zhang, Y. Zhu and Y. Qian, Mater. Today Energy, 2020, 17, 100432 CrossRef.
- S. Boyd and V. Augustyn, Inorg. Chem. Front., 2018, 5, 999–1015 CAS.
- D. Bin, F. Wang, A. G. Tamirat, L. Suo, Y. Wang, C. Wang and Y. Xia, Adv. Energy Mater., 2018, 8, 1703008 CrossRef.
- C. Ding, Z. Chen, C. Cao, Y. Liu and Y. Gao, Nano-Micro Lett., 2023, 15, 192 CrossRef CAS PubMed.
- J. L. Sudworth, J. Power Sources, 1984, 11, 143–154 CAS.
- H. Li, X. Zhang, Z. Zhao, Z. Hu, X. Liu and G. Yu, Energy Storage Mater., 2020, 26, 83–104 Search PubMed.
- W. Tang, R. Qi, J. Wu, Y. Zuo, Y. Shi, R. Liu, W. Yan and J. Zhang, Electrochem. Energy Rev., 2024, 7, 23 CrossRef CAS.
- H. Zhu, F. Wang, L. Peng, T. Qin, F. Kang and C. Yang, Angew. Chem., Int. Ed., 2022, 62, e202212439 Search PubMed.
- J. Wang, Z. Wang, J. Ni and L. Li, Energy Storage Mater., 2022, 45, 704–719 Search PubMed.
- Y. Wu, W. Shuang, F. Chen, S. Tang, X. Wu, Z. Bai, L. Yang and J. Zhang, Electrochem. Energy Rev., 2024, 7, 17 CAS.
- Z. Guo, Y. Zhao, Y. Ding, X. Dong, L. Chen, J. Cao, C. Wang, Y. Xia, H. Peng and Y. Wang, Chem, 2017, 3, 348–362 CAS.
- B. He, P. Man, Q. Zhang, C. Wang, Z. Zhou, C. Li, L. Wei and Y. Yao, Small, 2019, 15, 1905115 CrossRef CAS PubMed.
- L. Yang, P. Wang, S. Zhang, Y. Wang, L. Zang, H. Zhu, J. Yin and H. Yang, J. Mater. Chem. A, 2020, 8, 22791–22801 Search PubMed.
- B. He, K. Yin, W. Gong, Y. Xiong, Q. Zhang, J. Yang, Z. Wang, Z. Wang, M. Chen, P. Man, P. Coquet, Y. Yao, L. Sun and L. Wei, Nano Energy, 2021, 82, 105764 CAS.
- M. Kumar and T. C. Nagaiah, Energy Storage Mater., 2022, 49, 390–400 Search PubMed.
- Y. Zhao, J. He, L. Hu, J. Yang, C. Yan and M. Shi, Small, 2023, 19, 2304182 Search PubMed.
- H. Ren, X. Zhang, Q. Liu, W. Tang, J. Liang and W. Wu, Small, 2024, 20, 2312207 CrossRef CAS PubMed.
- F. Mo, G. Liang, Z. Huang, H. Li, D. Wang and C. Zhi, Adv. Mater., 2019, 32, 1902151 CrossRef PubMed.
- B. Yao, J. Zhang, T. Kou, Y. Song, T. Liu and Y. Li, Adv. Sci., 2017, 4, 1700107 CrossRef PubMed.
- S. Li, R. Dong, Y. Li, X. Lu, J. Qian, F. Wu, C. Wu and Y. Bai, Mater. Today, 2024, 72, 207–234 CrossRef CAS.
- S. Jayakumar, P. C. Santhosh, M. M. Mohideen and A. V. Radhamani, J. Alloys Compd., 2024, 976, 173170 Search PubMed.
- J. Wang, F. Zheng, Y. Yu, P. Hu, M. Li, J. Wang, J. Fu, Q. Zhen, S. Bashir and J. Liu, Chem. Eng. J., 2021, 426, 131804 CAS.
- P. Ragupathy, H. N. Vasan and N. Munichandraiah, J. Electrochem. Soc., 2008, 155, A34–A40 CAS.
- Y. Liu, Y. Qiao, W. Zhang, H. Wang, K. Chen, H. Zhu, Z. Li and Y. Huang, J. Mater. Chem. A, 2015, 3, 7780–7785 RSC.
- L. Rakočević, S. Štrbac, J. Potočnik, M. Popović, D. Jugović and I. S. Simatović, Ceram. Int., 2021, 47, 4595–4603 Search PubMed.
- R. Chua, Y. Cai, Z. Kou, R. Satish, H. Ren, J. Chan, L. Zhang, S. A. Morris, J. Bai and M. Srinivasan, Chem. Eng. J., 2019, 370, 742–748 CrossRef CAS.
- X. Shan, F. Guo, D. S. Charles, Z. Lebens-Higgins, S. Abdel Razek, J. Wu, W. Xu, W. Yang, K. L. Page, J. C. Neuefeind, M. Feygenson, L. F. J. Piper and X. Teng, Nat. Commun., 2019, 10, 4975 CrossRef PubMed.
- Z. Hao, X. Shi, Z. Yang, X. Zhou, L. Li, C. Ma and S. Chou, Adv. Mater., 2024, 36, 2305135 CrossRef CAS PubMed.
- M. Zheng, Z. Bai, Y. He, S. Wu, Y. Yang and Z. Zhu, ACS Omega, 2020, 5, 5192–5201 CrossRef CAS PubMed.
- X. Cao, A. Pan, B. Yin, G. Fang, Y. Wang, X. Kong, T. Zhu, J. Zhou, G. Cao and S. Liang, Nano Energy, 2019, 60, 312–323 Search PubMed.
- J. Peng, W. Zhang, Q. Liu, J. Wang, S. Chou, H. Liu and S. Dou, Adv. Mater., 2022, 34, 2108384 CrossRef CAS PubMed.
- Y. Xiao, J. Xiao, H. Zhao, J. Li, G. Zhang, D. Zhang, X. Guo, H. Gao, Y. Wang, J. Chen, G. Wang and H. Liu, Small, 2024, 20, 2401957 CrossRef CAS PubMed.
- G. Du and H. Pang, Energy Storage Mater., 2021, 36, 387–408 Search PubMed.
- H. Yi, R. Qin, S. Ding, Y. Wang, S. Li, Q. Zhao and F. Pan, Adv. Funct. Mater., 2020, 31, 2006970 CrossRef.
- Y. You, X. Wu, Y. Yin and Y. Guo, Energy Environ. Sci., 2014, 7, 1643–1647 RSC.
- X. Lamprecht, F. Speck, P. Marzak, S. Cherevko and A. S. Bandarenka, ACS Appl. Mater. Interfaces, 2022, 14, 3515–3525 CrossRef CAS PubMed.
- S. Jiao, J. Tuo, H. Xie, Z. Cai, S. Wang and J. Zhu, Mater. Res. Bull., 2017, 86, 194–200 CAS.
- C. Xu, J. Peng, X. Liu, W. Lai, X. He, Z. Yang, J. Wang, Y. Qiao, L. Li and S. Chou, Small Methods, 2022, 6, 2200404 CAS.
- T. Shao, C. Li, C. Liu, W. Deng, W. Wang, M. Xue and R. Li, J. Mater. Chem. A, 2019, 7, 1749–1755 RSC.
- H. Gao, S. Xin, L. Xue and J. B. Goodenough, Chem, 2018, 4, 833–844 Search PubMed.
- M. Zhong, L. Kong, N. Li, Y. Liu, J. Zhu and X. Bu, Coord. Chem. Rev., 2019, 388, 172–201 CAS.
- X. Li, C. He, J. Zheng, W. Ye, W. Yin, B. Tang and Y. Rui, J. Alloys Compd., 2020, 842, 155605 CrossRef CAS.
- J. Jiang, Y. Jiang, Z. Chen, C. Tang, Y. Cheng, Q. Zhuang and Z. Ju, Mater. Lett., 2023, 352, 135092 Search PubMed.
- Q. Zhou, C. Xia, J. Li, A. Cheng, J. Liu, H. Liu, L. Li and S. Wang, Solid State Ionics, 2024, 411, 116553 CrossRef CAS.
- Y. Liu, D. Liu, P. Liu, C. Liu and J. Zhou, J. Colloid Interface Sci., 2024, 666, 118–130 CrossRef CAS PubMed.
- L. Chen, Y. Li, X. Wang, J. Wu, Y. Ding, S. Ren, L. Zhang, Z. Xu, B. Chen, D. Han and Y. Wu, Chem. Eng. J., 2023, 464, 142658 CrossRef CAS.
- L. Wang, Y. Ni, X. Hou, L. Chen, F. Li and J. Chen, Angew. Chem., Int. Ed., 2020, 59, 22126–22131 CrossRef CAS PubMed.
- T. Jin, X. Ji, P. Wang, K. Zhu, J. Zhang, L. Cao, L. Chen, C. Cui, T. Deng, S. Liu, N. Piao, Y. Liu, C. Shen, K. Xie, L. Jiao and C. Wang, Angew. Chem., Int. Ed., 2021, 60, 11943–11948 CrossRef CAS PubMed.
- X. Wang, H. Huang, F. Zhou, P. Das, P. Wen, S. Zheng, P. Lu, Y. Yu and Z. Wu, Nano Energy, 2021, 82, 105688 CrossRef CAS.
- X. Shen, M. Han, X. Li, P. Zhang, C. Yang, H. Liu, Y. Hu and J. Zhao, ACS Appl. Mater. Interfaces, 2022, 14, 6841–6851 CrossRef CAS PubMed.
- L. Sharma, K. Nakamoto, R. Sakamoto, S. Okada and P. Barpanda, ChemElectroChem, 2018, 6, 444–449 CrossRef.
- H. Liu, W. Lei, Z. Tong, X. Li, Z. Wu, Q. Jia, S. Zhang and H. Zhang, Adv. Mater. Interfaces, 2020, 7, 2000494 CrossRef CAS.
- Y. Zhang, L. Tao, C. Xie, D. Wang, Y. Zou, R. Chen, Y. Wang, C. Jia and S. Wang, Adv. Mater., 2020, 32, 1905923 CrossRef CAS PubMed.
- J. Wu, Y. Faiz, S. Hussain, F. Faiz, N. Zarshad, A. U. Rahman, M. A. Masood, Y. Deng, X. Pan and M. Ahmad, Electrochim. Acta, 2023, 443, 141927 CrossRef CAS.
- M. S. Chae, A. Chakraborty, S. Kunnikuruvan, R. Attias, S. Maddukuri, Y. Gofer, D. T. Major and D. Aurbach, Adv. Energy Mater., 2020, 10, 2002077 CrossRef CAS.
- G. Chen, Q. Huang, T. Wu and L. Lu, Adv. Funct. Mater., 2020, 30, 2001289 CrossRef CAS.
- R. S. Kate, H. S. Jadhav, U. P. Chothe, K. Bhattacharjee, M. V. Kulkarni, R. J. Deokate, B. B. Kale and R. S. Kalubarme, J. Mater. Chem. A, 2024, 12, 7418–7451 RSC.
- X. Zhang, X. Rui, D. Chen, H. Tan, D. Yang, S. Huang and Y. Yu, Nanoscale, 2019, 11, 2556–2576 RSC.
- K. Wang, H. Li, G. Guo, L. Zheng, S. Passerini and H. Zhang, ACS Energy Lett., 2023, 8, 1671–1679 CrossRef CAS.
- B. Mai, B. Xing, Y. Yue, N. Cai, C. Cai, S. Lian, H. Fan, M. Yan, T. Zhu, P. Hu, X. Wang and L. Mai, J. Mater. Sci. Technol., 2023, 165, 1–7 CrossRef CAS.
- H. Yi, M. Ling, W. Xu, X. Li, Q. Zheng and H. Zhang, Nano Energy, 2018, 47, 340–352 CrossRef CAS.
- X. Jiang, C. Liu, Q. Huang, W. Cao, Y. Chen and L. Guo, J. Alloys Compd., 2022, 928, 167119 CrossRef CAS.
- C. Guo, J. Yang, Z. Cui, S. Qi, Q. Peng, W. Sun, L. Lv, Y. Xu, Y. Wang and S. Chen, J. Energy Chem., 2022, 65, 514–523 CAS.
- Z. Yang, G. Li, J. Sun, L. Xie, Y. Jiang, Y. Huang and S. Chen, Energy Storage Mater., 2020, 25, 724–730 CrossRef.
- Y. Chen, X. Liao, P. Wang, J. Chen, X. Zhang, X. Wu, S. C. Smith, D. Lin, X. Tan and Q. Zheng, J. Colloid Interface Sci., 2024, 653, 1–10 CrossRef CAS PubMed.
- W. Liu, H. Yi, Q. Zheng, X. Li and H. Zhang, J. Mater. Chem. A, 2017, 5, 10928–10935 RSC.
- W. Song, X. Cao, Z. Wu, J. Chen, Y. Zhu, H. Hou, Q. Lan and X. Ji, Langmuir, 2014, 30, 12438–12446 Search PubMed.
- T. Pan, Ruqia, C. Wu, C. Ni, S. Gull, A. Haider and H. Chen, Electrochim. Acta, 2022, 427, 140778 CrossRef CAS.
- S. Ullah, A. Rehman, T. Najam, I. Hossain, S. Anjum, R. Ali, M. Shahid, S. Shah and M. Nazir, J. Ind. Eng. Chem., 2024, 137, 87–105 CrossRef CAS.
- Y. Yuan, D. Bin, X. Dong, Y. Wang, C. Wang and Y. Xia, ACS Sustainable Chem. Eng., 2020, 8, 3655–3663 CrossRef CAS.
- G. Yuan, J. Xiang, H. Jin, Y. Jin, L. Wu, Y. Zhang, A. Mentbayeva and Z. Bakenov, Electrochim. Acta, 2018, 259, 647–654 CAS.
- L. Shao, J. Hong, S. Wang, F. Wu, F. Yang, X. Shi and Z. Sun, J. Power Sources, 2021, 491, 229627 CrossRef CAS.
- X. Yang and A. Rogach, Adv. Energy Mater., 2020, 10, 2000288 CAS.
- Z. He, Y. Huang, H. Liu, Z. Geng, Y. Li, S. Li, W. Deng, G. Zou, H. Hou and X. Ji, Nano Energy, 2024, 129, 109996 CrossRef CAS.
- D. Xiao, L. Zhang, Z. Li, H. Dou and X. Zhang, Energy Storage Mater., 2022, 44, 10–28 CrossRef.
- Y. Niu, Y. Guo, Y. Yin, S. Zhang, T. Wang, P. Wang, S. Xin and Y. Guo, Adv. Mater., 2020, 32, 2001419 Search PubMed.
- S. Kandula, B. Sik Youn, J. Cho, H. K. Lim and J. Gon Son, Chem. Eng. J., 2022, 439, 135678 Search PubMed.
- D. Chen, X. Miao, J. Liu, J. Geng, L. Zhang, Z. Dai, H. Dong, J. Yang and H. Geng, Chem. Eng. J., 2022, 446, 136772 CrossRef CAS.
- Y. Wang, M. Li, Y. Zhang and N. Zhang, Nano Res., 2024, 17, 6038–6057 CAS.
- Y. Zhu, Y. Wang, Y. Wang, T. Xu and P. Chang, Carbon Energy, 2022, 4, 1182–1213 CrossRef CAS.
- Y. Chen, X. Li, K. Park, W. Lu, C. Wang, W. Xue, F. Yang, J. Zhou, L. Suo, T. Lin, H. Huang, J. Li and J. B. Goodenough, Chem, 2017, 3, 152–163 CAS.
- E. Wang, M. Chen, X. Guo, S. Chou, B. Zhong and S. Dou, Small Methods, 2019, 4, 1900163 CrossRef.
- B. Cho, H. Lim, H. N. Lee, Y. M. Park, H. Kim and H. J. Kim, Surf. Coat. Technol., 2021, 407, 126797 CrossRef CAS.
- M. Kumar, A. K. Padhan, D. Mandal and T. C. Nagaiah, Energy Storage Mater., 2022, 45, 1052–1061 CrossRef.
- Z. Liu, Y. An, G. Pang, S. Dong, C. Xu, C. Mi and X. Zhang, Chem. Eng. J., 2018, 353, 814–823 CAS.
- Y. Cao, Y. Liu, D. Zhao, X. Xia, L. Zhang, J. Zhang, H. Yang and Y. Xia, ACS Sustainable Chem. Eng., 2020, 8, 1380–1387 CAS.
- X. Yan, Y. Zhou, W. Zhou, K. H. Lam and X. Hou, Electrochim. Acta, 2022, 429, 140925 CAS.
- Q. Meng, J. Shao, X. Dou and H. Chi, Small, 2024, 20, 2308483 CAS.
- G. Pang, P. Nie, C. Yuan, L. Shen, X. Zhang, J. Zhu and B. Ding, Energy Technol., 2014, 2, 705–712 CrossRef CAS.
- M. Kumar, N. Thakur, A. Bordoloi, A. Kumar Yadav, S. N. Jha, D. Bhattacharyya, D. Mandal and T. C. Nagaiah, J. Mater. Chem. A, 2022, 10, 11394–11404 CAS.
- Y. He, H. Chen, Y. Wang, Y. Zhang, L. Hou, R. Jiang and C. Yuan, Electrochim. Acta, 2023, 447, 142128 CrossRef CAS.
- X. Yao, Y. Luo, Y. Li, W. Li, M. Fang, M. Shui, J. Shu and Y. Ren, Mater. Res. Bull., 2018, 104, 194–201 CAS.
- Y. Qiu, Y. Yu, J. Xu, Y. Liu, M. Ou, S. Sun, P. Wei, Z. Deng, Y. Xu, C. Fang, Q. Li, J. Han and Y. Huang, J. Mater. Chem. A, 2019, 7, 24953–24963 CAS.
- J. Wu, L. Yang, H. Liu, H. Bu, W. Wang, C. Zeng and S. Zhu, J. Appl. Electrochem., 2022, 52, 1563–1572 CAS.
- S. Liu, L. Wang, J. Liu, M. Zhou, Q. Nian, Y. Feng, Z. Tao and L. Shao, J. Mater. Chem. A, 2019, 7, 248–256 RSC.
- F. Malchik, N. Shpigel, M. D. Levi, T. R. Penki, B. Gavriel, G. Bergman, M. Turgeman, D. Aurbach and Y. Gogotsi, Nano Energy, 2021, 79, 105433 CAS.
- Y. Zhou, X. Shao, K. H. Lam, Y. Zheng, L. Zhao, K. Wang, J. Zhao, F. Chen and X. Hou, ACS Appl. Mater. Interfaces, 2020, 12, 30328–30335 CAS.
- T. Zhu, P. Hu, X. Wang, Z. Liu, W. Luo, K. A. Owusu, W. Cao, C. Shi, J. Li, L. Zhou and L. Mai, Adv. Energy Mater., 2019, 9, 1803436 Search PubMed.
- Y. Jiang, J. Shi, M. Wang, L. Zeng, L. Gu and Y. Yu, ACS Appl. Mater. Interfaces, 2015, 8, 689–695 Search PubMed.
- J. Yang, H. Wang, P. Hu, J. Qi, L. Guo and L. Wang, Small, 2015, 11, 3744–3749 CrossRef CAS PubMed.
- H. Gao and J. B. Goodenough, Angew. Chem., Int. Ed., 2016, 55, 12768–12772 CrossRef CAS PubMed.
- S. Qiu, X. Wu, M. Wang, M. Lucero, Y. Wang, J. Wang, Z. Yang, W. Xu, Q. Wang, M. Gu, J. Wen, Y. Huang, Z. Xu and Z. Feng, Nano Energy, 2019, 64, 103941 CrossRef CAS.
- Z. Tian, Y. Zou, G. Liu, Y. Wang, J. Yin, J. Ming and H. N. Alshareef, Adv. Sci., 2022, 9, 2201207 CrossRef CAS PubMed.
- X. Li, X. Wang, L. Ma and W. Huang, Adv. Energy Mater., 2022, 12, 2202068 CrossRef CAS.
- H. Cheng, Q. Sun, L. Li, Y. Zou, Y. Wang, T. Cai, F. Zhao, G. Liu, Z. Ma, W. Wahyudi, Q. Li and J. Ming, ACS Energy Lett., 2022, 7, 490–513 CrossRef CAS.
- C. Li, H. Xu, L. Ni, B. Qin, Y. Ma, H. Jiang, G. Xu, J. Zhao and G. Cui, Adv. Energy Mater., 2023, 13, 2301758 CrossRef CAS.
- L. Suo, O. Borodin, Y. Wang, X. Rong, W. Sun, X. Fan, S. Xu, M. A. Schroeder, A. V. Cresce, F. Wang, C. Yang, Y. Hu, K. Xu and C. Wang, Adv. Energy Mater., 2017, 7, 1701189 CrossRef.
- G. Plečkaitytė, M. Petrulevičienė, L. Staišiūnas, D. Tediashvili, J. Pilipavičius, J. Juodkazytė and L. Vilčiauskas, J. Mater. Chem. A, 2021, 9, 12670–12683 RSC.
- X. Zhao, Z. Xing and C. Huang, J. Mater. Chem. A, 2023, 11, 22835–22844 RSC.
- J. Kim, S. H. Yi, L. Li and S. E. Chun, J. Energy Chem., 2022, 69, 649–658 CrossRef CAS.
- R. Meija, V. Lazarenko, A. Skrastina, Y. Rublova, J. Andzane, V. Voikiva, A. Viksna and D. Erts, Batteries, 2022, 8, 25 CrossRef CAS.
- X. Li, X. Zhu, J. Liang, Z. Hou, Y. Wang, N. Lin, Y. Zhu and Y. Qian, J. Electrochem. Soc., 2014, 161, A1181–A1187 CrossRef CAS.
- B. Zhang, Y. Liu, X. Wu, Y. Yang, Z. Chang, Z. Wen and Y. Wu, Chem. Commun., 2014, 50, 1209–1211 RSC.
- T. Zhu, H. Kong, T. Xia, Y. Shen, Y. Miao and X. Zhao, Mater. Res. Bull., 2021, 138, 111209 CrossRef CAS.
- M. K. Ramos, G. Martins, L. H. Marcolino-Junior, M. F. Bergamini, M. M. Oliveira and A. J. G. Zarbin, Mater. Horiz., 2023, 10, 5521–5537 RSC.
- Y. Wang, Z. Feng, D. Laul, W. Zhu, M. Provencher, M. L. Trudeau, A. Guerfi and K. Zaghib, J. Power Sources, 2018, 374, 211–216 CrossRef CAS.
- S. H. Jeong, B. H. Kim, Y. D. Park, C. Y. Lee, J. Y. Mun and A. Tron, J. Alloys Compd., 2019, 784, 720–726 CrossRef CAS.
- J. Liu, C. Yang, B. Wen, B. Li and Y. Liu, Small, 2023, 19, 2303896 CrossRef CAS PubMed.
- P. Marzak, P. Moser, S. Schreier, D. Scieszka, J. Yun, O. Schneider and A. S. Bandarenka, Small Methods, 2019, 3, 1900445 CrossRef CAS.
- S. Zhang, C. Zhao, K. Zhu, J. Zhao, Y. Gao, K. Ye, J. Yan, G. Wang and D. Cao, Energy Environ. Mater., 2022, 6, e12388 CrossRef.
- M. S. Chae, H. J. Kim, J. Lyoo, R. Attias, Y. Elias, Y. Gofer, S. T. Hong and D. Aurbach, ACS Appl. Energy Mater., 2020, 3, 10744–10751 CAS.
- H. Ao, Y. Zhao, J. Zhou, W. Cai, X. Zhang, Y. Zhu and Y. Qian, J. Mater. Chem. A, 2019, 7, 18708 RSC.
- Y. Zhou, Z. Zhang, Y. Zhao, J. Liu, K. H. Lam, X. Zheng, H. Lou and X. Hou, Chem. Eng. J., 2021, 425, 130459 CAS.
- H. Qin, Z. Song, H. Zhan and Y. Zhou, J. Power Sources, 2014, 249, 367–372 CAS.
- R. S. Kühnel, D. Reber and C. Battaglia, ACS Energy Lett., 2017, 2, 2005–2006 Search PubMed.
- L. Shen, H. Yang, Y. Jiang, J. Ma, T. Sun, M. Zhang and N. Zhu, ACS Sustainable Chem. Eng., 2021, 9, 3490–3497 CAS.
- I. Ahmad and A. Kumar, Chem. Eng. J., 2023, 477, 146972 CAS.
- M. S. Arsha and V. Biju, J. Energy Storage, 2023, 70, 108064 CrossRef.
- J. Han, A. Mariani, S. Passerini and A. Varzi, Energy Environ. Sci., 2023, 16, 1480–1501 CAS.
- H. Wu, J. Hao, Y. Jiang, Y. Jiao, J. Liu, X. Xu, K. Davey, C. Wang and S. Qiao, Nat. Commun., 2024, 15, 575 CrossRef CAS PubMed.
- Y. Dong, H. Hu, P. Liang, L. Xue, X. Chai, F. Liu, M. Yu and F. Cheng, Nat. Rev. Chem., 2025, 9, 102–117 CrossRef CAS PubMed.
- Q. Nian, J. Wang, S. Liu, T. Sun, S. Zheng, Y. Zhang, Z. Tao and J. Chen, Angew. Chem., 2019, 131, 17150–17155 CrossRef.
- Y. Sun, Y. Wang, L. Liu, B. Liu, Q. Zhang, D. Wu, H. Zhang and X. Yan, J. Mater. Chem. A, 2020, 8, 17998–18006 CAS.
- J. Hao, L. Yuan, C. Ye, D. Chao, K. Davey, Z. Guo and S. Qiao, Angew. Chem., Int. Ed., 2021, 60, 7366–7375 CAS.
- K. Zhu, Z. Sun, Z. Li, P. Liu, X. Chen and L. Jiao, Energy Storage Mater., 2022, 53, 523–531 Search PubMed.
- N. Chang, T. Li, R. Li, S. Wang, Y. Yin, H. Zhang and X. Li, Energy Environ. Sci., 2020, 13, 3527–3535 CAS.
- Y. Huang, J. Zhang, J. Liu, Z. Li, S. Jin, Z. Li, S. Zhang and H. Zhou, Mater. Today Energy, 2019, 14, 100349 Search PubMed.
- L. Zhong, Y. Lu, H. Li, Z. Tao and J. Chen, ACS Sustainable Chem. Eng., 2018, 6, 7761–7768 CrossRef CAS.
- J. Zhang, C. Cui, P. Wang, Q. Li, L. Chen, F. Han, T. Jin, S. Liu, H. Choudhary, S. R. Raghavan, N. Eidson, A. V. Cresce, L. Ma, J. Uddin, D. Addison, C. Yang and C. Wang, Energy Environ. Sci., 2020, 13, 2878–2887 RSC.
- Z. Wang, H. Li, Z. Tang, Z. Liu, Z. Ruan, L. Ma, Q. Yang, D. Wang and C. Zhi, Adv. Funct. Mater., 2018, 28, 180456 Search PubMed.
- T. Liu, X. Du, H. Wu, Y. Ren, J. Wang, H. Wang, Z. Chen, J. Zhao and G. Cui, Angew. Chem., Int. Ed., 2023, 62, e202311589 CrossRef CAS PubMed.
- Q. Gui, Y. Li and J. Liu, J. Colloid Interface Sci., 2024, 662, 119–128 CrossRef CAS PubMed.
- B. Dai, X. Shi, X. Pei, F. Xu and Y. Zhao, Energy Storage Mater., 2024, 66, 103231 CrossRef.
- Z. Liu, Q. Yang, D. Wang, G. Liang, Y. Zhu, F. Mo, Z. Huang, X. Li, L. Ma, T. Tang, Z. Lu and C. Zhi, Adv. Energy Mater., 2019, 9, 1902473 CrossRef CAS.
- Z. Wang, Y. Li, J. Wang, R. Ji, H. Yuan, Y. Wang and H. Wang, Carbon Energy, 2022, 4, 411–445 CrossRef CAS.
- X. Cao, L. Wang, J. Chen and J. Zheng, J. Mater. Chem. A, 2018, 6, 15762–15770 RSC.
- X. Wang, Z. Zhou, Z. Sun, J. Hah, Y. Yao, K. S. Moon, J. Di, Q. Li and C. P. Wong, Nano-Micro Lett., 2020, 13, 1–12 Search PubMed.
- C. Sun, Z. Han, X. Wang, B. Liu, Q. Li, H. Li, J. Xu, J. Cao and X. Wu, Adv. Funct. Mater., 2023, 33, 2305606 CrossRef CAS.
- D. Yu, H. Wang, J. Yang, Z. Niu, H. Lu, Y. Yang, L. Cheng and L. Guo, ACS Appl. Mater. Interfaces, 2017, 9, 21298–21306 CrossRef CAS PubMed.
- Y. Zhou, Z. Wang and Y. Lu, Mater. Today Energy, 2021, 19, 100570 CrossRef CAS.
- G. Tang, X. Zhang, B. Tian, P. Guo, J. Liang and W. Wu, Chem. Eng. J., 2023, 471, 144590 CrossRef CAS.
- J. Liang, B. Tian, X. Zhang, C. Jiang, D. He and W. Wu, J. Mater. Chem. A, 2023, 11, 3493–3503 RSC.
- B. Zhao, P. Jia, L. Yu, Y. Song, Z. Li, Y. Wang, R. Feng, H. Li, X. Cui, H. Cui, Y. Wang, M. Zhao, X. Zhao, X. Fang and Y. Pan, J. Energy Storage, 2023, 73, 109174 CrossRef.
- M. K. Shobana, J. Alloys Compd., 2020, 831, 154844 CrossRef CAS.
- J. Dong, G. Zhang, X. Wang, S. Zhang and C. Deng, J. Mater. Chem. A, 2017, 5, 18725–18736 RSC.
- S. Zhao, G. Li, Z. Li, K. Zhang, X. Chen, X. Dong, Y. Wang, Y. Cao and Y. Xia, Adv. Funct. Mater., 2024, 18, 2411007 Search PubMed.
| This journal is © The Royal Society of Chemistry 2025 |





