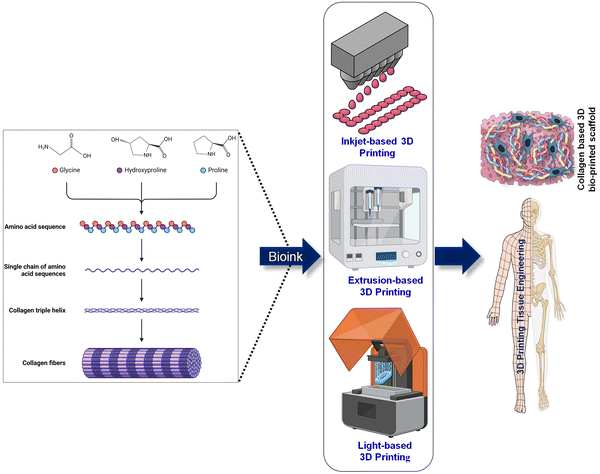
Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Collagen as a bio-ink for 3D printing: a critical review
Souvik
Debnath
 a,
Akhilesh
Agrawal
b,
Nipun
Jain
a,
Akhilesh
Agrawal
b,
Nipun
Jain
 a,
Kaushik
Chatterjee
a,
Kaushik
Chatterjee
 *ab and
Darren J.
Player
*ab and
Darren J.
Player
 *c
*c
aDepartment of Materials Engineering, Indian Institute of Science, C.V. Raman Avenue, Bangalore 560012, India. E-mail: kchatterje@iisc.ac.in
bDepartment of Bioengineering, Indian Institute of Science, C.V. Raman Avenue, Bangalore 560012, India
cCentre for 3D Models of Health and Disease, Division of Surgery and Interventional Science, Faculty of Medical Sciences, University College London, London, UK. E-mail: d.player@ucl.ac.uk
First published on 8th January 2025
Abstract
The significance of three-dimensional (3D) bioprinting in the domain of regenerative medicine and tissue engineering is readily apparent. To create a multi-functional bioinspired structure, 3D bioprinting requires high-performance bioinks. Bio-inks refer to substances that encapsulate viable cells and are employed in the printing procedure to construct 3D objects progressive through successive layers. For a bio-ink to be considered high-performance, it must meet several critical criteria: printability, gelation kinetics, structural integrity, elasticity and strength, cell adhesion and differentiation, mimicking the native ECM, cell viability and proliferation. As an exemplar application, tissue grafting is used to repair and replace severely injured tissues. The primary considerations in this case include compatibility, availability, advanced surgical techniques, and potential complications after the operation. 3D printing has emerged as an advancement in 3D culture for its use as a regenerative medicine approach. Thus, additive technologies such as 3D bioprinting may offer safe, compatible, and fast-healing tissue engineering options. Multiple methods have been developed for hard and soft tissue engineering during the past few decades, however there are many limitations. Despite significant advances in 3D cell culture, 3D printing, and material creation, a gold standard strategy for designing and rebuilding bone, cartilage, skin, and other tissues has not yet been achieved. Owing to its abundance in the human body and its critical role in protecting and supporting human tissues, soft and hard collagen-based bioinks is an attractive proposition for 3D bioprinting. Collagen, offers a good combination of biocompatibility, controllability, and cell loading. Collagen made of triple helical collagen subunit is a protein-based organic polymer present in almost every extracellular matrix of tissues. Collagen-based bioinks, which create bioinspired scaffolds with multiple functionalities and uses them in various applications, is a represent a breakthrough in the regenerative medicine and biomedical engineering fields. This protein can be blended with a variety of polymers and inorganic fillers to improve the physical and biological performance of the scaffolds. To date, there has not been a comprehensive review appraising the existing literature surround the use of collagen-based bioink applications in ‘soft’ or ‘hard’ tissue applications. The uses of the target region in soft tissues include the skin, nerve, and cartilage, whereas in the hard tissues, it specifically refers to bone. For soft tissue healing, collagen-based bioinks must meet greater functional criteria, whereas hard tissue restoration requires superior mechanical qualities. Herein, we summarise collagen-based bioink's features and highlight the most essential ones for diverse healing situations. We conclude with the primary challenges and difficulties of using collagen-based bioinks and suggest future research objectives.
1. Introduction
Since the conception and development of 3D printing, the globe has seen a tremendous rise in the field of bioprinting technology and tissue engineering.1 3D printing was first introduced in 1984 by Charles Hull, who printed 3D objects using stereolithography, which signified the birth of 3D printing. Following this, bioprinting by cytoscribing technology was first demonstrated in 1988 by Klebe using an inkjet printer. It was not until the year 2002 when extrusion-based bioprinting came into existence, with the development of bioplotter. After that, 3D bioprinting was explored in a wide spectrum of biomedical applications. The fabrication of constructs with complex geometries by 3D bioprinting enables the rapid production of structures loaded with biological molecules or cells,2 and is now used for the fabrication of highly complex tissues and organs. It is possible for 3D bioprinting to bio-imitate true tissue architecture, which makes it valuable in disease modelling, drug research and testing, high-throughput screening, and regenerative medicine. This is due to the tuneable bioink formulations and the applied manufacturing processes.3 The amalgamation of cell biology, material science, and health sciences has been pivotal in bridging 3D printing with tissue engineering to form a new area of research and development: 3D bioprinting.4 The advent of this rapidly developing field follows the adoption of ‘conventional’ 3D printing for use in medical devices, such as stents, implants, splints, etc., which are now commonly used in clinical practice.5,6An important development associated with 3D bioprinting is the enhancement of structural and functional indicators utilised in tissue engineering and regenerative medicine.7,8 However, there are still apparent shortfalls. In order to achieve in vivo translation, it is necessary to address the issues of lengthy processing durations, postprocessing modification, and the poor mechanical characteristics of the scaffold, which are important considerations for many clinical applications.9 3D bioprinting, an innovative technique performed in situ within the operating room on patient tissues, is designed to address specific medical requirements. Consequently, it signifies a novel paradigm in personalised medicine.8,10,11
A highly suitable 3D bio-printed scaffold requires a biomaterial ink with substantially good, necessary rheological, structural, chemical, and biological properties to build a new extracellular matrix (ECM) similar to the native tissue.12–15 Several future biomaterial ink qualities must be considered to create a 3D bio-printed scaffold that mimics biological tissue's ECM. These properties allow the 3D printing scaffold to enable cell development and tissue creation while mimicking the target tissue's mechanical and metabolic environment. The ink's viscosity must allow smooth extrusion during printing and retain the scaffold's form after deposition. For example, shear-thinning ink makes it simpler to flow through the printer nozzle and regains viscosity after printing. Post-printing, a controlled gelation period allows the ink to solidify quickly enough to maintain structural integrity without clogging the printer or adhering to previously printed layers. In order to support suitable tissue scaffold development, the bioink needs to have an appropriate storage modulus (G′) for elasticity and mechanical strength. Furthermore, optimising yield stress prevents the material from flowing under its own weight after printing, retaining the scaffold's intended design. For in vivo applications and to represent native physiology, the scaffold must be strong enough to withstand target tissue physiological stresses (e.g. mechanical loading).
The bioink must also be biocompatible, facilitate cellular adhesion, proliferation, and differentiation, and not release cytotoxins. The scaffold should deteriorate at the same rate as new tissue creation, to facilitate tissue regeneration and progressively shift load to newly produced tissue. A bioink with balanced rheological, structural, chemical, and biological characteristics is needed to create a 3D bioprinted scaffold that replicates the natural tissue ECM. These factors must be carefully adjusted to fit the target tissue's needs so the scaffold can support tissue regeneration, retain its structural integrity, and integrate with the host tissue.
To functionally customise 3D bioprinting scaffolds for human tissues, topologies and bioinks must be chosen. The efficacy of the 3D bioprinted scaffolds relies on their structural arrangement, the selection of bioinks, and the particular needs of the desired tissue. 3D bioprinted scaffolds have a network of pores for nutrition transport, and cell migration. For example, for bone scaffolds to facilitate osteogenesis, tissue vascularization and integration requires the use of a porous material. To this end, controlling layer-by-layer material deposition permits sophisticated architectures that mimic biological tissue hierarchies.
Using 3D bioprinting, it is also possible to develop scaffold structures with micro- and nano-scale characteristics, to improve cell adhesion and proliferation. These characteristics can mimic the natural ECM's topography, guiding cell behaviour and tissue development.
Since 3D printing technology has improved, scaffold building has been supported in numerous ways. All 3D printing methods require a bioink, which can be formulated and deployed in numerous ways.16,17 By definition, bioinks must be biocompatible for tissue culture and transplantation applications, however, such inks must also satisfy the requirements for the printing process directly. Bioinks that possess multiple functions in order to accommodate the intricate and diverse nature of printed scaffolds will be highly sought after. Such bioinks would facilitate appropriate pre-clinical evaluation and in vivo tissue regeneration.18–21 Indeed, an optimum bioink specific to each tissue will revolutionise 3D bioprinting, and with researchers, doctors, and patients eagerly anticipating the impact.
In order to design an appropriate bioink for 3D printing, the choice of material becomes important as it should represent the properties of native ECM. One of the most promising candidates is collagen, which constitutes 25% of the proteins in mammals and is a salient component of the ECM.21,22 Collagen organises itself into a highly ordered 3D network to support cellular behaviour and tissue function. As of now, a comprehensive understanding of the human body's tissues and organs has yielded 28 distinct types of collagen, of which Types I, II, III, and V constitute the principal extracellular matrix (ECM) components of diverse structures, including cartilage, skin, tendons, bone, muscles, and cartilage.23,24 The outstanding properties of collagen make it an excellent biomaterial in regenerative medicine with its innate ability to facilitate cell signalling, dynamic mechanical behaviours, constant remodelling to guide physiological functions, etc.25–29
Since the 1950s, collagen has been investigated and received widespread attraction in the field of biotechnology as a natural biomaterial. Historical context points towards palaeontologists, who found collagen in Tyrannosaurus rex fossils from around 68 million years ago.30,31 Thus, it underscores the evolutionary conserved nature of this structural protein. In 1881, surgeons Joseph Lister and William Macewen developed sheep intestine-based collagen sutures, introducing collagen as a biomaterial. Collagen was first used as a cell growth matrix to help cells grow in 1956, but it was not until 1993 that the FDA approved the first bone graft made of collagen.30,31 The biological milestones associated with collagen research (Fig. 1, as a means to provide context on the developments in this area). It has constantly emerged as an outstanding biomaterial, owing to its excellent biocompatibility and ability to modulate the physiological processes of the cells.32 More recently, 3D printing of collagen and its composites through various have supported the development of bioactive scaffolds with a high potential for clinical translation. The synergistic properties of the biological function of collagen and 3D printing technology to construct a scaffold mimicking the structure and composition of a specific tissue, becomes a potent strategy to achieve desired outcomes.
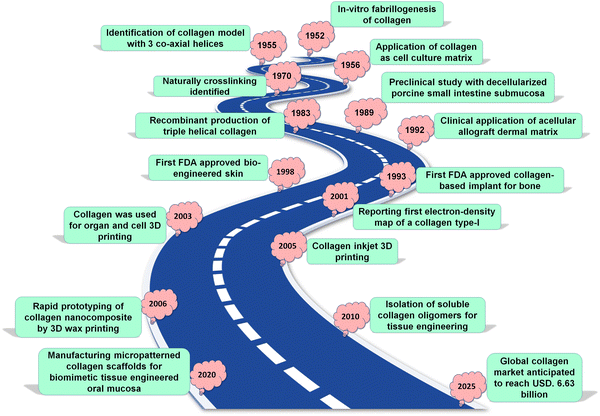 | ||
| Fig. 1 Historical perspective: significant achievements linked to collagen in the biomedical engineering domain. | ||
Collagen-based bioink's photocuring properties enable scaffolds with more precise geometries for tissue fidelity. Biodegradable collagen-based bioink controls tissue regeneration and degradation.33 In this review, we aim to summarise state of the art surrounding the use of collagen in 3D bioprinting applications. In particular, the focus will be on collagen-based bio-inks and collagen-based derivatives and the 3D bioprinting parameters to support physicochemical, mechanical, and biological qualities.
Previous literature reviews have mainly emphasised the sources of collagen and various properties of collagen34 and the utility of collagen-based scaffolds for tissue engineering and regenerative medicine.35,36 There still remains a scope to summarise the current 3D printing technologies and how collagen can be manipulated to develop the next generation bioinks for constructing natural tissues and organs. In this review, we have particularly focused on the development of such bioinks, as well as various 3D printing techniques of collagen bioinks for engineering hard and soft tissue regeneration, including bone, cartilage skin, and neural tissue. Ultimately, the opportunities and challenges to orchestrate collagen for 3D bioprinting of biomimetic constructs are also discussed, to pave the way forward in leveraging collagen as an advanced biomaterial for clinically relevant outcomes.
2. Source of the collagen
Many different methods have been researched by scientists in order to purify or synthesise chemicals that are based on collagen. A comparison of natural and synthetic collagen synthesis is shown in (Fig. 2A). In addition to humans, bovines and pigs are the primary sources of collagen, particularly collagen type I. The abundance of collagen can be attributed to the fact it is a large structural component of organs and tissues. The dermis, tendon, and bone are all examples of fibrous collagen-rich tissues that are considered to be the best providers of collagen although purified collagen has also been obtained from human peripheral nerve tissue37 and placenta.38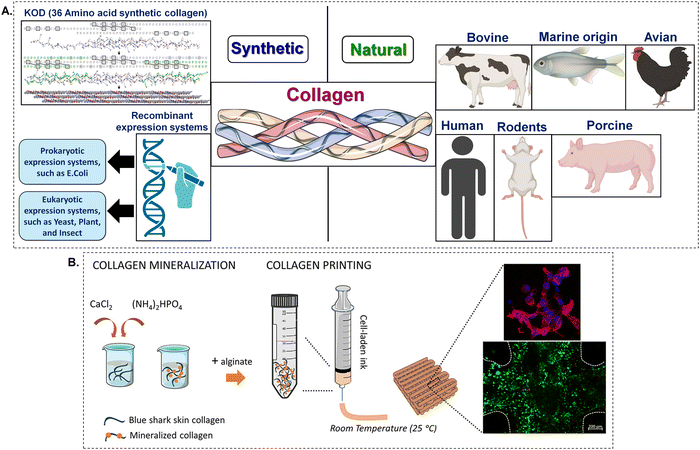 | ||
| Fig. 2 (A) The sources of collagen (B) 3D printed hydrogels made from shark-skin collagen, which are biomimetically mineralised and include living cells, are used for engineering hard tissues.39 Created with BioRender.com. | ||
Some of the other animal sources of collagen include chicken, sheepskin, alligator bone and skin, rat tail tendons, horse tendons, bird feet, duck feet, and frog skin. In spite of the fact that these sources are relatively inexpensive and simple to collect, there may be concerns over allergic reactions and infections if applied to in vivo contexts. There are a number of fundamental problems connected with collagen generated from animals, such as the fact that the quality of the collagen varies from batch to batch, the necessity of adhering to good manufacturing procedures (GMP) in order to support quality and prevent infections, and the moral and ethical concerns that are associated with harvesting tissues from animals.38,40 Therefore, the biological use of collagen derived from animal tissue may be limited due to the presence of disease-causing impurities and the potential for allergic responses.41
Thus, researchers have investigated recombinant systems and polymer manufacturing for collagen molecules.41–45 Recombinant human collagen has been produced in yeast, E. coli, mammalian cells, insect cells, tobacco plants, and maize seeds, but replicating proline hydroxylation has been the hardest obstacle. Recombinant human collagens provide bulk collagen production promise. It was in 1980 that Uitto et al. were able to manufacture type I and type III pro-collagen from human skin fibroblasts that had been grown in vitro under suitable conditions.46,47 Synthetic collagen may be manipulated to possess a diverse array of viscosities, rendering it versatile for various 3D printing and bioprinting methodologies. This is especially crucial in extrusion-based 3D bioprinting, since the material needs to have sufficient fluidity to be printed, while also maintaining enough solidity to retain its shape after being deposited. Synthetic collagen can also demonstrate shear-thinning characteristics, wherein its viscosity lowers when subjected to shear stress, hence enabling seamless extrusion in the process of 3D printing. Once the tension is alleviated, the viscosity subsequently rises, so aiding in the preservation of the structure's integrity.
Adjusting synthetic collagen concentration and gelling conditions like temperature and pH can change its rheology, with applications that require specified gelation periods and consistencies need such control of the biomaterial. Synthetic collagen can be engineered to possess precise mechanical properties, varying from pliable and resilient frameworks suitable for soft tissues to sturdier constructions suitable for bone and cartilage. Synthetic collagen can also match natural collagen's triple-helical structure, which is necessary for its mechanical qualities and cell contact and with this can provide a supporting ECM that closely resembles biological tissue due to structural similarity. Through the utilisation of recombinant technology, an additional synthetic collagen source is able to provide collagen that is of superior quality, obtained from animals, and free of contaminants. One of the enzymes that is required for the folding of collagen polypeptide chains that have been generated into triple helical structures is called prolyl-4-hydroxyproline, which is sometimes referred to as P4H: a heterotetramer.48 P4H activity is modest in insect cells and non-existent in bacteria and yeast. However, recombinant collagen polypeptide chains generate unstable triple helices at low temperatures, rendering them non-functional. There are a number of downsides associated with recombinant technology, including high costs, low yields, and the absence of cofactors and enzymes. Similarly, the utilisation of accessible bovine and porcine collagen also present a number of challenges, which include (i) the possibility of zoonotic transmission, (ii) the possibility of inducing an immunological response, and (iii) the possibility of generating an allergic reaction, as well as (iv) a purification process that is both complicated and expensive.49,50 Although recombinant human collagen has the ability to overcome the majority of these restrictions, its utilisation is limited due to expensive production costs and poor output. Given these constraints and an increasing desire for collagen, it is imperative to find other means of obtaining collagen.
Marine collagen is appealing because it has fewer health concerns than mammalian collagen.51,52 Additionally, solid marine trash is rich in collagen. This source of collagen is biocompatible and functions like mammalian collagen without constraints because the collagen genetic sequence is preserved and comparable across species.53 However, because marine tissues do not contain mammalian antigens like alpha-gal, persons who are allergic to mammalian proteins should avoid using marine collagen sources.54
Recently, there have been efforts made to utilise marine collagen for the purpose of extrusion-based three-dimensional bioprinting. Investigations have been conducted into a wide variety of techniques in order to enhance the capability of printing bioinks that are derived from marine collagen. The addition of an additional polymer network for support and the modification of the original collagen to offer chemical cross-linking, are two examples of these improvements.55
Bioinks have been developed by two different research groups by the combination of collagen derived from blue sharks and eels with the biocompatible polymer alginate. Due to of the ionic crosslinking of alginate, this combination makes it possible to use bioprinting to create structures that have improved stability and mechanical strength56,57 (Fig. 2B). In addition, it has been demonstrated that the incorporation of fish skin collagen into a bioink formulation that also includes methacrylated hydroxybutyl chitosan results in the formation of a surface that is advantageous for the adherence and proliferation of cells.58 In contrast, Sanz et al. created UV-cross-linkable collagen from Red Snapper by attaching methacrylate functional groups. The researchers showed that the use of a chemical crosslinking technique following extrusion bioprinting greatly improved the ability to print and the structural integrity of the collagen material.59 Furthermore, chemical crosslinking will make bio-printed scaffolds with cells more robust. This is due to the fact that the Td (denaturation, temperature) of marine collagen is typically lower than the temperature of the human body.
Different sources of collagen significantly affect printability. These discrepancies result from changes in the molecular structure, purity, biocompatibility, and extraction and preparation processes. The triple-helical structure of collagen derived from animals is often well-preserved and resembles that of human collagen, which makes it ideal for use in biomedical applications. Its ability to maintain structural integrity makes it appropriate for printing applications as it generally exhibits good printability. Nevertheless, the method used for extraction and purification can affect the viscosity and gelation characteristics. In contrast, marine collagen is more thermally sensitive, which affects its stability and printability at wider temperature ranges. Although it poses challenges in maintaining the shape and integrity of the printed constructs. It also makes it easier to print at lower concentrations. However, this can result in weak mechanical properties if not suitably cross-linked. The printability of recombinant collagen can be manipulated, but for the formulation to replicate the mechanical characteristics of natural collagen, several modifications could be necessary. For example, the amino acid sequence can be changed to improve stability or crosslinking potential.
2.1. Challenges of animal-derived collagen
Animal-derived collagen is commonly utilized in biomedical tissue-engineering applications because it is compatible with living organisms, may naturally break down over time, and has the capacity to enhance the attachment and development of cells. Nevertheless, its utilization is accompanied with several challenges and disadvantages:Collagen generated from animals has the potential to elicit an immunological response in humans, resulting in inflammation or rejection. This issue becomes more troublesome when the collagen is not well purified and still contains non-collagenous proteins or other contaminants that are identified as foreign by the human immune system. Collagen obtained from animals has a potential danger of transmitting zoonotic illnesses, such as bovine spongiform encephalopathy (BSE) or prions, particularly when received from bovine origins.
This gives rise to safety problems in medical applications. The variability of collagen obtained from animals can differ considerably among different batches, contingent upon the origin of the animal, its age, and the method of extraction. This unpredictability has the potential to impact the repeatability and performance of biomedical products. The utilization of products obtained from animals gives rise to ethical considerations pertaining to the well-being of animals. Moreover, certain animal products may be subject to religious constraints, hence restricting their suitability within specific communities. Collagen originating from animals frequently has worse mechanical qualities in comparison to synthetic alternatives or collagen obtained from different sources. This constraint can restrict its utilization in load-bearing applications or situations that need significant mechanical strength.
Collagen generated from animals may undergo rapid degradation in the body, depending on the specific location where it is applied. The fast deterioration can result in a decline in structural strength and functional characteristics prior to complete tissue regeneration. Cross-linking is frequently necessary to improve the mechanical characteristics and stability of collagen. Nevertheless, attaining precise and consistent cross-linking in collagen obtained from animals can be difficult, and incorrect cross-linking can lead to diminished biocompatibility and heightened toxicity. The process of producing collagen from animals requires a large amount of resources and has a substantial negative impact on the environment due to activities like animal husbandry, which contribute to issues like land usage, water consumption, and greenhouse gas emissions. This creates apprehensions over the long-term viability of using collagen obtained from animals on a significant magnitude. To improve the mechanical qualities and stability of collagen obtained from animals, it is common to use chemical cross-linking. However, if not well managed, this process can lead to cytotoxicity and decreased biocompatibility. Moreover, when collagen is cross-linked, it can acquire enhanced resistance to breakdown, thereby impeding its ability to integrate with the surrounding host tissues.
The manufacturing of animal-derived collagen is a laborious process that requires significant resources, as it entails the breeding, killing, and processing of animals. This not only gives rise to worries about sustainability but also adds to the environmental impact of biomedical goods.
2.2. Recombinant collagen technologies
Recombinant human collagen is used in 3D bioprinting because to its biocompatibility, minimal immunogenicity, and adaptable biochemical and mechanical characteristics. Through the use of tailored supramolecular assemblies, crosslinking densities, and matrix stiffness, research has modified recombinant human collagen to imitate natural extracellular matrices. This alteration allows for the manipulation of cellular microenvironments and cell destiny.Furthermore, the use of recombinant human collagen allows for the integration of cell-adhesive peptides, growth factors, and cytokines to influence cellular activity. At present, bioinks derived from recombinant human collagen are employed for the purpose of bioprinting various tissue architectures, including skin, cartilage, bone, and blood vessels. Recombinant human collagen bioinks hold significant potential for developing complex and varied tissues that closely resemble the structures, compositions, and functions observed in nature. Nevertheless, there are still obstacles to overcome in order to achieve large-scale production of recombinant human collagen and to create universally applicable crosslinking procedures that can improve the precision of printing. Continuing multidisciplinary research is crucial for enhancing the designs of recombinant human collagen, comprehending crosslinking mechanisms, and advancing printing techniques. This is necessary to effectively use 3D bioprinted tissues and organs in clinical environments.
Using recombinant human collagen instead of animal-derived collagen has many advantages: (1) safety: recombinant human collagen is generated in a controlled environment without animal tissues, reducing the risk of zoonotic disease transmission and immunogenic responses; (2) uniformity: recombinant human collagen can be rigorously managed to maintain batch-to-batch uniformity, unlike animal-derived collagen, which has intrinsic biological variability; (3) customisation: recombinant human collagen may be genetically changed to incorporate specific amino acid sequences or post-translational changes, unlike animal-derived collagen. This enables for collagen with tailored properties for certain uses. (4) Ethical considerations: the creation of recombinant human collagen eliminates the ethical issues linked to the use of animal-derived goods.
The use of recombinant human collagen in 3D bioprinting has gained significant attention because of its biocompatibility, minimal immunogenicity, and ability to be customised in terms of biochemical and mechanical characteristics. Researchers have manipulated recombinant human collagen to create customised supramolecular structures, crosslinking densities, and matrix stiffnesses that closely mimic natural extracellular matrices. This allows for accurate manipulation of microenvironments to guide cell fate processes. Furthermore, the use of recombinant collagen enables the inclusion of cell-adhesive peptides, growth factors, and cytokines to regulate cellular behaviours. At now, scientists have used bioinks made from recombinant human collagen to 3D print tissue structures including cartilage, bone, and blood vessels.
In the future, the use of recombinant human collagen bioinks shows enormous potential for creating intricate and diverse tissues that closely resemble natural structures in terms of their design, composition, and functionality. Nevertheless, there are still obstacles to overcome in achieving large-scale manufacturing of recombinant collagen and in creating universally applicable methods for improving the accuracy of 3D printing. It is crucial to do more multidisciplinary research to enhance the design of recombinant human collagen, understand crosslinking mechanisms, and improve printing techniques. This research is necessary to facilitate the widespread use of 3D bioprinted tissues and organs in clinical settings.
2.3. Advantages of marine collagen for 3D bio-printing
Marine collagen is becoming increasingly important in the field of 3D printing for tissue engineering because of its distinct features and benefits compared to collagen obtained from mammals.53 The features of the scaffolds, both mechanical and biological, have an impact on the shape, behaviour, and function of cells. Marine collagen, which is collagen obtained from marine species, has several benefits compared to collagen from mammals.50,54 These advantages include its capacity to work well with living tissues, biocompatibility, ease of extraction, ability to dissolve in water, safety, biodegradability, low likelihood of causing an immune response, and low costs of manufacturing.Marine collagen, similar to collagen found in mammals, aids in the attachment, growth, and specialisation of cells, which are essential for the renewal of tissues. Due to its decreased immunogenicity in comparison to mammalian collagen, it is a safer choice for incorporating into 3D-printed tissue structures.52 This helps to minimise the likelihood of adverse immune responses. While marine collagen often has a reduced likelihood of causing allergic responses, it remains crucial to carefully evaluate the origin and processing techniques to minimise any potential allergenicity, particularly in persons with heightened sensitivity.54
Marine collagen is commonly obtained from the leftover parts of fish, such as their skin, scales, and bones, which makes it a more environmentally friendly choice when compared to collagen generated from mammals.52,54 This is consistent with the increasing focus on ecologically sustainable techniques in the field of biomedical research and industry. Utilising marine collagen circumvents the ethical dilemmas linked to materials generated from animals, rendering it more suitable for certain uses, particularly in areas or societies where the utilisation of animal products is limited or disapproved. Marine collagen is becoming more often employed as a bioink in 3D printing because it has the capacity to build stable gels that can be accurately placed to construct intricate tissue formations. It can be utilised independently or in conjunction with other biomaterials to improve mechanical characteristics and biological performance.53
Marine collagen is utilised in 3D printing to fabricate scaffolds that replicate the ECM of different tissues. These scaffolds have the ability to facilitate the development and specialisation of cells, making them well-suited for use in the field of tissue engineering for skin, cartilage, and bone.51,53,55 Marine collagen's adaptability in 3D printing enables the production of tissue constructions that are tailored to individual patients. This is especially advantageous for personalised medicine, since it allows for the development of customised therapies and implants that are specifically designed to meet the unique demands of each individual patient.56
An obstacle in using marine collagen for 3D printing is its worse thermal stability in comparison to mammalian collagen. This constraint can restrict its use in applications that need high-temperature processing. Current research is dedicated on improving the thermal characteristics of marine collagen by means of chemical changes or combining it with other substances.
Although marine collagen is appropriate for soft tissue engineering, its mechanical characteristics may not meet the requirements for load-bearing applications. Scientists are investigating methods to enhance the strength and durability of marine collagen scaffolds by using other biomaterials. Marine collagen has great promise in the field of 3D printing for tissue engineering due to its benefits in terms of biocompatibility, sustainability, and ethical issues.51,52,54
The use of bioinks and 3D scaffold construction is facilitating the progress of creating sophisticated tissue constructs, which have the potential to significantly transform the field of regenerative medicine.54 Its distinctive characteristics render it well-suited for a variety of 3D bioprinting applications, specifically in the regeneration of skin, bone, and cartilage.53,56 Nevertheless, it is imperative to tackle the obstacles associated with thermal stability and mechanical qualities in order to fully exploit its capabilities in more rigorous applications.
3. 3D bioprinting methods for fabricating collagen-based scaffolds
3D bioprinting techniques have been effectively employed to construct several distinctive scaffolds for tissue modelling, replacement, and regeneration. It is predicated on its distinctive capability to manipulate intricate 3D structures into useful scaffolds for cells to reside and tissue to form.39,57 For this to be a success, bioinks need to display several basic features such as accuracy in printability, structural integrity, stability, and biocompatibility.58,59 Also, each bioink composition has its unique set of “optimal” printing settings such as printing temperature, pressure, print speed, flow rate, layer thickness, the cross-linking time, exposure to light intensity, and bed adhesion to produce complex 3D architectures with appropriate precision. The 3D scaffold created by careful deposition and assembly of biological and non-biological materials can design a structure according to the requirements of a given tissue. Furthermore, it is also possible to customise the manufacture of tissue for patient-specific requirements – e.g. personalised bone repair in the context of trauma. This method has created opportunities beyond traditional ones by creating functional, customisable, and reproducible constructs that can eventually support the regeneration of various tissues. As a suitable biomaterial, collagen fibres offer high surface area and elasticity, supporting alignment of collagen extrusion threads during printing processes.Beyond conventional programming of printing parameters, Machine learning (ML) is emerging as a powerful tool for optimizing 3D bioprinting, helping to address the complexity and variability inherent in bioprinting processes. ML algorithms can support the analysis of large printing parameter repertoires (temperature, pressure, speed) and associated outcome datasets. This allows predictive algorithms to optimise these parameters in real time, to increase print fidelity, eliminate errors, and strengthen printed constructions. ML can predict bioink behaviour under diverse settings, helping to choose the optimum materials and conditions for applications.
Real-time printing can also be monitored using ML, using image analysis which can help to identify print flaws and make rapid adjustments, accordingly. Therefore, although still emerging, ML will become indispensable in 3D bioprinting applications, offering tools that enhance precision, reduce costs, and improve the overall success of tissue engineering projects.
With the considerations of printing parameters in focus, the rheological characteristics of any biomaterial play a crucial role in determining its behaviour and suitability for use. Collagen has important rheological characteristics such as viscosity, gelation behaviour, shear-thinning behaviour, and viscoelasticity. Furthermore, the viscosity of collagen is affected by several parameters including concentration, temperature, pH, and the presence of crosslinking agents. In this regard, these features impact the behaviour of collagen when subjected to stress, its flow characteristics, and structural integrity once printed.
Gelation is the transformation of collagen from a liquid state (sol) to a solid state (gel). The gelation time is a critical factor as it dictates the speed at which the printed collagen scaffold can harden and retain its shape. For optimal 3D printing of collagen, both temperature and pH must be carefully controlled to ensure the material maintains its integrity and functionality. Collagen also changes its structure according to temperature and will likely deteriorate with time.60 To avoid gelation, collagen is stored and handled at 4 °C, with extrusion also controlled at 4 °C to during printing To gel and crosslink, collagen can be warmed to physiological temperatures (about 37 °C) at neutral pH to attain a fibrous structure after 3D printing.61 When using isolated collagen in vitro, a large drop in temperature changes it to a liquid form, whereas high temperature. This temperature range preserves the triple-helical structure of collagen, which is essential for bioactivity and mechanical qualities.
Collagen is most stable and keeps its native structure at pH 7.2 to 7, with bioactivity and cell interaction supported in this pH range. Some 3D printing processes dissolve collagen in acidic conditions (pH 3–4) to keep it in solution, with neutralisation to physiological levels after printing to promote gelation and biocompatibility. Therefore, the pH and temperature must be carefully adjusted during printing and ink preparation before employing collagen as a bioink61 (Fig. 3).
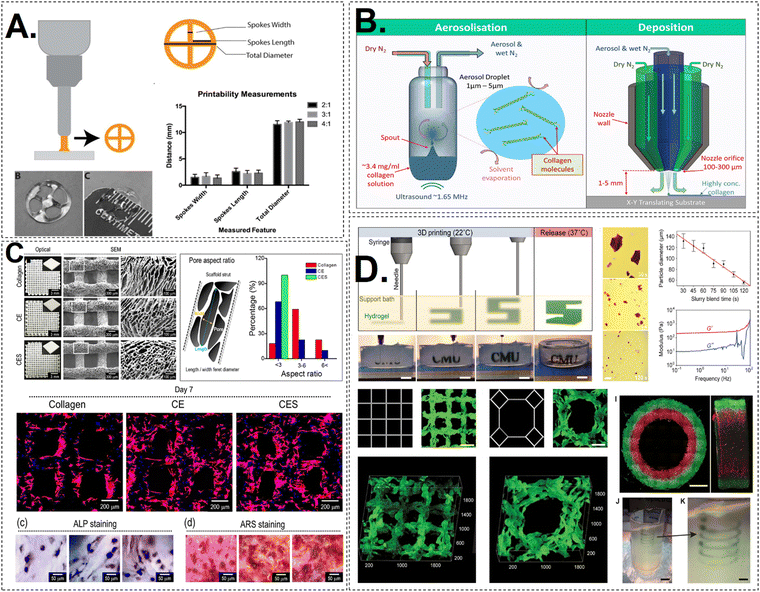 | ||
| Fig. 3 (A) A schematic representation of extrusion printing of a wheel structure. Different formulations of bioink were studied and they showed same uniformity and printing ability.62 (B) SEM images of the printed constructs of collagen and COL/dECM/Silk fibroin. The surface pore size and cellular morphology was studied and compared accordingly.63 (C) An illustration of Free form printing of collagen bioink in the suspension bath of gelatin particles. The 3D construct is printed in a layer by layer fashion. Samples stained with ALP and ARS probes after 7 days of cell culture.64 (D) A diagrammatic representation of aerosol jet printing. A ultrasound wave is used to produce aerosol particles which are then transferred on the printing substrate which results in deposition of highly concentration of ink. The FRESH material is produced in fluorescent alginate (green) using a conventional square lattice pattern designed for infill in 3D printing. It is then viewed in three dimensions from the top down. An octagonal infill pattern designed for FRESH printing in fluorescent alginate (green) and visualised in three dimensions. Illustrative of a two-material print consisting of coaxial cylinders made of red and green fluorescently labelled alginate, with a seamless interface, as seen in both top-down and lateral cross sections. A freeform, nonplanar FRESH print of a helix is shown submerged in a gelatin support bath. An enlarged perspective of the helix illustrating that FRESH has the capability to print in really freeform shapes and is not restricted to the conventional method of layer-by-layer planar manufacturing. Scale bars with dimensions of 1 mm, 500 μm, 2 mm, 10 mm, and 2.5 mm.65 | ||
In order to enable 3D printing, it is crucial to meticulously regulate the viscosity of collagen so that it can be smoothly extruded through the printer nozzle and retain its form once it is deposited. Collagen commonly displays shear-thinning behaviour, meaning that its viscosity reduces as the shear rates increase. The attribute facilitates its use in 3D printing with the ability to exhibit fluidity while passing through the nozzle, yet promptly restore its thickness and structural integrity upon deposition. In contrast, collagen offers limited mechanical strength and has slow self-assembly, causing a delay in gelation speed, ultimately leading to the collapse of the printed structure. Thus, an effective cross-linking method is required with limited use of toxic reagents to preserve the structure that can work in harmony to promote cell growth and construct stability.62,66
Another important mechanical property to consider is the storage modulus (G′), which quantifies a material's elastic behaviour or its capacity to accumulate energy while undergoing deformation. In the context of collagen in 3D printing, a greater storage modulus signifies a scaffold with increased stiffness, enabling it to effectively retain its shape when subjected to mechanical stress. In 3D printing applications of collagen, an adequate yield stress keeps the material immobile until enough pressure is applied, allowing accurate layer-by-layer deposition. Collagen's viscosity, gelation time, elasticity, and yield stress determine its appropriateness for tissue engineering 3D printing. The printability and mechanical performance must be carefully balanced by tuning these features.
Collagen also changes its structure according to temperature and will likely deteriorate with time.66 When using isolated collagen in vitro, a large drop in temperature changes it to a liquid form, whereas high temperature or neutral pH forces it to attain a fibrous structure.65 Therefore, the pH and temperature must be carefully adjusted during printing and ink preparation before employing collagen as a bioink.67 Collagen also has very low viscosity, although this can be manipulated using various processing methodologies. Thus, an effective cross-linking method is required with limited use of toxic reagents to preserve the structure that can work in harmony to promote cell growth.66 Also, special attention should be paid to the elastic modulus, which can again strike a balance between mechanical integrity and viability. As a result, producing highly accurate 3D bio-printed collagen scaffolds becomes difficult.65,68
Collagen scaffolds are often mixed with certain natural or synthetic polymers to enhance the rheological parameters for developing an efficient structure in a reproducible way and scalability at a minimal cost. Printing onto a sacrificial support gel is another strategy that can be employed63 (Fig. 3). The flexibility within the materials presents a standardisation challenge as multiple combinations are possible with various cross-linkers, alterations, and distinct constituent ratios. Next, optimisation must be done to find effective printing conditions, cell concentration, cross-linking time, etc.69,70
3.1. Extrusion-based printing
This technique offers high flexibility as it allows a vast number of materials that can be printed with multiple cell densities. The bioink is squeezed from the printer nozzle, and fibres align due to the extensional motion brought on by the applied pressure and shear flow.6 Process factors, such as vessel diameter, flow rate, and needle gauge, must be modified to get characteristics that closely resemble those of the desired extracellular matrix (ECM).71 High resolution is achieved using small-diameter nozzles; however, this exacerbates the shear stress applied, which can reduce cell viability. It is also necessary to prevent structure loss and manage gel characteristics like swelling and degradation.69,70 Collagen, when extruded, undergoes fibrillogenesis that allows deliberate gel transformation. However, during this process, there is a time delay that causes the deposited print line to expand and flow, resulting in inadequate print quality, making it challenging to accomplish several layers.54 However, it is possible to print several overlying layers when the collagen bioink concentration is high. The knowledge of the rheological parameters and associated printing conditions forms the basis of successful extrusion bioprinting.With extrusion bioprinting, it is also possible to combine collagen with other ECM components, such as Matrigel, which can be seen in (Fig. 4). Collagen and Matrigel 3D printing in hard tissue engineering uses their capabilities to generate more effective and biomimetic tissue constructions. Matrigel, a gelatinous protein mixture made from Engelbreth–Holm–Swarm (EHS) murine sarcoma basement membrane proteins, contains laminin, collagen IV, entactin, and growth factors. It mimics native ECM, promoting cell adhesion, differentiation, and proliferation. The combination of collagen and Matrigel yields a bio-ink structure that offers enhanced cellular support. The incorporation of growth factors and ECM proteins in Matrigel contributes to the augmentation of the collagen bioactivity, hence facilitating improved cell survival and proliferation. In certain instances, collagen alone may exhibit insufficient mechanical qualities to provide robust 3D printing. The incorporation of Matrigel into the bio-ink has the potential to augment its rheological characteristics, hence enhancing its printability without compromising its biocompatibility. The integration of collagen and Matrigel results in the establishment of a microenvironment that is more physiologically appropriate for cells, closely resembling the ECM present in natural tissues.
Cell-embedded constructs can survive the shear forces generated and formed cell clusters, while the collagen appeared as a fibril structure.63,72 Similar to the previous example, a collagen and Pluronic F-127 mixture was extruded in a temperature-controlled system, which enabled the crosslinking process to be tuned by varying the temperature and the amount of time it was permitted to take place. In this, the collagen fibres are able to orient along the direction of the printed filament.73
Aerosol jet printing (AJP) is another bioprinting technology that can be used to prepare scaffolds from both collagen types I and II to replicate the ECM of native tissue. Aerosol jet printing uses a carrier gas to atomise a liquid material like nanoparticle-containing ink into a fine aerosol mist that is directed through a nozzle. The aerosol is focussed into a narrow jet and placed on a substrate precisely. Aerosol jet printing can deposit thin lines and complicated patterns with excellent resolution. Aerosol jet printing differs from extrusion-based 3D printing in principle, application, and resolution. In this method, the gas flow confines and collimates the aerosol as it gets closer to the nozzle, thus creating an intense stream of droplets. This gives the structure a stiffness that cannot be achieved through covalent cross-linking. Collagen type II mixtures are less viscous and less vulnerable to structural deformities when any stress is applied during printing when compared to type I collagen. Subsequently, chemical cross-linking could be utilised to increase the structures' stiffness in order to mimic the ECM tissue. A more thorough comprehension of the post-print neutralisation and other print programs could be used to replicate the weaker collagenous tissues more closely74 (Fig. 3).
Hybrid collagen/chitosan bio-inks are highly printable and possess good shear-thinning properties. These bioinks have regulated temperature-sensitive characteristics with alterations in viscosity (forms a gel at 10 °C) and controllable stiffness. Therefore, this hybrid structure emerges as an ECM mimic that inhibits fast scaffold degradation and increased strength,73 which suits various regenerative medicine applications. An integrated cryogenic system with a 3D plotting capability may generate a three-dimensional scaffold that exhibits both high porosity and predetermined pore structures. Nevertheless, the formed constructs periodically collapsed because of the weak adhesion between the collagen strands. These collagen-chitosan-based scaffolds must be comprehensively studied for their wide range of applications in many tissue engineering fields, as they possess remarkable rheological characteristics with adjustable physiochemical and biological properties.74
Chemical cross-linking is an essential process for enhancing the stability of collagen structures following 3D printing. However, it can have a substantial effect on the bioactivity of the final scaffold. Cross-linking strengthens collagen scaffolds against enzymatic degradation and mechanical stress. This stability is necessary for scaffold integrity in biological contexts. Chemical cross-linking can hide collagen bioactive regions needed for cell adhesion, proliferation, and differentiation. Peptide sequences like the RGD (Arg–Gly–Asp) motif are essential for cell adherence at these places. Cross-linking decreases collagen degradation, which is useful in long-term scaffold applications. However, delayed breakdown may prevent tissue regeneration by not leaving enough area for new tissue. Chemical cross-linking can alter the scaffold's pH and ionic strength, affecting cell behaviour and bioactivity. In collagen 3D printing, chemical cross-linking is risky. Mechanical stability and scaffold longevity are improved. 3D printed collagen scaffolds for tissue engineering require optimal cross-linking conditions to balance mechanical characteristics and bioactivity.
3D collagen scaffolds have been created by directly plotting a highly viscous solution of fibrils at room temperature. The results showed the production of strands that do not collapse, enabling the construction of 3D scaffolds without needing instant cross-linking. At temperatures lower than 0 degrees Celsius, a cryogenic plotting approach that is combined with electrospinning makes it possible to create a highly porous three-dimensional collagen scaffold by employing a solution with a low viscosity.
Applications requiring scaffold stability benefit from the slower degradation rate of 3D printed collagen. 3D printed GelMA degrades faster than photocurable collagen, making it ideal for scaffold resorption applications. However, it is possible to adjust crosslinking to fine-tune degradation. It has strong mechanical qualities and shape integrity, although 3D printing may require more rheological tweaking.
In order to overcome a lot of the challenges associated with 3D printing of collagen, a self-made printer has been developed with the potential to fabricate scaffolds with minimal loss of fibrillar structure. This printer facilitates successful printing without additional conditions such as low temperature, hybrid inks, cross-linking, or electrospinning.75 Deposition of collagen onto a gelatine slurry bath is also used to preserve the scaffold structure, which helps to maintain a constant pH and temperature needed for collagen assembly and is useful in attaining a spatial resolution of 200 mm. It also overcomes associated problems, such as clogging of the extrusion nozzle, thus allowing the printing at higher concentrations similar to native tissues.
Collagen has also been combined with agarose, which can be used for a wide variety of tissues as it provides tunability in structure and stiffness after printing. It directly regulates the cell morphology, consequently affecting the differentiation status of the cell.76 As an alternative, strategies have also been employed to cross-link collagen in an effort to improve shape integrity physically. This could be achieved by incubating within physiological circumstances following the printing of each layer or by subjecting it to sodium bicarbonate, which neutralises the collagen and forms a solid gel.77
3.2. Light-based printing
Light-dependent bioprinting has grown in popularity because of its excellent precision, speed, and resolution, but is limited to photoreactive materials. This requires certain chemical modifications that may impact cell viability through UV exposure.78 A bioink should be rapidly cured, possess limited viscosity, and be highly fluid for the remaining ink to flow. In addition, the contactless feature allows for the plane-by-plane projection of complicated constructions with hollow morphology.Due to their biocompatibility and capacity to produce hydrogels upon light-induced crosslinking, photocurable collagen and Gelatin methacryloyl (GelMA) are frequently employed in 3D bioprinting and tissue engineering. Crosslinking photocurable collagen density and mechanical qualities can be controlled by methacrylation and UV irradiation. Collagen's triple-helical structure creates a denser crosslinked network, but it may limit material property adjustment. With 3D printed GelMA, gelatin methacrylation introduces photocurable groups, although denaturation reduces the bioactivity compared to native collagen. However, 3D printed GelMA allows for better crosslinking density control and a wider spectrum of mechanical and degrading properties for tissue engineering applications.
Photocurable collagen 3D printed under shear stress reduces viscosity during extrusion. This provides smoother printing, but slower recovery than 3D printed GelMA. Due to the increased viscosity and slower recovery, 3D printed photocurable collagen may require additional fine-tuning. Once successfully printed, crosslinked photocurable collagen generates hydrogels with strong mechanical properties but that are less rigid than GelMA scaffolds.
It is possible to create perpetual cellularised constructs with tissue-level characteristics and smooth topographies that were not possible with layer-by-layer deposition.78 The stiffness of the scaffold may be adjusted by manipulating the level of cross-linking, the degree of methacrylation, the concentration of collagen, the intensity of UV radiation, and the duration of exposure (Fig. 4).
While 3D printed photocurable collagen has great shape fidelity after crosslinking, its higher viscosity and longer crosslinking kinetics may make it difficult to maintain fine features in complicated constructions. In contrast, 3D printed GelMA has great shape fidelity due to its rapid crosslinking response and lower viscosity, making intricate features easier to preserve. The chemical alteration of 3D printed photocurable collagen to introduce photocurable groups like methacrylate preserves bioactive regions like RGD sequences, which are essential for cell adhesion. Therefore, photocurable collagen is appropriate for applications that need close tissue imitation due to its higher bioactivity and more natural ECM environment. Largely to accessibility and cost, GelMA is used in many tissue engineering applications due to its printability, mechanical characteristics, and controlled degradation. Despite having less bioactivity than natural collagen, it stimulates cell function and tissue regeneration across a range of applications.
3D bioprinting requires photoinitiators to polymerise photocurable materials like hydrogels when exposed to light. Several things can make them cell-toxic: common photoinitiators like Irgacure 2959 or lithium phenyl-2,4,6-trimethylbenzoylphosphinate (LAP) may emit hazardous chemicals during or after polymerisation, disrupting biological activities. The photoinitiator concentration and post-printing removal or neutralisation efficiency affect cytotoxicity, as well as the byproducts of photopolymerization (i.e. reactive oxygen species, ROS) causing further damage. Further to this, it is well known that UV radiation, employed in photopolymerization, damages DNA and induces apoptosis. Polymerisation can also affect pH locally, which can stress or kill implanted cells.
To ensure cell viability and function in 3D bioprinted constructions with photocurable materials, photoinitiators must be carefully considered for cytotoxicity. These dangers can be reduced by choosing the right photoinitiators, controlling light exposure, and post-processing.
To demonstrate the capability of light-based printing, collagen has been functionalised to prepare collagen methacrylamide (CMA) bioinks that preserve the fibrillar assembly and thermoreversible properties whilst also allowing for photo-cross-linking by radiating UV light at 365 nm.78 In this report, using free-form fabrication technology, this functionalised ink was first allowed to form a hydrogel at 37 °C, accompanied by UV exposure to cross-link the required structure. Next, the prepared scaffold was brought to 4 °C to remove the non-crosslinked regions, yielding a scaffold possessing stiffness five times that of the non-crosslinked one.78 In an alternative approach, recombinant collagen (RCPhC1) has been made photocurable by adding groups such as methacrylamide, norbornene, or thiol separately for printing using two-photon polymerisation (2PP). These functionalised chains allowed for preparing stem cell-embedded constructs that do not lose their proliferative capacity (Fig. 4).79
Photocurable bioinks of collagen can be used with procyanidins (PA), in which the former governs fluidity, compatibility, and native ECM structure. At the same time, PA serves as a cross-linker to obtain better mechanical characteristics through increasing printing accuracy, resolution, and structure fidelity. Using this technique, cells can be inserted with minimal damage due to lack of shearing force, and it has been shown that cells within such scaffolds adhere and proliferate easily with minimum death.80 Further evidence using a multiphoton 3D printing strategy provides insight into high-precision printing, achieving resolutions up to a micrometre level that allows for the creation of complex designs. This strategy used 5′-phosphorylated flavin mononucleotide (FMN, a photosensitiser) and acid-solubilized collagen as the bioink to achieve these outcomes.78 Different sources of collagen have also been used, including marine-based collagens, where collagen methacrylate has been formed and bioprinting parameters optimised to support different conditions for effective cell survival and growth after encapsulation.58
Several 3D printing techniques utilize collagen-based bio-inks, each with pros and cons. The advantages of extrusion-based 3D printing of collagens include high cell survival, precision cell and material deposition, and heterogeneous architectures. To maintain structural integrity after printing, bio-ink viscosity and cross-linking must be optimised. Inkjet printing deposits collagen-based bio-ink droplets onto a substrate using piezoelectric or thermal actuators. This method is utilised for high-resolution of collagen printing thin layers or patterns. Advantages include high resolution and quick printing speeds for detailed constructions. However, this is confined to low-viscosity bio-inks, which may influence printed structure mechanical strength. DLP cures collagen-based bio-inks with digital light. Curing the entire layer at once speeds up printing. High resolution, superb surface quality, and detailed designs are possible with DLP-based collagen printing. This requires photosensitive collagen or collagen coupled with a photoinitiator, which may raise biocompatibility difficulties.
4. Crosslinking strategies for collagen constructs
Native collagen is dominated by a series of inter- and intra-molecular interactions, which reinforces the network with structural strength and durability in different biological environments. During the extraction and purification of collagen from the natural tissues, it loses its intrinsic crosslink density and assembly with a consequential negative impact on mechanical properties, thermal stability, enzymatic degradation, and bioactivity. This significantly limits the biomedical application of collagen. To address these issues and functionally modify the mechanical, degradation, and biological properties of collagen, efforts have been made to introduce external crosslinks in collagen by means of chemical and physical methods. The most common approach to stabilise the collagen network by chemical crosslinking is to use glutaraldehyde, which promotes interactions between the aldehyde and amino functional groups. However, glutaraldehyde raises a major concern about cytotoxicity. For this reason, other potentially non-toxic chemical crosslink agents such as genipin, 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide/N-hydroxysuccinimide (EDC/NHS) are being employed as candidates to stabilise collagen-based scaffolds. Genipin, a natural crosslinker, forms inter-and intramolecular networks in the collagen chains by bridging the lysine and hydroxylysine amino acids. In contrast to genipin, EDC/NHS forms zero-length crosslinks without adding a crosslinker in the collagen network. It functions through condensation reactions between carboxylic moieties of aspartic and glutamic acids of one chain and primary amino moieties of adjacent amino acids and these. crosslinkers are considered to be biocompatible and non-toxic. Physical methods are generally regarded as safe, efficient, and simple for introducing crosslinks in the collagen matrix. Such methods include hydrothermal (DHT), ionising radiation, or ultra-violet (UV) techniques for crosslinking. During DHT treatment, the collagen is subjected to high temperatures under a vacuum, which loses water molecules, resulting in the intermolecular assembly of the chains either by esterification or amide bond formation between the carboxylic and amino groups present in the collagen. Exposure to radiation often introduces bonds between the aromatic residues of the collagen, although. thermal or light treatments may induce denaturation of the collagen peptides to some extent. Hence, a combination of approaches should be taken into consideration to obtain collagen scaffolds with the desired properties.5. Utilisation of collagen-based bio-ink in the regeneration of rigid and soft tissues
Clinical trials have revealed that collagen helps repair, maintain, and regenerate damaged tissues, which support its use as a bioink in regenerative medicine applications.81 Collagen scaffolds are used in wound dressings, skin transplants, nerve regeneration, and orthopaedics. To suit the purpose, polymers are often modified, and additional components added. Tables 1 and 2 summarise the potential application of various collagen-based bioinks for bone, cartilage, skin and neural tissue engineering.| Bioprinting technique | Crosslinking factor/gelation | Mechanical properties | Biocompatibility | Characteristic feature | Tissue application | Ref. | |
|---|---|---|---|---|---|---|---|
| Collagen type I/HA | Extrusion based printing | Glutaraldehyde | Young modulus 7.9 ± 0.3 MPa | Compatible with hBMSCs | • Regular porous structure | Bone regeneration | 82 |
| • High expression of SOX9, OCN and CollagenIA1 genes | |||||||
| Collagen type I/β-TCP | Extrusion based printing | Genipin | Compressive modulus 5.94 ± 0.50 MPa | Compatible with MC3T3 cells and hASCs | • Good printability and cell viability at 20 wt% β-TCP | Bone regeneration | 83 |
| • Enhanced cell mineralisation | |||||||
| Collagen methacrylate/45S bioglass | Extrusion based printing | Photoinitiator | Compressive modulus 3.32 ± 0.11 kPa | Compatible with hMSCs | • Increased degradation rate of scaffolds | Bone regeneration | 84 |
| • Enhanced rheological properties | |||||||
| • Improved recovery of the inks | |||||||
| Collagen/dECM/SF | Low-temperature-based extrusion printing | EDC | Compressive modulus 0.30 ± 0.036 | Compatible with MC3T3 cells | • Uniform round and oval pores in the scaffold | Bone regeneration | 85 |
| Collagenlagen/α-TCP | Extrusion-based: Dongbu Robot | Tannic acid | Elastic modulus 0.55 ± ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.10 0.10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MPa MPa |
Compatible with MC3T3 cells | • Increased cell metabolism | Bone regeneration | 86 |
| • New strategy to develop a hybrid structure with channels for vasculature | |||||||
| Collagen | Extrusion-based printing | — | Compressive modulus from 10–30 kPa across multidomain construct | Compatible with fibrochondrocytes | • Able to create multi-domain constructs with varying stiffness | Cartilage regeneration | 87 |
| • Support cell growth | |||||||
| Collagen/alginate | Extrusion-based printing | CaCl2 (for alginate) | Compressive modulus ∼55 kPa and tensile strength ∼40 kPa | Compatible with articular chondrocytes | • Increase integration and proliferation of cells with the scaffold. | Cartilage regeneration | 88 |
| • Increased GAG production |
| 3D Printing methods | Bioinks | Advantages | Ref. | |
|---|---|---|---|---|
| Soft tissue | ||||
| Cartilage | 3D Printing with extrusion | A hydrogel composed of high-density collagen | Constructions that are diverse, or bear loads | 87 |
| 3D Printing with extrusion | Hematopoietic stem cells, telocollagen, and hyaluronic acid | Mechanical stability, osteochondral structure, and two kinds of extracellular matrix | 89 | |
| FRESH bioprinting | Gel composed of collagen and suspension of human neural crest cells (hNCs) | Adjustable proportions, anatomically formed, and sizeable | 90 | |
| 3D Printing with extrusion | Gel composed of collagen and suspension of human neural crest cells (hNCs) | Adjustable proportions, anatomically formed, and sizeable | 91 | |
| Skin | Microfluid based printing | Collagen precursor, fibroblasts, and keratinocytes. | Construction in a hierarchical system, freeform fabrication | 92 |
| Laser-assisted printing | Collagen gel, fibroblasts, and HaCaT keratinocytes | Absence of any damage to cells, relationships between cells, and layout of cells | 93 | |
| 3D Printing with extrusion | Collagen, fibroblasts, endothelial cells, and pericytes, keratinocytes | Stratified structure with a multicellular channel environment | 94 | |
| 3D Printing with extrusion | GelMA, collagen, and tyrosinase are the specific substances mentioned. | The presence of several crosslinks and structural constancy | 95 | |
| Hard tissue | ||||
| Femur | FRESH printing | Matrigel, Collagen, fibrinogen, hyaluronic acid, and myoblasts are the substances mentioned. | performance that is high in strain and elastically recoverable, high in accuracy | 96 |
| 3D Printing with extrusion | Hydroxyapatite and collagen | Printing at low temperatures, pores that link to one another, and the absence of chemical solvents | 97 | |
| 3D Printing with extrusion | Hypoxorylol, polyvinyl alcohol, collagen, and hydroxyapatite | prolonged release, local distribution, and favorable mechanical qualities to consider | 98 | |
| 3D Printing with extrusion | The combination of collagen, tween 80. and calcium phosphate | High bending strength, printing at low temperatures, and defects that are very small | 99 | |
A considerable body of research has been dedicated to investigating the robust biological properties of collagen in relation to its diverse applications in tissue engineering. As described previously, the utilisation of collagen as a standalone bioink exhibits limited printability, necessitating its incorporation with other polymers to enhance the printability of collagen-based inks. Previous research has predominantly focused on investigating the biological properties of 3D printed collagen structures, neglecting to assess the printing precision of collagen-based inks.
5.1. Collagen-based scaffold for the engineering of bone tissue
A defect in bone tissue can be caused by various factors such as a congenital disorder, infections (osteomyelitis), mechanical damage and inflammation (osteoarthritis, osteoporosis), bone cancer, bone graft harvesting, traumatic injuries, etc.100–104 Collagen-based biopolymer scaffolds are considered a promising approach to creating a favourable microenvironment for the attachment, migration, and differentiation of bone precursor/stem cells. Being a major part of the organic phase of natural bone, collagen alone is not able to mediate an osteogenic response. Therefore, multiple groups of materials have frequently been investigated to improve and redesign the properties of collagen to induce osteoinduction,105 but with limited success.Several studies have discovered that the hierarchical arrangement of bone is responsible for its remarkable mechanical characteristics. In the past, macromolecular compounds were considered fundamental constituents of bone tissue, and their hierarchical arrangement was not investigated. Stevens and Kroger examined material hierarchies to characterise the nanoscale 3D structure of bone.106 The researchers discovered intricate collagen and HA patterns arranged in a nested, helix-like structure within the bone. Furthermore, they expanded the hierarchical organisation of the structure to include twelve levels.107 Mineralised collagen fibres, formed by constant HA distribution, are critical in bone tissue development from microstructure to macrostructure and alter bone mechanical properties. Collagen fibres have apatite crystal nucleation sites; therefore, they can drive mineral crystal formation and organise them along the fibre-long axis. This sequence renders bone tissue as anisotropic.107
With this in mind, collagen and HA have frequently been used to engineer scaffolds for bone healing.107,108 Using pure COL scaffolds directly for bone healing is disadvantageous due to the poor mechanical characteristics. Furthermore, pure apatite scaffolds also are not fit for purpose due to the incompatible mechanical properties of bone. Mineralised collagen laden scaffolds (MCSs), which are made of cemented collagen, help bones heal better than other supports.107,109 Besides mimicking native bone tissue, MCSs may be functionally reprocessed to improve bone defect healing. Determinants of bone defect repair include mechanical environment, cross-linking, form, and content of MCS. In addition, adding various cells and growth agents diversifies scaffold functionalities. These crucial elements work together to make MCSs more relevant in osteogenesis. MCSs duplicate the structure and composition of natural bone, with the inclusion of appropriate cells and growth factors, in order to provide an environment that is similar to the extracellular matrix (ECM) and has a high capacity for bone repair. The structural, biological, and mechanical features of MCSs allow them to heal bone more effectively than composite scaffolds. MCSs are compatible with natural bone and imitate the structure of bone.107,109
Notable progress has been achieved in the lengthy process of creating collagen-based bioink for constructing tissue-engineered scaffolds used in femoral rehabilitation (Table 3). Previous research has reported results on the creation of a reduced-size model of the femur, the development of tissue that promotes the growth of blood vessels in bone regeneration, the incorporation of drugs that aid in bone healing, and the promotion of femur restoration (Fig. 5). However, the existing research on femoral repair did not focus on a comprehensive, multi-scale approach to regenerating the entire organ. Instead, it was conducted as a separate issue or just considering some aspects of the femur.110 Simultaneously, with the advancement of regenerative medicine, the designed scaffold was progressively moving from the laboratory to the operating table; thus, the femoral repair research needed to address the pertinent clinical issues.110,111 In light of this, more research is required to fully comprehend, devise novel tissue engineering techniques, and use collagen laden-based bioinspired scaffolds, which successfully restore whole femurs and other major bone defects in clinical femur instances.
| Tissue application | Manufactured or treated substances | Bioprinting technology | Key results | Ref. |
|---|---|---|---|---|
| Cartilage tissue | Combination of Genipin and collagen are both | Extrusion-based: system that utilises a syringe for extrusion. | The embedded cells were shown to be adequately alive and proliferate at a high rate in in vitro studies. Compared to traditional bioinks based on alginate, the cells displayed higher osteogenic activities. | 112 |
| Collagen hydrogels with a high density | The Fab@Home 3D printer utilises extrusion-based technology. | The highest level of accuracy was achieved when the collagen content ranged from 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) mg ml−1 to 17.5 mg ml−1 to 17.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) mg ml−1. The constructions exhibited mechanical stability and were capable of supporting and sustaining cell development. mg ml−1. The constructions exhibited mechanical stability and were capable of supporting and sustaining cell development. |
87 | |
| A combination of alginate and collagen or agarose | The 3D Bioplotter system is based on extrusion technology. | The inclusion of collagen resulted in enhanced mechanical strength, cell adhesion, proliferation, and expression of genes unique to cartilage. | 88 | |
| Collagen that has been chemically bonded using riboflavin and light exposure | The Fab@Home 3D printer utilises an extrusion-based method. | When collagen bioinks are crosslinked with riboflavin, their storage modulus and printability are both greatly enhanced. The rate of gelation and the final gel moduli were quite pH dependent, reaching their highest points at about 8 pH. | 113 | |
| Bone tissue | By using tannic acid, collagen and α-TCP are crosslinked. | Dongbu Robot utilises extrusion-based technology. | The approach involves creating a scaffold with several layers utilising a two-step printing method, where the ceramic material makes up more than 70% of the volume. The scaffold printed with α-TCP/collagen, including cells, exhibited markedly superior mechanical characteristics and cellular activities. | 86 |
| Organotypic hydrogels composed of collagen and agarose | Drop system: based on an inkjet printer (Robocell) | The incorporation of agarose allowed for a more precise measurement; elevating the collagen solids content in the hydrogel mixture caused morphological alterations in the cells by increasing cell spreading and directing MSC osteogenic development. | 114 | |
| Tannic acid that has been crosslinked with collagen | Extrusion-based: system for extruding bioink | The scaffolds that were crosslinked with 0.5% tannic acid exhibited improved mechanical characteristics. MC3T3-E1 cells have a high level of vitality. | 115 | |
| Collagen, fibroin, and decellularised extracellular matrix | Extrusion-based: printing method that operates at low temperatures | When it comes to collagen/DECM and collagen/dECM/SF, the surface of each strut and micropore seems to be mostly round or oval in form. On the other hand, the collagen structure appears to be long in shape. | 85 | |
| Dense collagen | The BioScaffolder 2.1 model is an extrusion-based 3D charting solution. | With or without stimulation, the scaffolds encourage adipogenic and osteogenic differentiation. | 116 | |
| Tannic acid-crosslinked collagen and alginate | Dongbu Robot is specialised on extrusion-based robotics. | A novel approach to the production of hybrid structures that incorporate the vascular channel, the inspiration for which comes from the actual bone; an increase in the metabolic activity of the collagen structure that is packed with cells | 117 | |
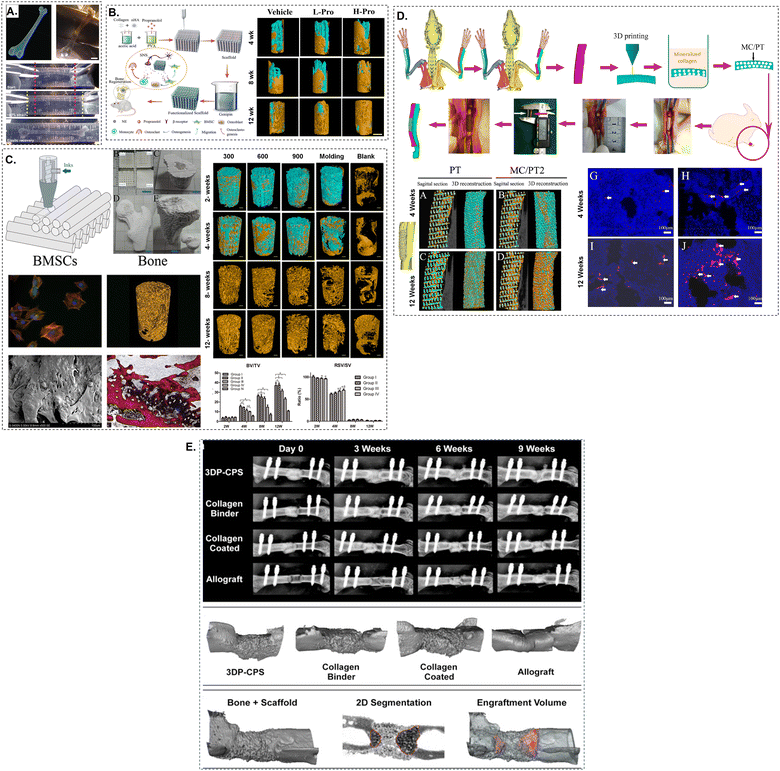 | ||
| Fig. 5 Schematic of a collagen-based bioink-printed scaffolds and femur treatment. (A) The 3D CT picture was used to create a human femur model via FRESH bioprinting.65 The printed femur was elastically recoverable after enduring 40% strain. (B) Micro-CT imaging was used to rebuild the development of bone at the defect location over a period of 4, 8, and 12 weeks.118 The propranolol group had much greater bone growth than the scaffold group. (C) Either BV/TV or RSV/SV can be utilised in order to provide a concise summary of the microstructural properties.119 (D) X-rays illustrating the development of the bone healing process at various times and three-dimensional micro-CT renderings at nine weeks.120 In most cases, the intramedullary canal was the primary route via which new bone development occurred, and periosteal bone production was frequently observed. (E) A radius defect was filled with the manufactured collagen-based composite scaffold (MC/PT2). After the in vivo experiment, the PT and MC/PT2 scaffolds were stained with immunofluorescence and micro-CT at 4 and 12 weeks.121 The incorporation of mineralised collagen not only enhanced the development of blood arteries in the scaffold, but it also aided the production of new bone. | ||
Growth factors, namely bone morphogenetic proteins (BMPs) like BMP-2 and BMP-7, are crucial in the process of osteoinduction. They stimulate stem cells to undergo differentiation into osteoblasts. ALP functions as an early predictor of osteogenic differentiation, whereas OPN and OSC are linked to subsequent phases, indicating the development and mineralisation of bone tissue. Conversely, BMP-2 is a powerful agent that stimulates the whole process of bone formation, making it a fundamental component in the fields of bone tissue engineering. The progress in 3D printing has made it possible to produce scaffolds with a strong ability to promote bone formation by including substances that stimulate bone growth. This allows for the production of personalised structures that closely resemble the natural bone's architecture.
Due to the lack of mechanical robustness in processed collagen, bioactive inorganic compounds like hydroxyapatite crystals and tri-calcium phosphates have been found to mediate bone regeneration when introduced in collagen scaffolds. Other inorganic ions, such as strontium (Sr2+), zinc (Zn2+), magnesium (Mg2+), etc., have also been widely used with collagen to improve the performance of the scaffolds.122,123 The addition of ceramic materials not only improves the mechanical qualities of the scaffolds but also maintains their ability to promote bone formation while ensuring the structural stability of the 3D structures.124–126 This section describes the studies related to the preparation of collagen-based bioceramic scaffolds for bone tissue engineering applications.
Hydroxyapatite (HA) is a widely used calcium phosphate ceramic as it forms the inorganic portion of bone. As collagen forms most of the organic portion, blends of HA and collagen are extensively utilised for bone regeneration.118,127 The compressive modulus of collagen scaffolds can be significantly increased by incorporating HA particles. Furthering, adding HA can increase the surface area of the scaffold which can lead to incremental cell adhesion and migration on the scaffolds. The interaction between collagen and HA can cause nucleation of HA crystals on collagen fibers by releasing high concentrations of Ca2+ and PO43− locally.127 3D bioprinting of HA and collagen composites reinforced with deproteinised bovine bone inks have shown to be biocompatible and orchestrate the differentiation of human bone marrow-derived mesenchymal stem cells (hBMSCs) into osteoblasts.128,129 Such composites could prove to be effective in bone recovery in clinics.118,127 Tricalcium phosphate (TCP) is another class of calcium phosphate-based ceramics with evident biocompatibility, biodegradability, and osteoinductivity that has been used as a bone substitute for decades.130
TCP has been demonstrated to enhance the structural stability of collagen scaffolds. It also helps in neo-angiogenesis in the newly formed bone tissue. It supports attachment, differentiation and proliferation of osteoblast cells. Additionally, TCP have a faster degradation rate as compared to HA.131,132 The collagen-I/β-TCP scaffold exhibited a roughly 2.7-fold increase in compressive modulus compared to the Collagen-I scaffold when 20 wt% β-TCP was added to a 5% collagen solution.131 By varying the wt% of TCP from 10–45% in the Collagen-I solution, it was observed that the flowability and printability of Collagen-I/TCP bioinks decreased with increased weight fractions of TCP.131,132 The cell viability also reduced substantially with higher concentrations of TCP due to the generation of shear forces which might have damaged the human Adipose-derived stem cells (hASCs) loaded in the inks during 3D bioprinting. The bioink containing 20 wt% of TCP was found to have optimal printability and cell viability. Moreover, this optimised hASC-laden Collagen-I/β-TCP scaffold assisted cell-mediated mineralisation two-fold that of pure Collagen scaffolds.131
The osteogenic potential of this composite was further confirmed by high expressions of ALP, OSP, OSC, and BMP-2 genes. The same group had earlier employed the α phase of TCP with Collagen-I to promote bone formation. An added advantage of α crystal structure is the faster degradation compared to β-TCP and it forms calcium-deficient hydroxyl apatite (CDHA) in the aqueous environment which results in faster bone healing.133,134 Similar results were obtained with α-TCP in terms of increased expression of osteogenic markers genes and increased elastic modulus with high α-TCP fractions in the scaffolds. Efforts have also been made to blend bioactive glass in collagen to benefit the bone healing process. The bioactive glass mainly contains three to four primary components, which are SiO2, CaO, Na2O, and P2O5, with silica being the predominant content in various editions of Bioglass ceramics.135–138 Bioactive glasses studied for bone regeneration have been reported to show comparatively better osteogenic properties due to their high surface reactivity along with biodegradability and biocompatibility.137,139–141 The blending of collagen and Bioglass is expected to corroborate superior bone regeneration coupled with improved stiffness.
Attempts have been made to include various inorganic ions such as (B3+), calcium (Ca2+), cobalt (Co2+), copper(II) (Cu2+), fluoride (F−), lithium (Li+), magnesium (Mg2+), niobium (Nb5+), phosphate (PO43−), silicate (Si4−), silver (Ag+), strontium (Sr2+), vanadium (V5+), and zinc (Zn2+) in the scaffolds to provide biological cues for osteogenesis. All of these ions have been reported to promote the osteogenic differentiation of stem cells and bone precursor cells through a growth signalling cascade or by supporting the growth of bone tissue. When blended with collagen-based bioink composites, it can aid in the sustained release of the ions, which can guide osteogenesis by providing biochemical factors to the precursor cells. The addition of bioglass also has a progressive effect on the bulk properties of the scaffold.137,139–141
Kajave et al. fabricated methacrylated collagen (CMA)/bioglass scaffolds by extrusion-based 3D printing method.142 The addition of Bioglass to CMA limited the degradation and swelling of the hydrogels with no significant improvement in the compressive strength and ameliorated the unfavorable rheological properties of these hydrogels. When incubated in simulated body fluid, the Bioglass-incorporated CMA scaffolds exhibited better apatite formation on day 3 as compared to CMA scaffolds which was progressively increased by day 7. Bioglass in the CMA matrix also enabled the cell-mediated Ca2+ ion deposition, indicating the mineralisation in hMSCs upon deposition on these scaffolds while maintaining cell viability and proliferation. The presence of Bioglass notably increased the alkaline phosphatase activity, which set forth the osteogenic potential of 3D-printed CMA/bioglass scaffolds.142 Although the incorporation of bioceramics in the collagen-based matrix has shown a significant increase in the mechanical and osteogenic properties of the scaffolds as compared to pure collagen, the increased strength of such materials does not meet the mechanical properties of a real bone. Manipulating the mechanical behaviour and addressing these obstacles continue to be significant obstacles in the production of bone mimetic scaffolds via ceramic and collagen composites.
Studies have found that SF can differentiate stem cells towards osteogenic lineages and induce mineralisation.149,150 A group of authors have studied the effect of SF on osteoclast genesis and discovered that SF can reduce bone resorption by inhibiting osteoclasts as well.151,152 Lee et al. attempted to fabricate tissue scaffolds mimicking the real organic composition of bone by using blends of collagen (4 wt%), decellularised matrix, and SF (3 wt%) with a low-temperature printer.64 The 3D printed scaffolds' compressive modulus increased dramatically in the presence of SF, from 0.03 + 0.002 to 0.3 + 0.036 MPa.
The compressive modulus of 3D-printed scaffolds is an essential characteristic that indicates the rigidity and mechanical durability of the scaffold material. In tissue engineering applications, especially for load-bearing tissues like bone and cartilage, it is crucial for the scaffold to be able to endure physiological pressures. The compressive modulus of the scaffold is also affected by its porosity and pore size. Decreased porosity often diminishes the compressive strength of the 3D printed scaffold, since there is a less amount of material to support the load. The compressive modulus can be influenced by many particular factors employed in the 3D printing process, including layer thickness, print speed, and infill density. Increasing the density of infill often results in an increase in the compressive modulus values. In applications requiring a softer, more flexible scaffold, hydrogels have lower compressive moduli, usually 10 kPa to 1 MPa. Bone tissue engineering scaffolds with greater compressive moduli sustain mechanical stresses and promote bone tissue formation. Because cartilage is softer than bone, scaffolds with a compressive modulus of 100 kPa to 1 MPa can promote tissue growth without losing flexibility.
It was also observed that the presence of a decellularised matrix and SF had amplified the ALP activity and calcium deposition. GAG is also a natural ingredient of extracellular matrices of many tissues, including the bone. It is desired that the integration of GAG with collagen will accelerate the bone healing process and can efficiently tailor the adhesion and proliferation of osteoblasts.64
Numerous reports emphasise collagen-based natural polymer composites for bone tissue engineering. However, studies discerning the effect and advantages of 3D bioprinting of collagen-based inks with or without cells have yet to be implemented on a large scale. Additionally, biodegradable synthetic materials such as polycaprolactone (PCL), polyethylene glycollagen (PEG), polylactic acid (PLA), etc. are promising for bone tissue engineering129,153–155 Sometimes, a mineral phase is added to these blends to reinforce the mechanical and osteoinductive properties. For example, Ebrahimi et al. constructed a PCL scaffold with a fused deposition moulding (FDM)-type 3D printer, which was further coated with Collagen-I and HA to get osseointegration.129 Along with the increase in mechanical properties, Collagen-I and HA facilitated high expressions of osteogenic markers, ALP, and osteonectin, suggesting that the immobilisation of these materials on PCL scaffolds has significantly increased the osteogenic differentiation of hADSCs.129 It is worth noting that Collagen supported the absorption and retention of water in the scaffolds.
Another strategy was implemented by Sun, Tianze, et al. in which they 3D printed the PCL/HA/collagen scaffold loaded with BMP-2 and fibroblast growth factor-2 (FGF-2) to promote endogenous bone regeneration.156 The researchers created agome-shaped devices that simultaneously distribute growth factors and enhance the mechanical qualities of the scaffolds. The systemic release of growth factors from the scaffold facilitated effective bone regeneration in a calvarial defect model of 8 mm diameter in rats.156 The bone volume was measured at 8 weeks following the implantation of the scaffold using micro-CT. The average bone volume in the defect treated with PCL/HA/collagen scaffold printed in the radial pattern was 27.4 ± 3.9 mm3, which was significantly higher than the untreated group (4.6 ± 3.3 mm3). Expression of CD31, a marker protein of angiogenesis, was noted, indicating the recovery of bone tissue in rats.156,157
5.2. Cartilage
Cartilage is a specialised avascular connective tissue present between the articulating joints to provide smooth movements in the bones without friction. When an injury occurs to the cartilage, it takes a long-time span to heal due to the lack of vasculature in the native cartilage. Therefore, cartilage defects often require a reparative surgical intervention to mitigate the progressive loss of the tissue. Depending on the function and location of cartilage, it may be either elastic, fibrous, or hyaline cartilage. The hyaline cartilage found in the joints is mostly made of collagen type II.158,159The current treatment options for cartilage degradation are conservative measures such as non-steroidal anti-inflammatory medicines (NSAIDs), viscosupplementation, and surgical interventions such as microfracture, autologous chondrocyte implantation, and arthroscopy. Such treatments have a short-term effect and do not alleviate cartilage degeneration. Further, cutting-edge research on injectable hydrogels has opened new avenues for cartilage repair through minimal invasion in the body tissues. However, most of these strategies simplify the cartilage, especially the articular cartilage, into a single layer.
The native cartilage is a complex, multilayered structure with distinct cellular morphology, and the ECM composition is each layer.160 The building of such gradient structures with the above-mentioned methods is very difficult and challenging. 3D bioprinting technology enables the fabrication of such sophisticated biomimetic structures relevant to the geometric arrangement of cells and ECM in the scaffolds for effective cartilage regeneration.161,162 As collagen forms the structural framework of the cartilage tissue matrix and endows unique physical and mechanical properties to the tissue, 3D bioprinting of collagen-based materials can amplify the release of cartilage stimulating growth factors and expression of stems to chondrocytes in vivo.163,164 Rhee et al. studied the influence of varying collagen concentrations in the hydrogels for 3D bioprinting of cartilage tissue scaffolds.159 The gelling ability and printability of the collagen bioinks were improved using alginate and modulating the temperature during bioprinting. The researchers noted that the bioinks with collagen concentrations ranging from 12.5 to 17.5 mg ml−1 maintained a strong resemblance to their original structure. However, the concentration of collagen did not have a noticeable effect on the survival of cells. Moreover, the compressive strength was linearly correlated with the concentration of collagen. The study highlighted the potential of using high-density collagen to create scaffolds that had excellent form accuracy and mechanical characteristics for the purpose of cartilage tissue restoration. In another study by Xu et al., the mechanical properties of collagen-based hydrogel was tailored with PCL nanofibres.165 A hybrid architecture was created by integrating inkjet printing and electrospinning technologies, featuring alternating layers of chondrocyte-loaded collagen–fibrin hydrogels and PCL nanofibres. It was observed that the compressive modulus and ultimate tensile strength of the hybrid scaffold were 1.76 and 1.1 MPa, respectively, which was exceptionally higher than collagen-fibrin scaffolds.
Further, the cell–laden hybrid scaffold exhibited very high expression of collagen II and GAGs after subcutaneous implantation in the rats. PCL nanofibres have the ability to significantly enhance the mechanical characteristics of the 3D bioprinted scaffold while still preserving its bioactivity for cartilage regeneration. Shim et al. fabricated multi-layered scaffolds using hyaluronic acid and atelocollagen (pepsin-treated collagen) loaded with MSCs to mimic the osteochondral structure.166 In the osteochondral defect that was generated in the knee joints of rabbits, the scaffolds demonstrated the production of neocartilage tissue. Additionally, the newly produced cartilage was amazingly interwoven with the cartilage tissue of the host. In addition, Collagen l-II, a marker protein of cartilage, was expressed in the tissue, confirming the formation of cartilage in the defects created.
Medicine divides cartilage into hyaline, fibrocartilage, and elastic. The body has the greatest quantity of hyaline cartilage, and the topic of discussion in this article is articular cartilage. Articular cartilage is a smooth and flexible layer of connective tissue that encloses the joint. It consists of chondrocytes and extracellular matrix (ECM) and exhibits a high degree of anisotropy. The composition and organisation of the cartilage vary depending on its depth.167 The collagen gradient, proteoglycan concentration, and fibre alignment split cartilage into three layers: shallow, medium, and deep.168 Natural cartilage is nerveless and avascular, limiting self-repair. Articular cartilage transfers stress and reduces friction where bone meets bone.169 Overweight, excessive exercise, poor food, unintentional injuries, and other factors weaken articular cartilage's load-bearing and lubricating abilities. Thus, bone friction rises.170 With repetitive joint strain, articular cartilage degeneration increases, damaging subchondral bone and weakening joint function. Research indicates that cartilage lesions larger than 2 mm are challenging to self-repair.171,172 Scientists have tried for decades to cure articular cartilage damage.173 No therapy options fulfil clinical application requirements because of immunological rejection, inadequate donor sources, low cartilage synthesis, and poor long-term effectiveness.174 To circumvent donor scarcity and immunological rejection, tissue engineering uses 3D bioprinting scaffolds with cells to repair/regenerate cartilage.164 The scaffold produced in vitro and implanted in the problem location accelerates cartilage healing.175 As presented in (Fig. 6) and (Table 3), collagen-based bioink-printed bioinspired scaffolds mimicked natural cartilage structure and performed well in cartilage tissue repair and regeneration.
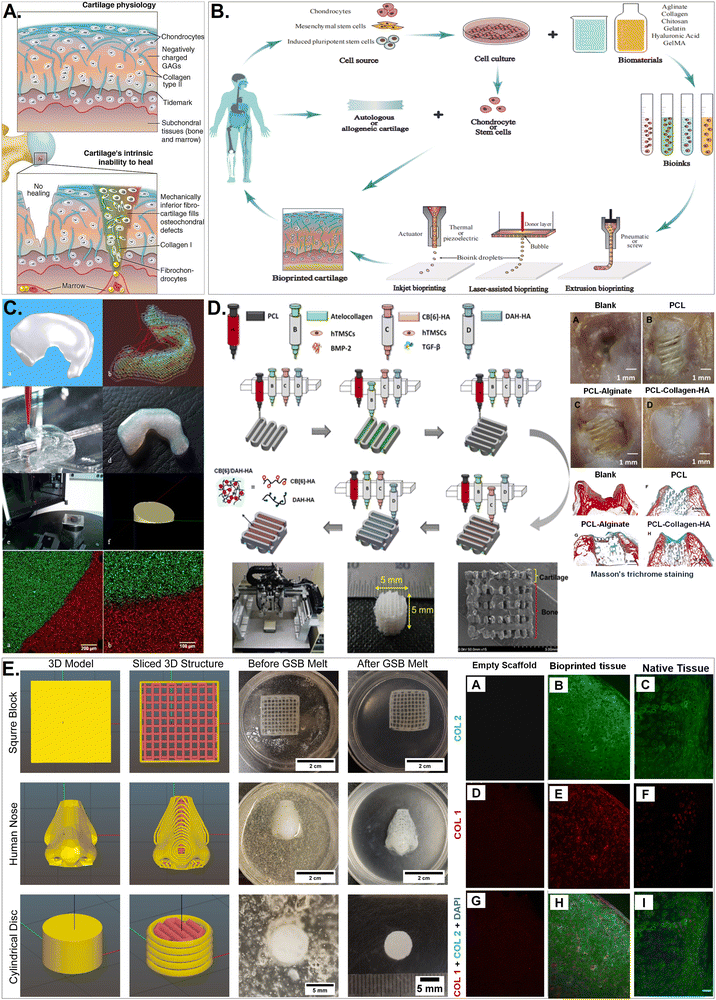 | ||
| Fig. 6 The composition of cartilage, the methods used to make scaffolds using collagen-based bioink, and the methods for treating them. (A) Variations in cartilage's physiological milieu, metabolic rate, and cellular composition have a significant impact on the possibility of creating tissue engineering.176 (B) Diagrammatic illustration of a cartilage regeneration plan. The various cell sources, materials, and printing techniques were chosen to create cartilage scaffolds that are bioinspired.164 (C) Tissue-engineered meniscus printed using high-density collagen hydrogel.164 The meniscus that was inspired possessed adequate mechanical stability and could sustain and uphold the proliferation of cells. Scale bar = 100 μm. (D) A diagram illustrating the overall structure of a scaffold created by 3D printing using a hydrogel made from collagen. This scaffold is used to repair osteochondral defects and the diagram also shows the appearance of the scaffold eight weeks after it was implanted.166 The collagen-based ink-containing groups' newly produced tissues were smooth and whiter than the nearby natural cartilage, indicating that collagen helped repair cartilage tissues. Scale bar = 10 μm. (E) The FRESH technique was used to create bioinspired tissues using a bioink made from collagen. After 6 weeks of being cultured in a laboratory setting, immunofluorescence was performed on the tissues.177 Together, the collagen-based bioink and hNCs enhanced type II collagen deposition and cartilage repair at the defect location. Scale bar = 100 μm. | ||
5.3. Skin
Skin is one of the few organs in direct contact with the external surroundings; thus, it plays a major role in protection, body temperature regulation, and responding to external stimuli.178 Chronic damage can occur due to physical wounds, surgical treatments, tumour resections, or substantial third-degree burns. 3D bioprinting of skin provides an opportunity to replicate the natural physiology and morphology of the skin and surrounding tissues179 ECM consists primarily of hyaluronic acid (HA), collagen, glycosaminoglycan, and elastin. Most tissue engineering applications related to skin growth use collagen as it is present in large concentrations, possesses inherent bioactive properties, and provide a moist microenvironment by holding a large percentage of water.180 Even though skin bioprinting has advanced, personalised modelling and vascularisation still provide difficulty for therapeutic use.181 Therefore, creating completely functional skin replicating the natural physiology and anatomy is the final objective of skin bioprinting. The cell density and collagen concentration in hydrogel must be optimised for perfect recreation, and the scaffold could be grown properly to produce mature skin or implanted right into the wound site.182With limited mechanical strength, various approaches have been incorporated to strengthen the easily manageable substrate, and it does not significantly contract in size.183 Implanted collagen hydrogels can cover the wound site throughout the healing process when resistant to contraction. Bioconjugate chemistries have been applied to cross-link and maintain the natural fibril structure throughout the gelation process. A four-armed PEG succinimidyl glutarate is often used for cross-linking, and the resultant hydrogel formed is highly biocompatible and resists contraction for at least twenty days.184 Revascularisation and host integration were two improved outcomes demonstrated by using low-density collagen.185
A collagen bioink has been used with fibroblasts to produce a skin model later cultured at air–liquid interface culture.186 The combination of collagen and alginate, used as a bioink, promotes the proliferation of keratinocytes and fibroblasts when exposed to air at the liquid interface. The cells could spread and multiply, producing a structure resembling human skin.187 Collagen has also been used where the pattern of gelatin was printed initially and then erased by selective liquefaction, resulting in the formation of a fluidic pathway.188 As such, laser bioprinting has also been used with fibroblast-embedded collagen scaffolds fabricated using collagen at a concentration of 3 mg ml−1 to develop human skin models.189
Collagen and polycaprolactone mesh was also used to print skin layers using extrusion on a microfluidic device of PDMS, and using inkjet printing, keratinocytes were layered to prepare a multilayered skin structure.190 The findings demonstrate that the bioprinted structure inhibits contraction during tissue maturation by promoting cell proliferation and protein release. A two-step drop-on-demand bioprinting has been used to produce skin biomimetic porous scaffolds.191 This model had consistent cell disposition, multiple layers, and distinct pigmentation, resulting in a high degree of similarities compared to human morphology. A blend of collagen (8 wt%) and GelMA (5 wt%) has been used to print an artificial skin with tyrosine as a cross-linker. The findings show that tyrosine can increase melanocyte growth and fibroblast migration, ultimately speeding up wound healing and reducing scarring.191 In a similar vein, tyrosine was employed to augment the mechanical rigidity of a composite material comprising hyaluronic acid, chitosan, and collagen.192 A skin made of collagen was created and cultivated at the boundary between air and liquid before being transplanted. This led to increased expression of tissue markers resembling the structure of human skin; porous layered structures with melanocytes can even induce pigmentation.
It is necessary for wound dressings and tissue-engineered skin substitutes to possess features such as biocompatibility, bioactivity, mechanical properties, and degradation. Many biomaterials have been employed. These healing methods control stem cell development and differentiation to reduce damage scarring.187 Yoo et al. developed tissue engineering-based multi-layer composites to imitate human skin stratification in 2008.193 For skin dermis and epidermis inspiration, collagen hydrogel precursors such as 3D printing bioink, fibroblasts, and keratinocytes were combined to produce two cell layers. Both flat and non-planar surfaces showed two-cell layer height increases in vitro. The creation of distinct dermis/epidermis layers within collagen gel scaffolds has enabled the engineering of skin tissue. This advancement has the potential for the development of skin transplants and artificial tissue assays, which may be utilised for disease modelling and pharmaceutical testing. Lothar Koch et al. organised FBs and KCs in collagen scaffold biomimetic layers using LaBP.193
In vitro cultivation revealed scaffold layer adhesions and gap junctions, which are important for tissue formation and cohesion. Karande et al. utilised a 3D printing technique to create a bioink composed of collagen, human dermal fibroblasts (HDFs), endothelial cells (ECs), and placental pericytes. This bioink was used to construct the dermis layer. Additionally, human foreskin keratinocytes (KCs) were employed to generate the epidermis layer.190 The dermis layer anastomosed with wound surface microvessels and promoted host microvessel invasion and epidermal rete formation when bioinspired skin was transplanted in the backs of immunodeficient mice. This occurred even though the mice did not have any immune system. GelMA and collagen were used to make the bioink for 3D skin bioprinting by Zhang et al.191 This framework kept human melanocytes, keratinocytes, and HDFs active and formed the epidermis and dermis.
Despite the growing interest, 3D bioprinting of skin is still new, and much work is needed to prepare these technologies for clinical translation. Most of the current study was focused on the large scale to make the skin's layered structure replicable. In the experiments that were discussed earlier (which are summarised in Fig. 7), various 3D printing processes, bioinks, and cell types were tested; nevertheless, the completely functional skin that consists of a stacked epidermis, blood arteries, nerves, and an elastic dermis has not yet been characterised. The next step in the creation of artificial skin is to figure out how to use 3D printing to faithfully mimic the complex structures and functions of bioinspired skin at the micrometre level.
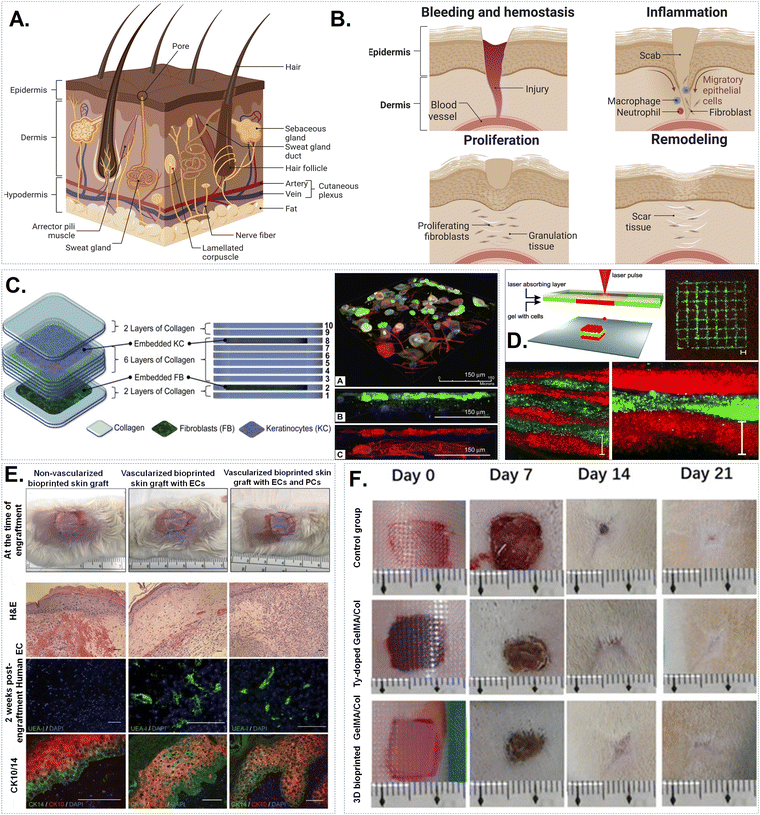 | ||
Fig. 7 Skin layers, collagen-based bioink scaffold restoration, and therapeutic techniques. (A) The three layers of the skin, as well as its appendages and primary cellular components, were all distinguishable. Created with BioRender.com (B) Hemostasis, inflammation, migration/proliferation, and maturation were the four steps in skin healing.185 (C) Developing a multi-layered synthetic skin and conducting cell culture experiments outside of a living organism. We successfully generated artificial dermis and epidermis layers in a laboratory setting, arranging them in a stratified manner and increasing their thickness.194 (D) Layer by layer, LaBP created collagen scaffold biomimic skin using FBs and KCs.189 (E) On immunodeficient mice, 3D bioprinted skin grafts were characterised at engraftment and 2 weeks later.195 Scale bar: 50 μm. (F) Bioinspired skin-treated SD rat wound closure. Significant differences across groups showed that 3D-printed GelMA/collagen heals skin wounds better. Each photo corresponds to an area of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cm2. cm2. | ||
5.4. Nerve
Trauma or neurodegenerative illnesses are the primary causes of peripheral nerve damage, frequently impairing motion, sensation, and sustenance. Therefore, 3D bioprinting has recently received much interest due to the numerous factors described herein, including the customisation of a graft, which could increase the likelihood of nerve healing.196–198 Collagen-based scaffolds can synergistically encourage nerve regrowth and restore the affected site functioning. It can minimise cysts packed with fluid, align longitudinally with the spinal cord, and prevent the degradation of connective tissue and muscle in the injured area. Together, this collagen scaffold prepared by 3D bioprinting can create an environment that accelerates tissue repair, axonal regeneration, vascularisation, recovery of nerve function and, prevents apoptosis, and decreases local inflammation.199 For a suitable nerve repair, the scaffold should provide a 3D interconnected architecture with high porosity that allows nutrients to diffuse inside and waste products to diffuse outside from the scaffold and can be achieved easily by 3D printing.The sciatic nerve defect was repaired by 3D bioprinting using a combination of collagen and poly(lactide-co-caprolactone) (PLCL). Axons could align and regenerate due to the nanostructures and comparable elastic properties.200 Schwann cells could also be integrated into the 3D system and have shown a substantial possibility in peripheral nerve repair. Collagen and hyaluronic acid have been utilised to regenerate neural tissue, causing the differentiation of induced pluripotent stem cells to neurons.201 This composite gel has been used by 3D bioprinting in enhanced differentiation of neural stem/progenitor cells, peripheral nerve regeneration, repair of neural damage, and neural disease models for glioblastoma.202
It is also possible to functionalise a collagen hydrogel with neutralising proteins such as CBD-EphA4LBD, CBD-PlexinB1LBD, and NEP1–40. These proteins facilitate the formation of axons and promote the regeneration of neurons. When seeded onto these collagen scaffolds, cerebellar granular neurons show neurite outgrowth by synthesising neurotrophic factors that help enhance the binding specificity.203 Utilizing this approach by 3D bioprinting allows for its usage in multiple applications in the future. 3D co-axial bioprinting of motor neuron-like cells and skeletal myoblasts was possible with collagen to develop a neuromuscular junction (NMJ). In this, a single organised platform was prepared in different microenvironments with tuneable conditions for enhanced differentiation. Such a model could be used to develop pre-clinical research into neuromuscular pathophysiology and for the development of therapeutic interventions.
Neurotrophin-3 (NT3) is a neurotrophic factor that can protect neurons and help them grow. Collagen and NT3 with a changed collagen-binding domain (CBD-NT3) connected to a poly(propylene fumarate) (PPF) structure were used to make a new way to treat spinal cord injury (SCI) (Fig. 8).204 In particular, the PPF scaffold's parallel-aligned multichannel structure prepared by degitail-light-based 3D printing helped neural tissues grow back in the spinal cord's axial orientation, while collagen filled the gaps to assist cells attach. When used on the transected SCI model, it led to successful cell regrowth, which made electrophysiological and locomotor healing much better. The results of the tests showed that a therapy consisting of collagen hydrogel containing NT3 spheres increased neuronal development, reduced glial scarring, and reduced inflammation in another hemisected spinal cord injury animal model. The advantages of 3D bioprinting, on the other hand, did not transfer into recovered functional capacity. Because the collagen fibres in this hydrogel are not arranged in a specific way, there are no guidance signs at the injury site, which may lead to slower functional healing as studied by collagen and heparin sulfate scaffolds.205 The arrangement of collagen-FB fibrous hydrogels was demonstrated upon the introduction of paclitaxel/stromal cell-derived factor-1α (SDF1α) printed via electrohydrodynamic jet printing technique (Fig. 8). Collagen-FB hydrogels were longer before they broke (230%), had a lower Young's modulus (17.93 ± 1.16 kPa), and were stronger at sticking things together (3.45 ± 0.48 kPa) than collagen hydrogels.206 This implies they can adhere to the host spinal cord more strongly and have the properties of soft tissue. The slow release of SDF1α and paclitaxel made a good setting for the quick recruitment of endogenous NSPCs, which helped severely injured rats recover their ability to move.206
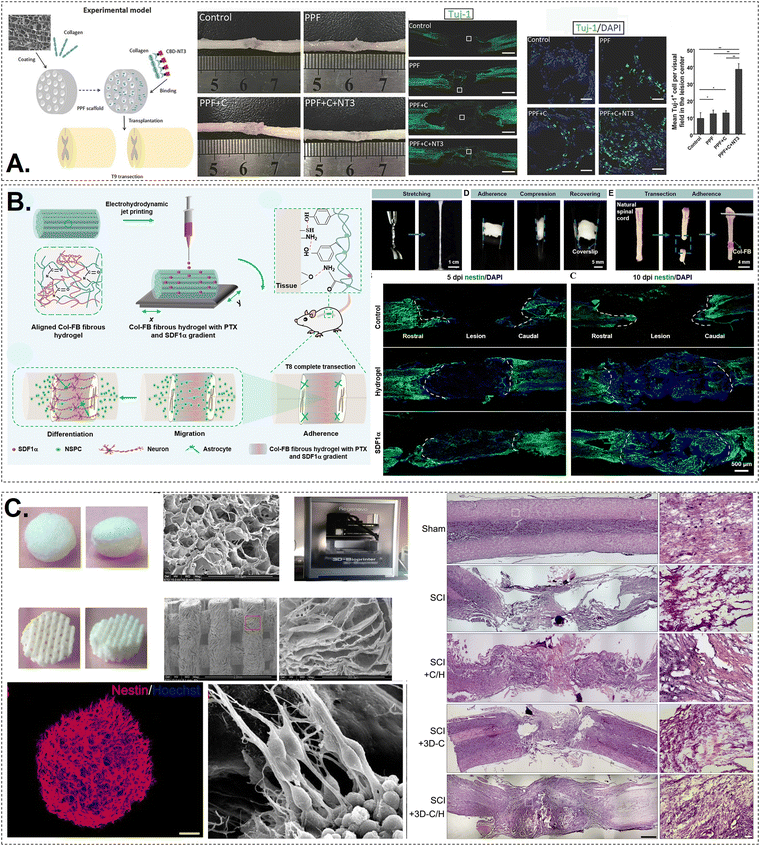 | ||
| Fig. 8 (A) PPF, collagen, and CBD-NT3 were used in the experimental model of the combinatorial therapy procedure.207 (B) Adhesive, stretchy, spatiotemporal delivery aligned Collagen-FB fibrous hydrogel schematic for SCI rat nerve reconnection. Collagen-FB fibrous hydrogel stretching photos. Pictures that show how Collagen-FB flexible gelatin sticks to smooth glass slides when they are pulled and pushed together. Photographs showing Collagen-FB fibrous hydrogel bio-adhesion in rat spinal cord transection gaps. Over the course of five and ten days following transplantation into the complete transection of the rat spinal cord, the migration of endogenous neural stem cell (NSPC) towards lesion regions was stimulated by aligned fibrous hydrogels containing SDF1α gradient.206 (C) The scaffold made of collagen and heparin sulfate that was created using the freeze-drying technique (C/H). Through the use of SEM, the morphology of the C/H scaffold was revealed. A 3D bioprinter will be used to build scaffolds. Visual representations of the structure of the scaffold made of collagen and heparin sulfate that was created using the 3D bioprinter (3D-C/H). Through the use of SEM images, the shape of the porous 3D-C/H scaffold was revealed. A red immunofluorescence picture of neural stem cells that have been immunostained with the Nestin antibody. Hoechst that was blue in color was used to stain the nucleus. Scaffolds that have been co-cultured with neural stem cells are seen in SEM pictures.205 | ||
Another example is where a collagen nerve multichannel scaffold was constructed with collagen and gelatine, which have a critical role in stimulating nerve growth. The effects of different concentrations were evaluated as the low concentration leads to structural collagen-lapse while high concentration creates voids in the printed construct.202
6. Presents limitations and strategies for overcoming the obstacles
Casting techniques that are more traditional are unable to make sophisticated three-dimensional scaffolds made of collagen, with limitations in developing scaffolds that account for native structure and function. Reconstituted collagen has been used to create unique scaffolds that allows for the engineering of hitherto unknown tissue defects. In recent years, 3D bioprinting and collagen solution adaption have advanced. It is undeniable that 3D bioprinting and collagen-based bioink can produce synthetic organs and tissues for use in regenerative medicine and tissue engineering.However, there has been slow progress in this direction largely due to the lack of freely accessible collagen bioinks that meet “perfect” bioink standards. Collagen solution concentration determines 3D bioprinting suitability. Printing accuracy is enhanced exclusively in single-component collagen bioinks when the collagen concentration exceeds 20 mg ml−1. There are only limited commercial options for high-concentration collagen – Lifeink (35 mg ml−1, Advanced Biomatrix, USA) and Viscollagen (80 mg ml−1, Imtek, Russia) are commercially available. Collagen-based acellular and cellular scaffolds have been printed using inkjet, extrusion, and laser-aided methods.159,208–210 Collagen solution concentration and printing conditions limit bioink usefulness. Generally, high collagen concentrations and low printing temperatures increase printability. To sustain the structure for cell development, the elastic modulus of the final constructions should be balanced between mechanical integrity and cell viability. Cell-laden collagen-based hydrogels may be printed, shear-thinned, and sequentially cross-linked to create self-supporting constructs.
3D printing can be fully utilized and precise printing can be achieved with the help of suspension bath printing, which offers a method for creating supporting structures from low viscous inks. Crosslinking within the bath allows for the retention of constructs after printing. Consequently, suspension medium have also been used as a 3D substrate, where elements like vessels can aid in the designed tissue's maturation. Most of the issues associated with direct printing in the air are eliminated by using suspension baths, which offer a semi-solid environment. It also offers an infrastructure for arranging physically feeble biological inks into intricate, precise patterns.211,212
Consequently, it reduces the requirement to choose different materials and thus, offer a paradigm change in bioprinting. When there is no applied stress or when there are very little forces, suspension bath behaves like solids. Applying a stress that surpasses the yield stress—a crucial stress—will start a flow and make the medium appear liquid-like. Secondly, the internal structure of a bath dynamically heals after being disturbed by a moving nozzle and being replaced by the deposited ink. The ability of the medium to mend itself allows it to change from a fluid-like state to a solid, encasing the ink that has been injected. Suspension bath technique can therefore be used to print lightweight substances or low-viscosity inks, which frequently have a high-water content.113,208,213
The higher viscosity medium sustains the extruded hydrogel and gives excellent shape fidelity, which may allow unaltered collagen to form fibrils over time. SBF or PILP components can prime the suspension medium to enable co-continuous surface and intrafibrillar mineralisation during 3D printing. This technique for next-generation 3D mineralised tissue manufacturing is intriguing but recreating the nanoscale organisation of collagen is difficult.214
Electro-compaction is one such another approach which creates complex 3D scaffolds with tightly coordinated and tightly packed collagen fibres.14,21 Additional mechanical stimulation may be needed to replicate the original environment as scaffolds mineralise over hours or days. Multiple tissue investigations have indicated that such stimulation improves regeneration approaches. This stimulation may replicate tissue mechanical stresses and help generate native collagen.
Integration characterisation techniques, which encompass sophisticated microscopy and biochemical analysis, are a hindrance to the creation of collagen scaffolds. These approaches offer a comprehensive understanding of collagen mineralisation at many scales, ranging from the nano- to macro-level. Studies must be studied on collagen fibre organisation pre- and post-mineralisation to comprehend the ultrastructure and increase the scaffold's performance.26,28 Collagen organisation and composition can be shown using cryo-transmission electron microscopy at nanoscale resolutions, focused ion beam milling with scanning electron microscopy, and secondary ion mass spectrometry. These measurements will help the field comprehend the hierarchical structure and compare other aspects.
7. Conclusions & future perspectives
As outlined in this review, advanced 3D bioprinting methods can create natural microenvironments with aligned and tightly packed collagen fibres. This technology is improving the process of hard tissue mineralisation and inherent properties such as biodegradation, cellular activity, mechanical properties, and biocompatibility are all governed by collagen. The properties of collagen-based scaffolds made them very suitable for applications such as wound healing, regenerative medicine, tissue engineering, and medical device surface coating. Collagen-based bioink with 3D printing can create prosthetic organs for regenerative medicine and defect repair. Over the past decade, collagen-based 3D bioprinting technology has advanced. We categorised the results of regenerative medicine using collagen-based bioinks into two groups: those applying to soft and hard tissues. Varied tissues have varied collagen-based bioink performance requirements and this evaluation of collagen-based bioink applications can help design the future generation applications.The production of sophisticated macro-3D scaffolds is now possible via the use of 3D manufacturing techniques like as electro-compaction and suspended extrusion printing. Microarchitecture may be controlled by the use of collagen that has not been chemically changed. This offers an unexplored opportunity in bone tissue engineering to integrate 3D printing methods with in vitro mineralisation strategies, allowing for the hierarchical organisation of collagen fibres and the production of mineralised hard tissues with intricate topologies on a large scale. Furthermore, advanced processing techniques that regulate the structures at the nano, micro, and macro levels may facilitate the development of a novel class scaffolds devoid of cells, which are utilised in hard tissue engineering.
In the field of regenerative medicine, 3D bioprinting has the potential to produce patient-specific scaffolds and devices that are both structurally complicated and highly customisable, thanks to the use of both acellular and cell–laden scaffolds. Tissue engineering applications have shown considerable potential in early research, with several in vitro and in vivo investigations indicating the possibility of bio-printed artificial organs. Due of the numerous advantages it offers, including cell encapsulation, high productivity, and limitless micro size, bioprinting is seeing rapid expansion. Although there are a number of obstacles to overcome, this novel approach to the distribution of cell-rich hydrogels has a promising future. Nevertheless, the progress in this field is being hindered by the scarcity of bioinks suitable for cell encapsulation and gelation processes that are compatible with cell growth. Over the years, more bioprinters and bioinks are expected to become commercially available, allowing research teams without the expertise to develop their own printing equipment to use as a fabrication tool for tissue engineering and biomedical system development.
Tissue engineering constructs for soft tissue regeneration requires collagen-based scaffolds to not lose their functional specifications and mechanical characteristics during 3D printing. During the process of using collagen-based bioink to mend skin, the priority was placed on multi-layer bioinspired skin for quicker regeneration. Bioinspired scaffolds for cartilage regeneration possess the ability to endure substantial stresses and provide lubrication. The collagen-based scaffold that is now in use focuses on the manufacture of big heart models or the preparation of various cardiac patches for the purpose of repairing damage. When it comes to blood vessels, it is necessary to take into consideration blood vessel networks of varying diameters as well as bioinspired tissue vascularisation. Collagen-based bioink is highly effective for shaping and has superior mechanical properties for promoting the healing of hard tissues. It shows significant potential in tissue engineering by creating scaffolds that facilitate good restoration. In the process of repairing the skull with collagen-based bioink tissue engineering scaffolds, it is necessary to match the shape of the injuries. It was difficult to manufacture large-size bone and integrate and regenerate surrounding tissues for femur restoration. Mechanical properties and scaffold layered structures that matched teeth were also essential. In SCI, nerve regeneration and motor function recovery were as important as form.
This study deliberates oriented collagen fibre scaffold fabrication and bone tissue healing applications. Some orientated collagen fibre scaffold fabrication methods have yielded excellent results, but bone tissue engineering has few uses. Future research should consider these three criteria: first, oriented collagen fibres can be prepared in several ways, however, bone biomimetic materials with natural bone collagen fibre arrangement are still challenging to make. First, biomimetic bone lamellae should be prepared to make more complicated bone biomimetic material. Second, there are several elements that impact the production of extracellular matrix with orientated structure, however, the mechanism of directional collagen fibres needs additional investigation. The process by which cells secrete directed collagen fibres is unknown. Can cells produce a directional extracellular matrix if they proliferate directionally? Third, there are several ways to regenerate bone tissue, but collagen fibre organisation in new bone has received little study. Future studies should focus on bone structure and function rehabilitation rather than bone development.
In conclusion, modern manufacturing and proteomics techniques allow us to treat collagen in a way that achieves more native biomimicry and creates a template for mineralisation. In vitro mineralisation methods have the potential to be more effective if they are able to approximate the original hierarchical collagen structure in three-dimensional structures. The hierarchical collagen template that corresponds to the initial creation remains elusive, despite the fact that these structures are becoming closer thanks to recent advancements in collagen processing and 3D printing technology. We believe that the PILP methodology is the most effective method for aligned mineral formations that have the greatest penetration depth; nevertheless, the dispersion of minerals on a macroscale is still challenging. The development of this field will occur through the achievement of biomimicry and the use of increased manufacturing techniques to replicate the hierarchical collagen structure. Subsequently, in vitro mineralisation technologies will be employed.
These studies summarised that collagen-based bioink has expanded its use in regenerative medicine. Because collagen-based bioink performs poorly, development has been slower than projected. The quality of collagen-based bioink is determined by its tissue regeneration function, cell loading capacity, printing performance, and intelligent response characteristic. In order to tackle the challenges associated with collagen-based bioinks and provide guidance for future research, we have compiled a summary of these requirements. Here are its main characteristics.
How to enhance collagen-based bioink printing in various applications using various methods? Biomineralisation is used to fix broken bones, multiple crosslinking is used to fix cartilage, and different polymers are used to fix heart defects. Improving collagen-based bioink 3D printing performance and making it unique in tissue regeneration procedures is an essential future development.
Using collagen-based bioink, how can complex multifunctional designed 3D scaffolds be intelligently generated? To fabricate an intelligent bioinspired scaffold, it is essential to incorporate controlled intelligent elements such as black phosphorus into a collagen-based bioink. Additionally, the scaffold's regional design should be printed, and different types of cells should be loaded into it. The intelligent, adaptable, and programmable collagen-based bioink broadens the applications of tissue engineering and regenerative medicine.
What part does bioink that is based on collagen play in the process of tissue regeneration? Through the manipulation of collagen to enable visualisation or grafting with alternative extracorporeal detection substances, this study aims to ascertain whether collagen-based bioink participates directly in the regeneration of defective tissue or whether it induces defect site cells to generate bioactive substances that facilitate tissue regeneration subsequent to collagen degradation. This explains collagen's role in regenerative medicine and guides collagen-based bioink use.
Due to its properties, collagen has promising applications, especially with new collagen-manufacturing methods. Within the realm of regenerative medicine, the collagen-based bio-ink-fabricated bioinspired tissue engineering scaffold that possesses ideal structure and function has the potential to substitute organ transplantation. This would allow for the advancement of scientific and medical research without the need for organ shortages. If the review's issues are resolved, collagen-based bioink will be created for existing uses. Bioink that is based on collagen has the potential to regenerate large-scale, complex tissue with many structures, which is the ultimate goal of this technology.
Data availability
No primary research results, software or code has been included and no new data were generated or analysed as part of this review.Conflicts of interest
The authors declare no competing financial interest.Acknowledgements
The authors thank the Science and Engineering Research Board (SERB), Government of India (IPA/2020/000025), for funding. DJP acknowledges H. I. Aaronson Fellowship from the Department of Materials Engineering, Indian Institute of Science and also the Royal Academy of Engineering/Leverhulme Trust for supporting his Research Fellowship (LTRF-2324-20-121). SD acknowledges Yusuf Olatunji Waidi for the insightful discussions. AA acknowledges the Nurturing Clinical Scientist fellowship from the Indian Council of Medical Research (ICMR).References
- S. Derakhshanfar, R. Mbeleck, K. Xu, X. Zhang, W. Zhong and M. Xing, Bioact. Mater., 2018, 3, 144–156 Search PubMed.
- L. G. Bracaglia, B. T. Smith, E. Watson, N. Arumugasaamy, A. G. Mikos and J. P. Fisher, Acta Biomater., 2017, 56, 3–13 CrossRef CAS PubMed.
- M. E. Prendergast and J. A. Burdick, Adv. Mater., 2020, 32, 1902516 CrossRef CAS.
- U. Jammalamadaka and K. Tappa, J. Funct. Biomater., 2018, 9, 22 CrossRef.
- A. J. Capel, R. P. Rimington, M. P. Lewis and S. D. Christie, Nat. Rev. Chem., 2018, 2, 422–436 CrossRef.
- J. K. Placone and A. J. Engler, Adv. Healthcare Mater., 2018, 7, 1701161 CrossRef.
- G. Gao and X. Cui, Biotechnol. Lett., 2016, 38, 203–211 CrossRef CAS.
- G. Saini, N. Segaran, J. L. Mayer, A. Saini, H. Albadawi and R. Oklu, J. Clin. Med., 2021, 10, 4966 CrossRef.
- W. Aljohani, M. W. Ullah, X. Zhang and G. Yang, Int. J. Biol. Macromol., 2018, 107, 261–275 CrossRef CAS.
- S. Vijayavenkataraman, W.-C. Yan, W. F. Lu, C.-H. Wang and J. Y. H. Fuh, Adv. Drug Delivery Rev., 2018, 132, 296–332 CrossRef CAS.
- M. A. Habib and B. Khoda, J. Manuf. Process., 2022, 76, 708–718 CrossRef PubMed.
- S. Kyle, Z. M. Jessop, A. Al-Sabah and I. S. Whitaker, Adv. Healthcare Mater., 2017, 6, 1700264 CrossRef PubMed.
- I. Donderwinkel, J. C. Van Hest and N. R. Cameron, Polym. Chem., 2017, 8, 4451–4471 RSC.
- R. R. Jose, M. J. Rodriguez, T. A. Dixon, F. Omenetto and D. L. Kaplan, ACS Biomater. Sci. Eng., 2016, 2, 1662–1678 CrossRef CAS PubMed.
- M. Taghizadeh, A. Taghizadeh, M. K. Yazdi, P. Zarrintaj, F. J. Stadler, J. D. Ramsey, S. Habibzadeh, S. H. Rad, G. Naderi and M. R. Saeb, Green Chem., 2022, 24, 62–101 RSC.
- A. Schwab, R. Levato, M. D’Este, S. Piluso, D. Eglin and J. Malda, Chem. Rev., 2020, 120, 11028–11055 CrossRef CAS PubMed.
- A. Parak, P. Pradeep, L. C. du Toit, P. Kumar, Y. E. Choonara and V. Pillay, Drug Discovery Today, 2019, 24, 198–205 CrossRef CAS PubMed.
- Y. Huang, X. F. Zhang, G. Gao, T. Yonezawa and X. Cui, Biotechnol. J., 2017, 12, 1600734 CrossRef.
- A. Fatimi, O. V. Okoro, D. Podstawczyk, J. Siminska-Stanny and A. Shavandi, Gels, 2022, 8, 179 CrossRef.
- A. Sorushanova, L. M. Delgado, Z. Wu, N. Shologu, A. Kshirsagar, R. Raghunath, A. M. Mullen, Y. Bayon, A. Pandit and M. Raghunath, Adv. Mater., 2019, 31, 1801651 CrossRef PubMed.
- M. E. Nimni and R. D. Harkness, Collagen, CRC Press, 2018, pp. 1–78 Search PubMed.
- J. Glowacki and S. Mizuno, Biopolymers, 2008, 89, 338–344 CrossRef CAS.
- C. Dong and Y. Lv, Polymers, 2016, 8, 42 CrossRef.
- E. Rezvani Ghomi, N. Nourbakhsh, M. Akbari Kenari, M. Zare and S. Ramakrishna, J. Biomed. Mater. Res., Part B, 2021, 109, 1986–1999 CrossRef CAS.
- T. Yuan, L. Zhang, K. Li, H. Fan, Y. Fan, J. Liang and X. Zhang, J. Biomed. Mater. Res., Part B, 2014, 102, 337–344 CrossRef.
- E. E. Antoine, P. P. Vlachos and M. N. Rylander, Tissue Eng., Part B, 2014, 20, 683–696 CrossRef CAS.
- R. Parenteau-Bareil, R. Gauvin and F. Berthod, Materials, 2010, 3, 1863–1887 CrossRef CAS.
- M. D. Shoulders and R. T. Raines, Annu. Rev. Biochem., 2009, 78, 929–958 CrossRef CAS PubMed.
- T. Miyata, T. Taira and Y. Noishiki, Clin. Mater., 1992, 9, 139–148 CrossRef CAS PubMed.
- S. Chattopadhyay and R. T. Raines, Biopolymers, 2014, 101, 821–833 CrossRef CAS PubMed.
- C. Marques, G. Diogo, S. Pina, J. Oliveira, T. Silva and R. Reis, J. Mater. Sci.: Mater. Med., 2019, 30, 1–12 CrossRef CAS.
- M. Gómez-Guillén, B. Giménez, M. E. López-Caballero and M. Montero, Food Hydrocolloids, 2011, 25, 1813–1827 CrossRef.
- E. J. Miller, Collagen, CRC Press, 2018, pp. 139–156 Search PubMed.
- C. Dhand, S. T. Ong, N. Dwivedi, S. M. Diaz, J. R. Venugopal, B. Navaneethan, M. H. Fazil, S. Liu, V. Seitz and E. Wintermantel, Biomaterials, 2016, 104, 323–338 CrossRef CAS PubMed.
- K. Fujii, M. Tsuji and K. Murota, Neurochem. Res., 1986, 11, 1439–1446 CrossRef CAS PubMed.
- M. Spira, B. Liu, Z. Xu, R. Harrell and H. Chahadeh, J. Biomed. Mater. Res., 1994, 28, 91–96 CrossRef CAS PubMed.
- D. Miranda-Nieves and E. L. Chaikof, ACS Biomater. Sci. Eng., 2017, 3, 694–711 CrossRef CAS PubMed.
- T. Saxena, L. Karumbaiah and C. M. Valmikinathan, Natural and synthetic biomedical polymers, Elsevier, 2014, pp. 43–65 Search PubMed.
- P. Jain, S. B. Rauer, M. Möller and S. Singh, Biomacromolecules, 2022, 23, 3081–3103 CrossRef CAS.
- T. Adachi, M. Tomita, K. Shimizu, S. Ogawa and K. Yoshizato, J. Biotechnol., 2006, 126, 205–219 CrossRef CAS PubMed.
- B. An, D. L. Kaplan and B. Brodsky, Front. Chem., 2014, 2, 40 Search PubMed.
- J. A. M. Ramshaw, J. Biomed. Mater. Res., Part B, 2016, 104, 665–675 CrossRef CAS PubMed.
- C. Yang, P. J. Hillas, J. A. Báez, M. Nokelainen, J. Balan, J. Tang, R. Spiro and J. W. Polarek, BioDrugs, 2004, 18, 103–119 CrossRef CAS PubMed.
- F. W. Kotch and R. T. Raines, Proc. Natl. Acad. Sci. U. S. A., 2006, 103, 3028–3033 CrossRef CAS.
- D. Olsen, C. Yang, M. Bodo, R. Chang, S. Leigh, J. Baez, D. Carmichael, M. Perälä, E.-R. Hämäläinen and M. Jarvinen, Adv. Drug Delivery Rev., 2003, 55, 1547–1567 CrossRef CAS PubMed.
- O. Shoseyov, Y. Posen and F. Grynspan, Tissue Eng., Part A, 2013, 19, 1527–1533 CrossRef CAS PubMed.
- J. Uitto, J. R. Lichtenstein and E. A. Bauer, Biochemistry, 1976, 15, 4935–4942 CrossRef CAS PubMed.
- J. Myllyharju, M. Nokelainen, A. Vuorela and K. Kivirikko, Biochem. Soc. Trans., 2000, 28, 353–357 CrossRef CAS.
- S. Jin, F. Sun, Q. Zou, J. Huang, Y. Zuo, Y. Li, S. Wang, L. Cheng, Y. Man and F. Yang, Biomacromolecules, 2019, 20, 2058–2067 CrossRef CAS PubMed.
- Y.-S. Lim, Y.-J. Ok, S.-Y. Hwang, J.-Y. Kwak and S. Yoon, Mar. Drugs, 2019, 17, 467 CrossRef CAS.
- T. Maschmeyer, R. Luque and M. Selva, Chem. Soc. Rev., 2020, 49, 4527–4563 RSC.
- B. Hoyer, A. Bernhardt, A. Lode, S. Heinemann, J. Sewing, M. Klinger, H. Notbohm and M. Gelinsky, Acta Biomater., 2014, 10, 883–892 CrossRef CAS PubMed.
- A. Daugs, N. Lehmann, D. Eroglu, M. C. Meinke, A. Markhoff and O. Bloch, Tissue Eng., Part C, 2018, 24, 280–288 CrossRef CAS.
- J. M. Lee, S. K. Q. Suen, W. L. Ng, W. C. Ma and W. Y. Yeong, Macromol. Biosci., 2021, 21, 2000280 CrossRef CAS.
- G. S. Diogo, C. F. Marques, C. G. Sotelo, R. I. Pérez-Martín, R. P. Pirraco, R. L. Reis and T. H. Silva, Biofabrication, 2020, 6, 3664–3672 CAS.
- M. Govindharaj, U. K. Roopavath and S. N. Rath, J. Cleaner Prod., 2019, 230, 412–419 CrossRef CAS.
- Y. Liu, X. Luo, W. Wu, A. Zhang, B. Lu, T. Zhang and M. Kong, Int. J. Biol. Macromol., 2021, 182, 689–700 CrossRef CAS PubMed.
- B. Sanz, A. Albillos Sanchez, B. Tangey, K. Gilmore, Z. Yue, X. Liu and G. Wallace, Biomedicines, 2020, 9, 16 CrossRef.
- N. Cubo, M. Garcia, J. F. Del Cañizo, D. Velasco and J. L. Jorcano, 3D bioprinting of functional human skin: production and in vivo analysis, Biofabrication, 2016, 9, 015006 CrossRef.
- N. Liu, X. Zhang, Q. Guo, T. Wu and Y. Wang, Front. Mater., 2022, 9, 925321 CrossRef.
- M. Hospodiuk, M. Dey, D. Sosnoski and I. T. Ozbolat, Biotechnol. Adv., 2017, 35, 217–239 CrossRef CAS PubMed.
- A. Mazzocchi, M. Devarasetty, R. Huntwork, S. Soker and A. Skardal, Biofabrication, 2018, 11, 015003 CrossRef PubMed.
- R. Gibney and E. Ferraris, Front. Bioeng. Biotechnol., 2021, 9, 786945 CrossRef PubMed.
- H. Lee, G. H. Yang, M. Kim, J. Lee, J. Huh and G. Kim, Mater. Sci. Eng., C, 2018, 84, 140–147 CrossRef CAS PubMed.
- T. J. Hinton, Q. Jallerat, R. N. Palchesko, J. H. Park, M. S. Grodzicki, H.-J. Shue, M. H. Ramadan, A. R. Hudson and A. W. Feinberg, Sci. Adv., 2015, 1, e1500758 CrossRef.
- A. Oryan, A. Kamali, A. Moshiri, H. Baharvand and H. Daemi, Int. J. Biol. Macromol., 2018, 107, 678–688 CrossRef CAS.
- J. Gopinathan and I. Noh, Biomater. Res., 2018, 22, 11 CrossRef.
- Y. Zhang, Q. Yang, H. Wan, G. Zhu, Z. Xiao, Y. Zhang, L. Lei and S. Li, Int. J. Bioprint., 2024, 3006 CrossRef.
- D. M. Kirchmajer and R. Gorkin Iii, J. Mater. Chem. B, 2015, 3, 4105–4117 RSC.
- R. Attalla, C. Ling and P. Selvaganapathy, Biomed. Microdevices, 2016, 18, 1–12 CrossRef CAS PubMed.
- B. Zhang, R. Cristescu, D. B. Chrisey and R. J. Narayan, Int. J. Bioprint., 2020, 6, 211 CrossRef CAS PubMed.
- X. Cui, J. Li, Y. Hartanto, M. Durham, J. Tang, H. Zhang, G. Hooper, K. Lim and T. Woodfield, Adv. Healthcare Mater., 2020, 9, 1901648 CrossRef CAS PubMed.
- S. Zhong, Y. Zhang and C. Lim, Wiley Interdiscip. Rev.: Nanomed. Nanobiotechnol., 2010, 2, 510–525 CAS.
- H. Suo, J. Zhang, M. Xu and L. Wang, Mater. Sci. Eng., C, 2021, 123, 111963 CrossRef CAS PubMed.
- A. D. Nocera, R. Comín, N. A. Salvatierra and M. P. Cid, Biomed. Microdevices, 2018, 20, 1–13 CrossRef CAS.
- D. F. Duarte Campos, A. Blaeser, A. Korsten, S. Neuss, J. Jäkel, M. Vogt and H. Fischer, Tissue Eng., Part A, 2015, 21, 740–756 CrossRef CAS PubMed.
- W. L. Ng, W. Y. Yeong and M. W. Naing, Int. J. Bioprint., 2016, 2, 53–62 CrossRef CAS.
- C. Yu, J. Schimelman, P. Wang, K. L. Miller, X. Ma, S. You, J. Guan, B. Sun, W. Zhu and S. Chen, Chem. Rev., 2020, 120, 10695–10743 CrossRef CAS PubMed.
- L. Tytgat, A. Dobos, M. Markovic, L. Van Damme, J. Van Hoorick, F. Bray, H. Thienpont, H. Ottevaere, P. Dubruel and A. Ovsianikov, Biomacromolecules, 2020, 21, 3997–4007 CrossRef CAS PubMed.
- Z. Wu, J. Liu, J. Lin, L. Lu, J. Tian, L. Li and C. Zhou, Biomacromolecules, 2021, 23, 240–252 CrossRef PubMed.
- A. Dewle, N. Pathak, P. Rakshasmare and A. Srivastava, ACS Biomater. Sci. Eng., 2020, 6, 779–797 CrossRef CAS PubMed.
- Q. Li, X. Lei, X. Wang, Z. Cai, P. Lyu and G. Zhang, Tissue Eng., Part A, 2019, 25, 1261–1271 CrossRef CAS.
- W. Kim and G. Kim, Biofabrication, 2019, 12, 015007 CrossRef PubMed.
- N. S. Kajave, T. Schmitt, T. U. Nguyen, A. K. Gaharwar and V. Kishore, Biomed. Mater., 2021, 16, 035003 CrossRef CAS PubMed.
- H. Lee, G. H. Yang, M. Kim, J. Lee, J. Huh and G. Kim, Mater. Sci. Eng., C, 2018, 84, 140–147 CrossRef CAS PubMed.
- W. J. Kim, H. S. Yun and G. H. Kim, Sci. Rep., 2017, 7, 3181 CrossRef.
- S. Rhee, J. L. Puetzer, B. N. Mason, C. A. Reinhart-King and L. J. Bonassar, ACS Biomater. Sci. Eng., 2016, 2, 1800–1805 CrossRef CAS.
- X. Yang, Z. Lu, H. Wu, W. Li, L. Zheng and J. Zhao, Mater. Sci. Eng., C, 2018, 83, 195–201 CrossRef CAS.
- J. H. Shim, K. M. Jang, S. K. Hahn, J. Y. Park, H. Jung, K. Oh, K. M. Park, J. Yeom, S. H. Park, S. W. Kim, J. H. Wang, K. Kim and D. W. Cho, Biofabrication, 2016, 8, 014102 CrossRef PubMed.
- X. Lan, Y. Liang, E. J. N. Erkut, M. Kunze, A. Mulet-Sierra, T. Gong, M. Osswald, K. Ansari, H. Seikaly, Y. Boluk and A. B. Adesida, FASEB J., 2021, 35, e21191 CrossRef CAS PubMed.
- X. Lan, Y. Liang, M. Vyhlidal, E. J. Erkut, M. Kunze, A. Mulet-Sierra, M. Osswald, K. Ansari, H. Seikaly, Y. Boluk and A. B. Adesida, J. Tissue Eng., 2022, 13 DOI:10.1177/20417314221086368.
- W. Lee, J. C. Debasitis, V. K. Lee, J. H. Lee, K. Fischer, K. Edminster, J. K. Park and S. S. Yoo, Biomaterials, 2009, 30, 1587–1595 CrossRef CAS.
- L. Koch, A. Deiwick, S. Schlie, S. Michael, M. Gruene, V. Coger, D. Zychlinski, A. Schambach, K. Reimers, P. M. Vogt and B. Chichkov, Biotechnol. Bioeng., 2012, 109, 1855–1863 CrossRef CAS PubMed.
- T. Baltazar, J. Merola, C. Catarino, C. B. Xie, N. C. Kirkiles-Smith, V. Lee, S. Hotta, G. Dai, X. Xu, F. C. Ferreira, W. M. Saltzman, J. S. Pober and P. Karande, Tissue Eng., Part A, 2020, 26, 227–238 CrossRef CAS PubMed.
- Y. Shi, T. L. Xing, H. B. Zhang, R. X. Yin, S. M. Yang, J. Wei and W. J. Zhang, Biomed. Mater., 2018, 13, 035008 CrossRef CAS PubMed.
- T. J. Hinton, Q. Jallerat, R. N. Palchesko, J. H. Park, M. S. Grodzicki, H. J. Shue, M. H. Ramadan, A. R. Hudson and A. W. Feinberg, Sci. Adv., 2015, 1, e1500758 CrossRef PubMed.
- K. F. Lin, S. He, Y. Song, C. M. Wang, Y. Gao, J. Q. Li, P. Tang, Z. Wang, L. Bi and G. X. Pei, ACS Appl. Mater. Interfaces, 2016, 8, 6905–6916 CrossRef CAS PubMed.
- H. Wu, Y. Song, J. Li, X. Lei, S. Zhang, Y. Gao, P. Cheng, B. Liu, S. Miao, L. Bi, L. Yang and G. Pei, Cell Proliferation, 2020, 53, e12725 CrossRef.
- J. A. Inzana, D. Olvera, S. M. Fuller, J. P. Kelly, O. A. Graeve, E. M. Schwarz, S. L. Kates and H. A. Awad, Biomaterials, 2014, 35, 4026–4034 CrossRef CAS PubMed.
- W. Wang and K. W. Yeung, Bioact. Mater., 2017, 2, 224–247 Search PubMed.
- D. P. Lew and F. A. Waldvogel, The Lancet, 2004, 364, 369–379 CrossRef CAS PubMed.
- B. Li and R. M. Aspden, J. Bone Miner. Res., 1997, 12, 641–651 CrossRef CAS PubMed.
- K. N. Weilbaecher, T. A. Guise and L. K. McCauley, Nat. Rev. Cancer, 2011, 11, 411–425 CrossRef CAS PubMed.
- K. E. Yu, K. D. Alder, M. T. Morris, A. M. Munger, I. Lee, S. V. Cahill, H.-K. Kwon, J. Back and F. Y. Lee, Ther. Adv. Musculoskeletal Dis., 2020, 12, 1759720X20966135 CrossRef CAS PubMed.
- T. Albrektsson and C. Johansson, Eur. Spine J., 2001, 10, S96–S101 CrossRef PubMed.
- N. Reznikov, M. Bilton, L. Lari, M. M. Stevens and R. Kröger, Science, 2018, 360, eaao2189 CrossRef PubMed.
- Z. Li, T. Du, C. Ruan and X. Niu, Bioact. Mater., 2021, 6, 1491–1511 CAS.
- M. M. Villa, L. Wang, J. Huang, D. W. Rowe and M. Wei, J. Biomed. Mater. Res., Part B, 2015, 103, 243–253 CrossRef PubMed.
- G. Thrivikraman, A. Athirasala, R. Gordon, L. Zhang, R. Bergan, D. R. Keene, J. M. Jones, H. Xie, Z. Chen and J. Tao, Nat. Commun., 2019, 10, 3520 CrossRef PubMed.
- C. Hu, D. Ashok, D. R. Nisbet and V. Gautam, Biomaterials, 2019, 219, 119366 CrossRef CAS PubMed.
- L. Zhang, G. Yang, B. N. Johnson and X. Jia, Acta Biomater., 2019, 84, 16–33 CrossRef CAS PubMed.
- Y. B. Kim, H. Lee and G. H. Kim, ACS Appl. Mater. Interfaces, 2016, 8, 32230–32240 CrossRef CAS PubMed.
- N. Diamantides, L. Wang, T. Pruiksma, J. Siemiatkoski, C. Dugopolski, S. Shortkroff, S. Kennedy and L. J. Bonassar, Biofabrication, 2017, 9, 034102 CrossRef PubMed.
- D. F. Duarte Campos, A. Blaeser, K. Buellesbach, K. S. Sen, W. Xun, W. Tillmann and H. Fischer, Adv. Healthcare Mater., 2016, 5, 1336–1345 CrossRef CAS PubMed.
- J. Lee, M. Yeo, W. Kim, Y. Koo and G. H. Kim, Int. J. Biol. Macromol., 2018, 110, 497–503 CrossRef CAS PubMed.
- A. Lode, M. Meyer, S. Brüggemeier, B. Paul, H. Baltzer, M. Schröpfer, C. Winkelmann, F. Sonntag and M. Gelinsky, Biofabrication, 2016, 8, 015015 CrossRef PubMed.
- M. G. Yeo and G. H. Kim, Biofabrication, 2017, 9, 025004 CrossRef PubMed.
- K.-F. Lin, S. He, Y. Song, C.-M. Wang, Y. Gao, J.-Q. Li, P. Tang, Z. Wang, L. Bi and G.-X. Pei, ACS Appl. Mater. Interfaces, 2016, 8, 6905–6916 CrossRef CAS PubMed.
- J. A. Inzana, D. Olvera, S. M. Fuller, J. P. Kelly, O. A. Graeve, E. M. Schwarz, S. L. Kates and H. A. Awad, Biomaterials, 2014, 35, 4026–4034 CrossRef CAS PubMed.
- L. Ma, X. Wang, N. Zhao, Y. Zhu, Z. Qiu, Q. Li, Y. Zhou, Z. Lin, X. Li and X. Zeng, ACS Appl. Mater. Interfaces, 2018, 10, 42146–42154 CrossRef CAS PubMed.
- L. Ma, X. Wang, N. Zhao, Y. Zhu, Z. Qiu, Q. Li, Y. Zhou, Z. Lin, X. Li, X. Zeng, H. Xia, S. Zhong, Y. Zhang, Y. Wang and C. Mao, ACS Appl. Mater. Interfaces, 2018, 10, 42146–42154 CrossRef CAS PubMed.
- M. Sayahi, J. Santos, H. El-Feki, C. Charvillat, F. Bosc, I. Karacan, B. Milthorpe and C. Drouet, Mater. Today Chem., 2020, 16, 100230 CrossRef CAS.
- C. Garbo, J. Locs, M. D’Este, G. Demazeau, A. Mocanu, C. Roman, O. Horovitz and M. Tomoaia-Cotisel, Int. J. Nanomed., 2020, 1037–1058 CrossRef CAS.
- A. K. Teotia, K. Dienel, I. Qayoom, B. van Bochove, S. Gupta, J. Partanen, J. Seppälä and A. Kumar, ACS Appl. Mater. Interfaces, 2020, 12, 48340–48356 CrossRef CAS PubMed.
- X. Pei, L. Ma, B. Zhang, J. Sun, Y. Sun, Y. Fan, Z. Gou, C. Zhou and X. Zhang, Biofabrication, 2017, 9, 045008 CrossRef PubMed.
- A. Barba, A. Diez-Escudero, Y. Maazouz, K. Rappe, M. Espanol, E. B. Montufar, M. Bonany, J. M. Sadowska, J. Guillem-Marti and C. Ohman-Magi, ACS Appl. Mater. Interfaces, 2017, 9, 41722–41736 CrossRef CAS.
- L. Yu, D. W. Rowe, I. P. Perera, J. Zhang, S. L. Suib, X. Xin and M. Wei, ACS Appl. Mater. Interfaces, 2020, 12, 18235–18249 CrossRef CAS PubMed.
- A. K. Özenler, T. Distler, A. R. Akkineni, F. Tihminlioglu, M. Gelinsky and A. R. Boccaccini, Biofabrication, 2024, 16, 025027 CrossRef PubMed.
- Z. Ebrahimi, S. Irani, A. Ardeshirylajimi and E. Seyedjafari, Sci. Rep., 2022, 12, 12359 CrossRef CAS PubMed.
- J.-H. Shim, J.-Y. Won, J.-H. Park, J.-H. Bae, G. Ahn, C.-H. Kim, D.-H. Lim, D.-W. Cho, W.-S. Yun and E.-B. Bae, Int. J. Mol. Sci., 2017, 18, 899 CrossRef PubMed.
- E. Pugliese, A. Rossoni and D. I. Zeugolis, Biomater. Adv., 2023, 213740 Search PubMed.
- S. Swetha, K. Lavanya, R. Sruthi and N. Selvamurugan, J. Mater. Chem. B, 2020, 8, 9836–9862 RSC.
- J. Lee and G. Kim, ACS Biomater. Sci. Eng., 2018, 4, 278–289 CrossRef CAS PubMed.
- Y. Raymond, M. Bonany, C. Lehmann, E. Thorel, R. Benítez, J. Franch, M. Espanol, X. Solé-Martí, M.-C. Manzanares and C. Canal, Acta Biomater., 2021, 135, 671–688 CrossRef CAS PubMed.
- M. Bini, S. Grandi, D. Capsoni, P. Mustarelli, E. Saino and L. Visai, J. Phys. Chem. C, 2009, 113, 8821–8828 CrossRef CAS.
- M. Kairon Mubina, S. Shailajha, R. Sankaranarayanan and M. Iyyadurai, J. Sol-Gel Sci. Technol., 2022, 103, 151–171 CrossRef CAS.
- R. G. Ribas, V. M. Schatkoski, T. L. do Amaral Montanheiro, B. R. C. de Menezes, C. Stegemann, D. M. G. Leite and G. P. Thim, Ceram. Int., 2019, 45, 21051–21061 CrossRef CAS.
- L. Li, H. Hu, Y. Zhu, M. Zhu and Z. Liu, Ceram. Int., 2019, 45, 10997–11005 CrossRef CAS.
- J. S. Fernandes, P. Gentile, R. A. Pires, R. L. Reis and P. V. Hatton, Acta Biomater., 2017, 59, 2–11 CrossRef CAS PubMed.
- M. Rizwan, M. Hamdi and W. J. Basirun, J. Biomed. Mater. Res., Part A, 2017, 105, 3197–3223 CrossRef CAS.
- H. R. Fernandes, A. Gaddam, A. Rebelo, D. Brazete, G. E. Stan and J. M. Ferreira, Mater. Des., 2018, 11, 2530 CAS.
- N. S. Kajave, T. Schmitt, T.-U. Nguyen, A. K. Gaharwar and V. Kishore, Biomed. Mater., 2021, 16, 035003 CrossRef CAS PubMed.
- G. A. Rico-Llanos, S. Borrego-González, M. Moncayo-Donoso, J. Becerra and R. Visser, Polymers, 2021, 13, 599 CrossRef CAS PubMed.
- L. Guo, Z. Liang, L. Yang, W. Du, T. Yu, H. Tang, C. Li and H. Qiu, J. Controlled Release, 2021, 338, 571–582 CrossRef CAS PubMed.
- V. Martin, I. A. Ribeiro, M. M. Alves, L. Gonçalves, R. A. Claudio, L. Grenho, M. H. Fernandes, P. Gomes, C. F. Santos and A. F. Bettencourt, Mater. Sci. Eng., C, 2019, 101, 15–26 CrossRef CAS PubMed.
- N. Celikkin, C. Rinoldi, M. Costantini, M. Trombetta, A. Rainer and W. Święszkowski, Mater. Sci. Eng., C, 2017, 78, 1277–1299 CrossRef CAS PubMed.
- S. Ullah and X. Chen, Appl. Mater. Today, 2020, 20, 100656 CrossRef.
- X. Xing, Y. Han and H. Cheng, Int. J. Biol. Macromol., 2023, 240, 124407 CrossRef CAS PubMed.
- J. Wang, Q. Yang, C. Mao and S. Zhang, J. Biomed. Mater. Res., Part A, 2012, 100, 2929–2938 CrossRef PubMed.
- G.-J. Lai, K. Shalumon, S.-H. Chen and J.-P. Chen, Carbohydr. Polym., 2014, 111, 288–297 CrossRef CAS PubMed.
- J. Melke, S. Midha, S. Ghosh, K. Ito and S. Hofmann, Acta Biomater., 2016, 31, 1–16 CrossRef CAS PubMed.
- G. L. Jones, A. Motta, M. J. Marshall, A. J. El Haj and S. H. Cartmell, Biomaterials, 2009, 30, 5376–5384 CrossRef CAS PubMed.
- S. Salehi, H. Ghomi, S. Hassanzadeh-Tabrizi, N. Koupaei and M. Khodaei, Int. J. Biol. Macromol., 2022, 221, 1325–1334 CrossRef CAS PubMed.
- B. Yedekçi, A. Tezcaner, B. Yılmaz, T. Demir and Z. Evis, J. Mech. Behav. Biomed. Mater., 2022, 125, 104941 CrossRef PubMed.
- Z. U. Arif, M. Y. Khalid, R. Noroozi, A. Sadeghianmaryan, M. Jalalvand and M. Hossain, Int. J. Biol. Macromol., 2022, 218, 930–968 CrossRef CAS PubMed.
- M. P. Prabhakaran, L. Ghasemi-Mobarakeh and S. Ramakrishna, J. Nanosci. Nanotechnol., 2011, 11, 3039–3057 CrossRef CAS PubMed.
- Y. S. Cho, M.-S. Ghim, M. W. Hong, Y. Y. Kim and Y.-S. Cho, Mater. Des., 2023, 229, 111913 CrossRef CAS.
- K. Martyniak, A. Lokshina, M. A. Cruz, M. Karimzadeh, R. Kemp and T. J. Kean, Acta Biomater., 2022, 152, 221–234 CrossRef CAS PubMed.
- S. Rhee, J. L. Puetzer, B. N. Mason, C. A. Reinhart-King and L. J. Bonassar, ACS Biomater. Sci. Eng., 2016, 2, 1800–1805 CrossRef CAS PubMed.
- X. Yang, S. Li, Y. Ren, L. Qiang, Y. Liu, J. Wang and K. Dai, Composites, Part B, 2022, 237, 109863 CrossRef CAS.
- J. Huang, J. Xiong, D. Wang, J. Zhang, L. Yang, S. Sun and Y. Liang, Gels, 2021, 7, 144 CrossRef CAS PubMed.
- J. H. Galarraga, M. Y. Kwon and J. A. Burdick, Sci. Rep., 2019, 9, 19987 CrossRef CAS PubMed.
- L. Sun, Y. Xu, Y. Han, J. Cui, Z. Jing, D. Li, J. Liu, C. Xiao, D. Li and B. Cai, Orthop. Surg., 2023, 15, 3026–3045 CrossRef PubMed.
- M. Li, D. Sun, J. Zhang, Y. Wang, Q. Wei and Y. Wang, Biomater. Sci., 2022, 10, 5430–5458 RSC.
- W. Jiang, L. Li, D. Zhang, S. Huang, Z. Jing, Y. Wu, Z. Zhao, L. Zhao and S. Zhou, Acta Biomater., 2015, 25, 240–252 CrossRef CAS PubMed.
- J.-H. Shim, K.-M. Jang, S. K. Hahn, J. Y. Park, H. Jung, K. Oh, K. M. Park, J. Yeom, S. H. Park and S. W. J. B. Kim, Biofabrication, 2016, 8, 014102 CrossRef PubMed.
- E. Steck, J. Fischer, H. Lorenz, T. Gotterbarm, M. Jung and W. Richter, Stem Cells Dev., 2009, 18, 969–978 CrossRef CAS PubMed.
- A. Armiento, M. Stoddart, M. Alini and D. Eglin, Acta Biomater., 2018, 65, 1–20 CrossRef CAS PubMed.
- J. L. Silverberg, A. R. Barrett, M. Das, P. B. Petersen, L. J. Bonassar and I. Cohen, Biophys. J., 2014, 107, 1721–1730 CrossRef CAS PubMed.
- J. Pan, X. Zhou, W. Li, J. E. Novotny, S. B. Doty and L. Wang, J. Orthop. Res., 2009, 27, 1347–1352 CrossRef PubMed.
- Y. Liu, L. Peng, L. Li, C. Huang, K. Shi, X. Meng, P. Wang, M. Wu, L. Li and H. Cao, Biomaterials, 2021, 279, 121216 CrossRef CAS PubMed.
- B. B. Christensen, C. B. Foldager, M. L. Olesen, L. Vingtoft, J. H. D. Rölfing, S. Ringgaard and M. Lind, J. Exp. Orthop., 2015, 2, 1–11 CrossRef PubMed.
- H. Gao, Q. Pan, W. Dong and Y. Yao, Ann. Biomed. Eng., 2022, 50, 1232–1242 CrossRef PubMed.
- C. Y. C. Montesdeoca, S. Afewerki, T. D. Stocco, M. A. F. Corat, M. M. M. de Paula, F. R. Marciano and A. O. Lobo, Colloids Surf., B, 2020, 194, 111192 CrossRef CAS PubMed.
- V. Smolinska, M. Debreova, M. Culenova, M. Csobonyeiova, A. Svec and L. Danisovic, Int. J. Mol. Sci., 2022, 23, 2490 CrossRef CAS PubMed.
- D. J. Huey, J. C. Hu and K. A. Athanasiou, Science, 2012, 338, 917–921 CrossRef CAS PubMed.
- X. Lan, Y. Liang, E. J. Erkut, M. Kunze, A. Mulet-Sierra, T. Gong, M. Osswald, K. Ansari, H. Seikaly and Y. Boluk, FASEB J., 2021, 35, e21191 CrossRef CAS PubMed.
- T. Hirsch, T. Rothoeft, N. Teig, J. W. Bauer, G. Pellegrini, L. De Rosa, D. Scaglione, J. Reichelt, A. Klausegger and D. Kneisz, Nature, 2017, 551, 327–332 CrossRef CAS PubMed.
- U. Wölfle, G. Seelinger, G. Bauer, M. C. Meinke, J. Lademann and C. M. Schempp, Skin Pharmacol. Physiol., 2014, 27, 316–332 CrossRef PubMed.
- F. Zarei and A. Abbaszadeh, J. Cosmetic Laser Therapy, 2018, 20, 193–197 CrossRef PubMed.
- G. Marfia, S. E. Navone, C. Di Vito, N. Ughi, S. Tabano, M. Miozzo, C. Tremolada, G. Bolla, C. Crotti and F. Ingegnoli, Organogenesis, 2015, 11, 183–206 CrossRef PubMed.
- E. M. Tottoli, R. Dorati, I. Genta, E. Chiesa, S. Pisani and B. Conti, Pharmaceutics, 2020, 12, 735 CrossRef CAS PubMed.
- S. T. Boyce and A. L. Lalley, Int. J. Burns Trauma, 2018, 6, 4 Search PubMed.
- L. Yildirimer, N. T. Thanh and A. M. Seifalian, Trends Biotechnol., 2012, 30, 638–648 CrossRef CAS PubMed.
- R. F. Pereira, C. C. Barrias, P. L. Granja and P. J. Bartolo, Nanomedicine, 2013, 8, 603–621 CrossRef CAS PubMed.
- K.-I. Jang, H. U. Chung, S. Xu, C. H. Lee, H. Luan, J. Jeong, H. Cheng, G.-T. Kim, S. Y. Han and J. W. Lee, Nat. Commun., 2015, 6, 6566 CrossRef CAS PubMed.
- H. S. Kim, X. Sun, J.-H. Lee, H.-W. Kim, X. Fu and K. W. Leong, Adv. Drug Delivery Rev., 2019, 146, 209–239 CrossRef CAS PubMed.
- S. Li, H. Li, X. Shang, J. He and Y. Hu, MedComm: Biomater. Appl., 2023, 2(3), e46 Search PubMed.
- L. Koch, A. Deiwick, S. Schlie, S. Michael, M. Gruene, V. Coger, D. Zychlinski, A. Schambach, K. Reimers and P. M. Vogt, Biotechnol. Bioeng., 2012, 109, 1855–1863 CrossRef CAS PubMed.
- T. Baltazar, J. Merola, C. Catarino, C. B. Xie, N. C. Kirkiles-Smith, V. Lee, S. Hotta, G. Dai, X. Xu and F. C. Ferreira, Tissue Eng., Part A, 2020, 26, 227–238 CrossRef CAS PubMed.
- Y. Shi, T. Xing, H. Zhang, R. Yin, S. Yang, J. Wei and W. Zhang, Biomed. Mater., 2018, 13, 035008 CrossRef CAS PubMed.
- Y. Liu and D. Fan, Int. J. Biol. Macromol., 2019, 141, 700–712 CrossRef CAS PubMed.
- Z. Li, C. Ruan and X. Niu, Med. Novel Technol. Devices, 2023, 17, 100211 CrossRef.
- W. Lee, J. C. Debasitis, V. K. Lee, J.-H. Lee, K. Fischer, K. Edminster, J.-K. Park and S.-S. Yoo, Biomaterials, 2009, 30, 1587–1595 CrossRef CAS PubMed.
- K.-Y. Song, W.-J. Zhang and M. Behzadfar, Biomed. Eng. Lett., 2024, 1–10 Search PubMed.
- T. Bedir, S. Ulag, C. B. Ustundag and O. Gunduz, Mater. Sci. Eng., C, 2020, 110, 110741 CrossRef CAS PubMed.
- S. J. Lee, T. Esworthy, S. Stake, S. Miao, Y. Y. Zuo, B. T. Harris and L. G. Zhang, Adv. Biosyst., 2018, 2, 1700213 CrossRef.
- J.-P. Jiang, X.-Y. Liu, F. Zhao, X. Zhu, X.-Y. Li, X.-G. Niu, Z.-T. Yao, C. Dai, H.-Y. Xu and K. Ma, Neural Regener. Res., 2020, 15, 959–968 CrossRef CAS PubMed.
- A. T. Mneimneh and M. M. Mehanna, Int. J. Pharm., 2021, 601, 120559 CrossRef CAS PubMed.
- J. Yoo, J. H. Park, Y. W. Kwon, J. J. Chung, I. C. Choi, J. J. Nam, H. S. Lee, E. Y. Jeon, K. Lee and S. H. Kim, Biomater. Sci., 2020, 8, 6261–6271 RSC.
- S. Suri and C. E. Schmidt, Tissue Eng., Part A, 2010, 16, 1703–1716 CrossRef CAS PubMed.
- W.-H. Huang, S.-L. Ding, X.-Y. Zhao, K. Li, H.-T. Guo, M.-Z. Zhang and Q. Gu, Mater. Today Bio, 2023, 20, 100639 CrossRef CAS PubMed.
- X. Li, J. Han, Y. Zhao, W. Ding, J. Wei, J. Li, S. Han, X. Shang, B. Wang and B. Chen, Acta Biomater., 2016, 30, 233–245 CrossRef CAS PubMed.
- X. Chen, Y. Zhao, X. Li, Z. Xiao, Y. Yao, Y. Chu, B. Farkas, I. Romano, F. Brandi and J. Dai, Adv. Healthcare Mater., 2018, 7, e1800315 CrossRef PubMed.
- C. Chen, M. l Zhao, R. K. Zhang, G. Lu, C. Y. Zhao, F. Fu, H. T. Sun, S. Zhang, Y. Tu and X. H. Li, J. Biomed. Mater. Res., Part A, 2017, 105, 1324–1332 CrossRef CAS PubMed.
- Z. Chen, H. Zhang, C. Fan, Y. Zhuang, W. Yang, Y. Chen, H. Shen, Z. Xiao, Y. Zhao and X. Li, ACS Nano, 2021, 16, 1986–1998 CrossRef PubMed.
- X. Chen, Y. Zhao, X. Li, Z. Xiao, Y. Yao, Y. Chu, B. Farkas, I. Romano, F. Brandi and J. Dai, Adv. Healthcare Mater., 2018, 7, 1800315 CrossRef PubMed.
- X. Yang, Z. Lu, H. Wu, W. Li, L. Zheng and J. Zhao, Mater. Sci. Eng., C, 2018, 83, 195–201 CrossRef CAS PubMed.
- J. Zhou, Q. Li, Z. Tian, Q. Yao and M. Zhang, Mater. Today Bio, 2023, 23, 2930–2940 Search PubMed.
- Y. B. Kim, H. Lee and G. H. Kim, ACS Appl. Mater. Interfaces, 2016, 8, 32230–32240 CrossRef CAS PubMed.
- Q. Li, X. Lei, X. Wang, Z. Cai, P. Lyu and G. Zhang, Tissue Eng., Part A, 2019, 25, 1261–1271 CrossRef CAS PubMed.
- J. Lee, M. Yeo, W. Kim, Y. Koo and G. H. Kim, Int. J. Biol. Macromol., 2018, 110, 497–503 CrossRef CAS PubMed.
- A. Lode, M. Meyer, S. Brüggemeier, B. Paul, H. Baltzer, M. Schröpfer, C. Winkelmann, F. Sonntag and M. Gelinsky, Biofabrication, 2016, 8, 015015 CrossRef PubMed.
- W. J. Kim, H.-S. Yun and G. H. Kim, Sci. Rep., 2017, 7, 3181 CrossRef PubMed.
| This journal is © The Royal Society of Chemistry 2025 |