Xanthene-based NIR organic phototheranostics agents: design strategies and biomedical applications
Xiao-Yun
Ran†
 a,
Yuan-Feng
Wei†
b,
Yan-Ling
Wu
a,
Li-Rui
Dai
c,
Wen-Li
Xia
a,
Pei-Zhi
Zhou
c and
Kun
Li
a,
Yuan-Feng
Wei†
b,
Yan-Ling
Wu
a,
Li-Rui
Dai
c,
Wen-Li
Xia
a,
Pei-Zhi
Zhou
c and
Kun
Li
 *a
*a
aKey Laboratory of Green Chemistry and Technology of Ministry of Education, College of Chemistry, Sichuan University, Chengdu 610064, P. R. China. E-mail: kli@scu.edu.cn
bDivision of Abdominal Tumor Multimodality Treatment, Cancer Center, West China Hospital, Sichuan University, Chengdu 610041, China
cDepartment of Neurosurgery, West China Hospital of Sichuan University, Chengdu, Sichuan, China
First published on 14th January 2025
Abstract
Fluorescence imaging and phototherapy in the near-infrared window (NIR, 650–1700 nm) have attracted great attention for biomedical applications due to their minimal invasiveness, ultra-low photon scattering and high spatial–temporal precision. Among NIR emitting/absorbing organic dyes, xanthene derivatives with controllable molecular structures and optical properties, excellent fluorescence quantum yields, high molar absorption coefficients and remarkable chemical stability have been extensively studied and explored in the field of biological theranostics. The present study was aimed at providing a comprehensive summary of the progress in the development and design strategies of xanthene derivative fluorophores for advanced biological phototheranostics. This study elucidated several representative controllable strategies, including electronic programming strategies, extension of conjugated backbones, and strategic establishment of activatable fluorophores, which enhance the NIR fluorescence of xanthene backbones. Subsequently, the development of xanthene nanoplatforms based on NIR fluorescence for biological applications was detailed. Overall, this work outlines future efforts and directions for improving NIR xanthene derivatives to meet evolving clinical needs. It is anticipated that this contribution could provide a viable reference for the strategic design of organic NIR fluorophores, thereby enhancing their potential clinical practice in future.
1. Introduction
Cancer remains a significant threat to human health, driven by the rapid proliferation, systemic dissemination, and metastasis of malignant cells. Early detection is crucial for improving treatment outcomes as it significantly increases the chances of successful intervention. Over the past several decades, medical imaging technologies have become central to cancer diagnosis, such as magnetic resonance imaging (MRI),1,2 X-rays,3 positron emission tomography (PET),4–6 computed tomography (CT),7–9 and ultrasound imaging.10,11 Despite their widespread adoption, these technologies face inherent limitations, including radioactivity, low sensitivity, high costs, and inadequate spatial–temporal resolution, which hinder their utility for real-time guidance during surgical and therapeutic procedures.At the same time, conventional cancer treatments, such as radiotherapy, chemotherapy, targeted therapy, immunotherapy, and surgical resection, often suffer from issues related to suboptimal efficacy and severe side effects.1,12,13 These challenges have driven the search for more effective, targeted, and personalized treatment strategies. The emergence of precision medicine presents an exciting opportunity to address these limitations by combining diagnostic and therapeutic functions in a single, unified platform, thus maximizing therapeutic outcomes while overcoming the drawbacks of isolated diagnostic or treatment modalities.14,15 Among the most promising solutions are phototheranostic strategies, which integrate diagnostic imaging (e.g., photoacoustic imaging and fluorescence imaging) with therapeutic approaches (e.g., photodynamic therapy (PDT) and photothermal therapy (PTT)) to deliver precise diagnostics and localized treatments, thereby minimizing toxicity and aligning with the principles of precision oncology.16–23
Recent advancements in near-infrared (NIR, 650–1700 nm) imaging and therapy systems have transitioned from theoretical concepts to clinical applications.24–30 NIR fluorophores, ranging from single-walled carbon nanotubes (SWNTs),26,31 quantum dots,32–34 and lanthanide nanoparticles35–37 to organic small-molecule dyes,38–40 have shown tremendous potential in biomedical applications, particularly in fluorescence imaging and phototherapy. These innovations have paved the way for the integration of NIR-based phototherapeutic and diagnostic strategies. Small-molecule NIR dyes are particularly appealing due to their tunable photophysical properties, ease of chemical modification, biocompatibility, stability, and cost-effectiveness. Consequently, they have emerged as key players in NIR phototherapy and diagnostic applications. These dyes can be broadly categorized into four main classes based on their molecular structures, namely, polymethine cyanines,40–42 BODIPY dyes,43–45 BBTD-based donor–acceptor oligomers (D–A–D or A–D–A),46,47 and J-aggregates.48 Each of these classes offers distinct advantages, including strong fluorescence, high photostability, and synthetic flexibility, making them highly versatile. By modifying their structures, these fluorophores can achieve enhanced fluorescence intensities, optimized photothermal properties, and improved photodynamic activity, all of which are crucial for their effectiveness in NIR phototherapy (Scheme 1).20,41,49–56
Despite their advantages, as small-molecule dyes, xanthene-based dyes face challenges when compared to traditional NIR dyes, such as cyanine, semi-cyanine, BODIPY, and squaraine. Their relatively short excitation and emission wavelengths limit their use in in vivo imaging and phototherapy. Nevertheless, xanthene dyes offer significant promise owing to their multiple functionalization sites, which allow for modifications that can extend their absorption into the NIR region. This ability to extend absorption into the NIR spectrum makes xanthene dyes some of the most promising photosensitizers for NIR-based applications.38,41,57 Despite considerable progress in the synthesis and functionalization of xanthene derivatives and numerous reviews addressing their biological applications in imaging and detection, there is a noticeable gap in the literature regarding the design and synthesis of NIR xanthene derivatives as well as strategies to enhance their therapeutic and diagnostic capabilities. This gap is critical as these aspects are essential for developing high-performance xanthene photosensitizers capable of meeting the demands of modern cancer theranostics. This review aims to address this gap by providing a comprehensive discussion on the core structure of traditional xanthene derivatives and the key strategies used to extend their absorption into the NIR region. These strategies include structural modifications, aromatic ring fusion, polymerization, and supramolecular approaches. Furthermore, we will summarize the recent advancements in the use of xanthene derivatives for fluorescence imaging, PTT, PDT, and theranostics, providing insights into the design and development of advanced xanthene-based photosensitizers with enhanced therapeutic and imaging capabilities.
2. Colourful dyes with tenable photophysics
In 1871, Adolf von Bayer first synthesized fluorescein by combining phthalic anhydride and resorcinol with zinc chloride, marking a significant breakthrough in the development of fluorescent dyes.1 This discovery laid the foundation for the development of fluorescein- and rhodamine-based dyes, such as Rhodamine 123,58 which have become indispensable tools in chemical, biological, and medical research.42,59 These dyes are highly valued for their high sensitivity, real-time detection, and non-destructive analysis.60,61 Their structural flexibility, combined with water solubility, stability, and brightness, makes them particularly effective in fluorescence imaging applications, allowing researchers to visualize and quantify various biological processes with great precision.62–64However, the fluorescence emission of xanthene-based dyes typically falls within the visible spectrum, which limits their applications in deep tissue imaging and in vivo studies.24,39,65–67 The fluorescence properties of a fluorophore are primarily determined by the energy gap between the highest occupied molecular orbital (HOMO) and the lowest unoccupied molecular orbital (LUMO). To overcome the limitations of the visible spectrum and extend their utility for deep tissue penetration, particularly in near-infrared (NIR) applications, several strategies have been developed. These included narrowing the energy gap through extended conjugation, enhancing the rigidity, reducing bond rotation, and improving the fluorescence yield. Another effective approach was replacing the oxygen atom in the xanthene backbone, which red-shifts both the absorption and emission wavelengths, thereby making these dyes more suitable for NIR imaging and therapy (Fig. 1).
While xanthene dyes are widely utilized, their fluorescence wavelengths typically lie within the visible range, which limits their application in deep tissue imaging and in vivo diagnostics. To overcome this limitation, numerous strategies have been employed to extend the absorption and emission wavelengths of xanthene derivatives into the NIR region (650–1700 nm), where tissue penetration is optimal for biomedical applications. These strategies include modifications at the 9-position and 10-position of the xanthene core and conjugation extension, each of which plays a critical role in enhancing the optical properties of the dyes. This section explores the various structural modifications of xanthene dyes to optimize their properties for phototheranostic applications, which combine both diagnostic and therapeutic functions in a single platform. The section is divided into three subsections, each discussing key strategies for modifying the xanthene structure to enhance its optical properties and biological applications.
2.1. 9-Position modification
The 9-position of xanthene dyes plays a pivotal role in altering the photophysical and biological properties. Modifications at this position have a significant impact on fluorescence emission, solubility, and biological targeting, all of which are crucial for their application in imaging and therapeutics. This section explores the key strategies for 9-position modification, including amino, carbon-based, and alternative group modifications.In 2008, Wu and Burgess reported a novel synthesis route for amino-xanthene fluorophores.68 They started with ditriflyl xanthone and treated it with piperidine derivatives to produce a diamine intermediate. This intermediate was then triflated and reacted with appropriate amines to yield a variety of amino-modified xanthene derivatives. The resulting compounds exhibited green fluorescence, with the absorption maxima ranging from 456 to 499 nm and emission maxima between 537 and 562 nm. Notably, the Stokes shift of these compounds ranged from 59 nm to 82 nm, which was significantly larger than that of previous rhodamine derivatives, thus enhancing their signal-to-noise ratio in cell imaging (Fig. 2, F2-1-3).69
Further work by the Klan group in 2015 introduced acylamino and sulfonamide groups at the 9-position of Si Rhodamine.70 These modifications led to Stokes shifts as large as 210 nm for amido-Rhodamine (F2-4) and 173 nm for sulfamide-Rhodamine (F2-5), significantly improving the fluorescence properties and biological targeting capabilities of the dyes. These lysosomal-targeting dyes exhibited excellent photostability and high pH stability, making them ideal for lysosomal imaging and pH sensing applications.
In 2017, Hell's research group developed a series of amino-x Rhodamine derivatives by introducing secondary amino groups at the 9-position of heteroatom-substituted Rhodamine (Fig. 2, F2-6-9).71,72 The resulting dyes exhibited large Stokes shifts, all exceeding 135 nm, and showed excellent lysosomal targeting and cellular imaging abilities. This series further demonstrated the potential of amino-modified xanthene dyes for biomedical applications.
In 2017, Guo et al. developed a lysosome-targeting probe (F3-1) based on an alkene-Si-xanthene scaffold.73 This probe exhibited longer absorption and emission wavelengths compared to amino-modified xanthene derivatives, making it more suitable for detecting reactive oxygen species (ROS) in lysosomal cell death studies. The carbon-based modification not only extended the fluorescence range but also enhanced the signal-to-noise ratio for more accurate imaging.
Following this approach, Frei et al. (2019) synthesized a photoactivatable fluorophore (F3-2a), which was initially non-fluorescent but converted into a red-emitting photoproduct (F3-2c) upon UV irradiation.64 The emission wavelength of 670 nm allowed for controlled fluorescence activation in biological systems, making it ideal for dynamic imaging and therapeutic monitoring.
In addition, alkynyl-xanthene derivatives exhibit bathochromic shifts due to the electron-withdrawing properties of the carbon–carbon triple bond. These derivatives also exhibit higher quantum yields as the coplanarity between the triple bond and the fluorophore facilitates electron delocalization. For example, in 2014, Pastierik et al., developed mitochondria-targeted fluorescent dyes (F3-3a, b) based on 9-phenylethynylpyronine scaffolds, which exhibited redshifted emissions at 708 nm and 738 nm.74 Notably, Wei et al. reported various novel dyes utilizing the rational design strategy by replacing the 9-position with other groups based on xanthene skeleton, which was conjugated to the triple bonds and employed to visualize DNA replication and protein synthesis activity via 16-color imaging (F3-4).75
Xanthone exhibited strong electronic-donor capability and rigid conjunction skeleton, which has been developed for constructing fluorescence dyes by extending the π-conjugation. Li's group modified the xanthene-based fluorophore by incorporating a carbon atom at the 9-position, which resulted in significant bathochromic shifts. The donor–acceptor (D–A) interaction, reorganization energy, and intersystem crossing (ISC) were optimized to achieve high photothermal conversion efficiency (PCE) and reactive oxygen species (ROS) generation, ultimately enhancing the antitumor efficacy (F3-5-7) (Fig. 3).20,76–78
For instance, in 2015, Liu et al., developed thiolpyronine (F4-1), which could discriminate between cysteine (Cys), homocysteine (Hcy), and glutathione (GSH) based on the direct conjugation of a thiol group with the xanthene fluorophore.87 The probe exhibited no fluorescence in its free form, but fluorescence was restored upon exposure to thiol-containing compounds, demonstrating its biothiol-responsive imaging capabilities. Similarly, Zhou et al. (2017) developed a ratiometric probe (F4-2) for ovarian cancer diagnosis based on the conjugation of GSH and a pyronine moiety.88 The probe displayed bright red fluorescence at 622 nm in its free form, and after interacting with γ-glutamyltranspeptidase (GGT), it converted into fluorescent derivatives (F4-2a), which enabled the selective detection of cancerous tissues. Zhang et al.89 reported oxygenpyronine (F4-3), which discriminated between cancerous and normal cells. The free probe F4-3 displayed weak fluorescence regardless of excitation at 450 or 580 nm but showed distinct emission at 570 nm under 520 nm excitation. Upon the addition of Cys and GSH, F4-3 converted into aminopyronine F4-3a and thiolpyronine F4-3b, emitting at 545 nm and 622 nm, respectively. When incubated in cancer cells, probe F4-3 exhibited bright green and red fluorescence through distinct channels (Fig. 4).
9-Position-substituted xanthene-based probes possess several intrinsic advantages, including high wavelength tunability (ranging from the green to the near-infrared (NIR) region), a large Stokes shift, and remarkable photostability.79,87,90 Due to these properties, the modification of xanthene-based probes at the 9-position successfully addressed many of the limitations associated with classical fluorophores, making them an exceptionally powerful platform for designing phototheranostic agents. Additionally, incorporating diverse functional groups allows these probes to detect a variety of targets, such as nitric oxide (NO),91 peroxynitrite,92 glutathione (GSH),87 pH,93 and viscosity.94
2.2. 10-Position modification
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) shifts the absorption maxima into the near-infrared region, extending both the absorption and emission wavelengths without significantly affecting the dye's conjugated system.
O) shifts the absorption maxima into the near-infrared region, extending both the absorption and emission wavelengths without significantly affecting the dye's conjugated system.
For instance, in 2001, Drexhage's group replaced the oxygen atom with carbon, redshifting the fluorescence emission by approximately 50 nm (X-2).98 In 2010, Hell, S. W. et al., improved the detailed synthesis of a carbopyronin scaffold that allows modifications on the final product, i.e., a photostable dye with large fluorescence quantum yield and the required absorption and emission bands in the red.99 On the basis of carbon Rhodamine F5-1, increasing the rigidity of 3 and 6 amino substituents or lengthening the π-conjugation system of its fluorescent parent nucleus could further redshift the fluorescence emission wavelength (F5-2). In addition, in 2017, Yang's research group obtained diphenyl carbon Rhodamine F5-3 by extending the structure of two benzene rings on the carbon Rhodamine parent nucleus, which showed an extremely long fluorescence emission wavelength, reaching 921 nm (Fig. 5).100
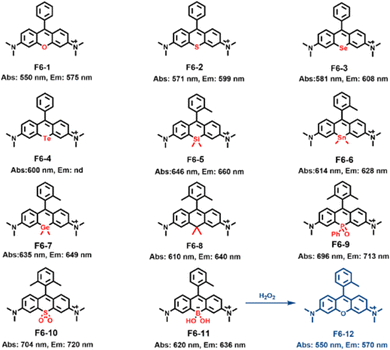
Other studies have focused on replacing oxygen with oxygen group, nitrogen group, and carbon group. In 2004, Detty's group reported Rhodamine dyes in which oxygen was replaced by sulfur and arsenic, resulting in redshifts of 24 and 33 nm, respectively.101 They also found that replacing O with S, Se, and Te enhanced the intersystem crossing (ISC) process, reducing the fluorescence quantum yield and promoting singlet oxygen production (F6-1-4).102,103 In 2008, Qian's group synthesized silicon-substituted Rhodamine, achieving a redshift of 90 nm.104 Later, Nagano and co-workers extended this strategy by replacing oxygen atom with germanium and tin, though their emission wavelengths were slightly lower than silicon Rhodamine, similar to carbon-substituted Rhodamine, all of which successfully extended the fluorescence emission wavelengths (F6-5-8).105 Next, a series of novel near-infrared (NIR) wavelength-excitable fluorescent dyes were prepared by modifying the Si-xanthene scaffold to obtain emission in the range suitable for in vivo imaging, which showed sufficiently high quantum efficiency and high tolerance to photobleaching in aqueous solution.106–111 In 2015, Wang's group used phosphorus to replace oxygen, where the strong electron-absorbing “P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O” group formed a σ*–π* conjugation with the Rhodamine core, successfully extending the emission wavelength to over 700 nm (F6-9).112 In 2016, Guo's research group synthesized sulfone Rhodamine by replacing oxygen with a highly electron-absorbing sulfone group. This resulted in emission wavelengths exceeding 720 nm, surpassing phosphorhodamine (F6-10). Additionally, sulfone Rhodamine exhibited high photostability and cell membrane penetration, making it a promising candidate for long-term biofluorescence imaging.113 In 2017, Cliff I. Stains et al., introduced a borinate functionality into the xanthene scaffold with the goal of leveraging the selective chemical reactivity of this group to afford a ratiometric sensor for H2O2, and they demonstrated the ability of F6-11 to yield robust and tunable ratiometric sensors (Fig. 6).114
O” group formed a σ*–π* conjugation with the Rhodamine core, successfully extending the emission wavelength to over 700 nm (F6-9).112 In 2016, Guo's research group synthesized sulfone Rhodamine by replacing oxygen with a highly electron-absorbing sulfone group. This resulted in emission wavelengths exceeding 720 nm, surpassing phosphorhodamine (F6-10). Additionally, sulfone Rhodamine exhibited high photostability and cell membrane penetration, making it a promising candidate for long-term biofluorescence imaging.113 In 2017, Cliff I. Stains et al., introduced a borinate functionality into the xanthene scaffold with the goal of leveraging the selective chemical reactivity of this group to afford a ratiometric sensor for H2O2, and they demonstrated the ability of F6-11 to yield robust and tunable ratiometric sensors (Fig. 6).114
More recently, heteroatom substitution with a ketone has been shown to enhance the photophysical properties of fluorophores (Fig. 7). In 2022, Schnermann's group replaced the oxygen bridge at the 10-position of the xanthene core with an electron-withdrawing ketone bridge, guided by computational design. This modification extended the absorbance maximum to 860 nm and the emission beyond 1000 nm (F7-1–F7-3).115 Although the quantum yield remains relatively low and stability is limited, the small molecular weight and good solubility offer significant opportunities for further exploration. Building on Schnermann's work, Evan W. Miller introduced cyclization to rigidify the nitrogen, resulting in a more stable and brighter analogue (F7-4).116
Among the various atom replacements in xanthenoid fluorophores, a prominent strategy involves the removal of the bridging atom. The removal of the bridging oxygen atom produces the xanthene fluorophore to achieve absorption in the near-infrared II (NIR-II) region. For example, in 1954, the Barker group synthesized a derivative from 3,6-dimethylaminofluorene, which exhibited long absorption wavelength up to 955 nm (F8-1).117 In 1991, Akiyama's group synthesized the 9-arylene-substituted aminofluorene derivative (F8-2), exhibiting an absorption wavelength beyond 1052 nm.118 Similarly, in 1999, Wainwright's group synthesized the fluorenylrhodamine analogue (F8-3), which also showed an absorption wavelength of 955 nm.119 Although this approach has been known for some time, it has only recently garnered significant attention. Until 2022, Garbacz et al.120 enriched the xanthenoid dye class by replacing nitrogen with oxygen, creating an asymmetric rhodol-like dye (F8-4) and symmetric fluorescein-like dyes (F8-5, 6). Building on Garbacz's work, in 2022, Fan Zhang et al.,121 developed diaminofluorene-based dyes with molecular weights of 299–504 Da, achieving NIR absorption from 700 to 1600 nm, suitable for in vivo NIR bioimaging and sensing applications (F8-7, 8) (Fig. 8).
2.3. Extending conjugated plane and chain
The extension of the π-conjugated system is a key strategy to enhance the absorption and emission properties of xanthene-based dyes, especially in the NIR region, where the tissue penetration and biological imaging capabilities are greatly improved. The extension of the conjugated system can be achieved through two main methods: extending the conjugated plane (e.g., by incorporating aromatic rings) and extending the conjugated chain (e.g., through the introduction of alkynyl or other conjugative linkages). These strategies lead to bathochromic shifts, increased fluorescence quantum yields, and enhanced photostability, all of which are essential for the application of these dyes in theranostics.While extending the absorption and emission wavelengths presents certain limitations, combining it with other modification strategies can lead to further redshifts. In 2012, Nagano's team developed silicon-based rhodamine dyes with emissions ranging from 660 nm to 740 nm through incremental modifications (F9-5-7).106 Applying 9-position modifications and expanding the π-conjugated system, Yang's group created a new family of brightly fluorescent dyes (F9-8) with absorption maxima in the deep-NIR spectral region of 800–1000 nm.100,128 Building on this, they reported high-performance NIR-II-active fluorochromic scaffolds, the tetra-benzannulated xanthene dyes (F9-9), which absorb at long wavelengths of ∼1200 nm.129 These dyes demonstrate remarkable resistance to dipole/H-bonding-induced symmetry breaking, possess structural rigidity, and exhibit superior photophysical properties along with good chemo- and photostability.
Drawing inspiration from the fluorescence switching mechanisms of donor–acceptor–donor (D–A–D) dyes such as CH 1055, techniques such as π-conjugation extension and donor–acceptor strength adjustment can lead to significant bathochromic shifts.46 In recent years, Colleen N. Scott's group has explored a D–A–D approach to develop far-red to NIR xanthene-based dyes. They initially coupled pyrrole and indole at the C-2 position of the xanthene core using the Suzuki–Miyaura reaction to create dye F10-1,130 which exhibited absorption and emission wavelengths between 665 nm and 717 nm. By introducing indolizine at the C-3 position via C–H activation, the absorption maxima were shifted to the NIR-II region (900–1100 nm) for dye F10-2.131 This extension of the conjugation system significantly enhanced the NIR fluorescence, and the dyes demonstrated excellent biological imaging capabilities.
Further modifications incorporated a thiophene spacer between the xanthene acceptor and amine donors, enhancing the donor strength and further extending the conjugation. This led to the development of two types of dyes: a thienylpiperidine donor (F10-3)132 and a series of thienyldibenzazepine donors (F10-4).91 These dyes exhibited absorption and emission maxima in the NIR-II region (890–1260 nm), and F10-4 was successfully used in a nano-receptor to detect nitric oxide (NO) in a mouse liver model. Jared H. Delcamp also contributed to the development of NIR-II fluorescent dyes by tuning indolizine donors with N,N-dimethylaniline (DMA) groups. His group synthesized FluIndz dyes (F10-5, 8),133–135 which exhibited absorption in the NIR-IIa and NIR-IIb regions and demonstrated “cyanine-like” behavior in CS2. These dyes had absorption ranging from 1590 to 2088 nm (F10-9), opening up new possibilities for deep-tissue imaging.136
Additionally, substituting silicon for oxygen in the xanthene core has been shown to induce bathochromic shifts of approximately 90 nm.104 Ellen M. Sletten's group investigated the combination of silicon-substituted xanthene with DMA-decorated indolizine donors to achieve longer absorption and emission wavelengths. This approach produced small-molecule organic fluorophores, with the emission extending over 300 nm beyond current capabilities and entering the NIR-II region.137 While indolizine has facilitated longer wavelength shifts compared to DMA, it remains unclear whether this is due to extended conjugation or increased donor strength from better overlap with the planar nitrogen atom. To distinguish these effects, vinyl aniline, which has a similar number of π-bonds and nitrogen donor electrons as indolizine, was appended as a donor. Recently, Huimin Ma et al. utilized xanthene to create the dye F10-6, which emits in the NIR-II range (720–1260 nm).138 Similarly, Jared H. Delcamp's group used DMA with silicon-substituted xanthene to develop NIR and NIR-II emitting materials (F10-7),139 comparing the photophysical properties of donor groups on a consistent Si-substituted xanthene-based core with varied structures and conjugation lengths (Fig. 10).
3. Biomedical applications
Xanthene-based functional dyes have been widely used in life science and materials science.140–142 Numerous studies have reviewed their functions in the field of materials science. Meanwhile, due to their excellent photophysical properties such as high fluorescence quantum yield, high molar extinction coefficients, good chemical stability, excellent photostability and easily modifiable backbones, they have been widely applied in the biomedical field.143,144 Previously, Oriola AO et al. reviewed their biological implications in antifungal, antibacterial, coagulant, antioxidant, anti-inflammatory, and insecticidal effects.145 Silva CFM et al. summarized xanthene as a core structure for the development of antileishmanial agents.146 Maia M et al. overviewed the design strategies and biological activities of xanthene in medicinal chemistry.147 In recent years, xanthene derivatives have been extensively studied and explored in the field of fluorescence bioimaging, biosensing and light-mediated therapy including phototherapy (PTT) and photodynamic therapy (PDT). For example, Dai M et al. presented an overview of strategies applied toward tuning the emission bathochromic shift to xanthene fluorophores and briefly exemplified its applications in bioimaging.148 Samanta S et al. overviewed recent trends in the development of contemporary super-resolution bioimaging strategies using common fluorophores including xanthene.149 Si D et al. reviewed the mechanism and design strategies of xanthene derivatives rhodamines and their applications in bioimaging and biosensing.150,151 Briefly, xanthene-based functional dyes have been used in bioimaging for some fields, but little has been summarized in cancer imaging and light-mediated PTT/PDT, as well as vascular imaging and neuroimaging; we will highlight in this review.FLI and phototherapy has been widely utilized in the diagnosis and treatment of solid tumors, skin diseases, and other diseases owing to its non-invasive detection, high spatiotemporal resolution, easy operation, and real-time monitoring. To accomplish high-performance diagnosis, the fluorescence probes should have good biocompatibility, large extinction coefficient in the NIR region, and specific tumor targeting.152,153 Several PSs such as indocyanine green and methylene blue have been approved by the U.S. Food and Drug Administration for clinical use and have been utilized for fluorescence-guided tumour resection during clinical surgery and therapy.154 Xanthene-based dyes have the advantages of strong absorption, excellent biocompatibility, and tunable optical properties. Thus, they are extremely popular in imaging and therapy.
3.1. FLI
FLI in the visible spectrum (400–650 nm) faces a constraint in terms of penetration depth due to light–tissue interactions.155 These interactions include substantial photon scattering, photon absorption, and the presence of tissue autofluorescence. Consequently, there is an urgent requirement for advancements in the field of deep tissue penetration and in vivo fluorescence imaging. The near-infrared spectrum is further categorized into two distinct channels: NIR-I (650–950 nm) and NIR-II (1000–1700 nm).21,24,35,156,157 While NIR-I fluorescence imaging is extensively used in both basic research and clinical applications, it also has some limitations. This method permits the observation of intricate biological processes only within a shallow depth region of about 0.2 mm, even though it provides micron-level spatial resolution.31,158 Recent research has showed the advantages of optical bioimaging within the NIR-II channel, which offers reduced tissue autofluorescence and signal attenuation. This translates into a significantly improved signal-to-noise ratio (SNR) and an approximate penetration depth of 1–3 mm. Particularly, when the emission wavelength falls within the NIR-IIb channel, autofluorescence becomes negligible. The exceptional characteristics of NIR-IIb materials, such as their brightness and high SNR, effectively synergize with 3D confocal imaging techniques, enabling the acquisition of intricate and detailed information from biological samples.159,160Urano et al. designed and synthesized a novel near-infrared fluorescent probe, FolateSiR-1,176 utilizing a Si-rhodamine fluorophore with a carboxy group at the benzene moiety. This probe is coupled to a folate ligand through a negatively charged tripeptide linker, resulting in very low background fluorescence and an SNR of up to 83 in folate receptor-expressing tumor-bearing mice within 30 minutes, allowing for precise tumor imaging (Fig. 11). Kanduluru et al.177 established strong binding and specificity of the NK1R-targeted ligand using a rhodamine conjugate, leading to the development of the NIR dye conjugate NK1RL-Peptide-LS288, which accurately images cancer and guides surgical removal in an NK1R-transfected HEK293 tumor xenograft model.
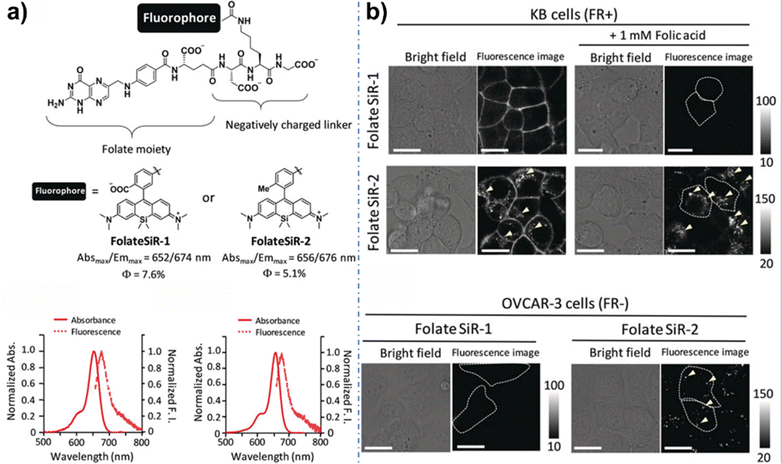 | ||
| Fig. 11 (a) Structures of FolateSiR-1 and FolateSiR-2 and their photophysical properties. (b) Fluorescence images of KB cells and OVCAR-3 cells incubated with FolateSiR-1 or FolateSiR-2 in the presence or absence of folic acid and 0.5% DMSO as a cosolvent. Reproduced with permission from ref. 176 Copyright 2020, Wiley. | ||
In 2020, Wang et al.178 developed an activatable two-photon NIR fluorescent probe, DHQ-Rd-PN (Fig. 12a). This probe exhibited increased NIR emission in response to peroxynitrite, facilitating the detection of ONOO− in both cells and in vivo. Moreover, it enabled the imaging of ONOO− production in xenograft 4T1 tumor-bearing mice. In 2023, Jiang et al.179 modified a rhodamine dye, observing different uptake and retention times across various cell types. Notably, cancer cells demonstrated greater uptake through active transport, allowing for prolonged retention of the fluorescent moiety, which enabled long-term tumor-specific imaging. They designed the probe NYL2-NQO1 to detect human nicotinamide adenine dinucleotide (phosphate) reduced (NAD(P)H): quinone oxidoreductase isozyme 1 (hNQO1), showing a higher signal contrast between cancerous and normal cells than its predecessor (Fig. 12b). Upon intravenous injection into A549-bearing nude mice, the tumor area was illuminated within two hours, revealing fluorescent signals 3.1-fold and 6.9-fold stronger than those in skin and muscle tissues, respectively. These results suggest that NYL2-NQO1 could be an effective tool for fluorescence-guided surgery, with broad potential in various NIR imaging applications, including photoacoustic imaging.
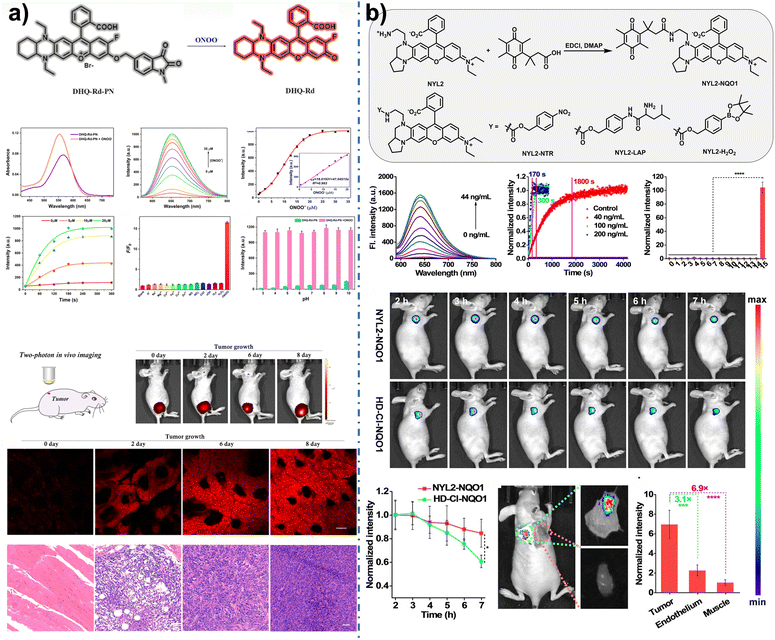 | ||
| Fig. 12 (a) Chemical structure of DHQ-Rd-PN and its two-photon in vivo fluorescence imaging of ONOO− in mice tumor. Reproduced with permission from ref. 178 Copyright 2020, American Chemical Society. (b) Synthesis pathway of NYL2-NQO1 and the structure of other probes and their photophysical properties and retention in mice tumors as well as their ability to distinguish tumor boundaries. Reproduced with permission from ref. 179 Copyright 2023, Wiley. | ||
Li et al. incorporated a pyridine group at the 9-position of xanthene to develop pyridine-Si-xanthene (Py-SiRh), a near-infrared fluorescent platform exhibiting good solubility and intrinsic targeting ability for lysosomes.180Py-SiRh showed a red-shifted emission wavelength and good modifiability compared to traditional Si-rhodamines, making it an excellent platform for studying lysosomal cell death. Additionally, a phosphorus-amino-rhodamine (Q-P-ARh) system was prepared, demonstrating exceptional penetration ability within one minute and photostability, enabling the monitoring of lipophagy and capturing its dynamic process, thus greatly facilitating the study of autophagy pathways (Fig. 13a).166 In 2023, we developed Si-NH2-Glu, a novel meso-amine Si-Rhodamine that incorporates γ-glutamyl transpeptidase and pH dual-responsive sites, making it suitable for orthotopic tumor imaging and fluorescence-guided surgery.86 Additionally, we introduced a combination modification method for optimizing sulfone–xanthone performance by incorporating an amino group at the meso-position and extending the π-system. This resulted in meso-amino-substituted sulfone–xanthone derivatives (J-S-LS301),181 which exhibited strong fluorescent signals in subcutaneous and orthotopic transplantation tumor models of hepatocellular carcinoma. In 2024, we designed multicoloured and pKa-tunable probes by inserting different heteroatoms on rhodamine X-10. Moreover, theoretical calculations verified the rationality and developability of the design strategy. Overall, a novel and versatile strategy was provided to construct a series of pyridinamine-functionalized rhodamine probes. Among them, Si-4Py possesses a near-infrared emission wavelength, a suitable pKa, and pH hypersensitivity. Moreover, Si-4Py was applied for high-contrast imaging and fluorescence-guided surgery of different tumours. It can distinguish peritoneally disseminated ovarian tumours from normal tissue within 10 minutes, with a high SNR > 4.0, and highly accurate tumour identification, together with its broad cancer specificity, making Si-4Py a particularly effective tool for fluorescence-guided tumour resection (Fig. 13b).93 In the same year, our group investigated the currently available xanthene dyes through a machine learning-assisted strategy and constructed a quantitative prediction model to facilitate the rational synthesis of novel fluorescent molecules with desired pH responsiveness. Next, we successfully synthesised two novel Si-rhodamine derivatives, and a series of experiments demonstrated that SiR-CTS-pH has a higher signal-to-noise ratio for fluorescence imaging. In addition, SiR-CTS-pH has a strong differentiation ability for tumour cells and tissues and could accurately distinguish complex liver cancer tissues from normal tissues, which shows its great potential for clinical application.182
 | ||
| Fig. 13 (a) Structures of P-P-ARh, I-P-ARh, and Q-P-ARh and dynamic movement images of the lipophagy process of 3T3-L1 preadipocytes. Reproduced with permission from ref. 166 Copyright 2022, Wiley. (b) Multi-color and pKa-tunable platform and the fluorescence images of different living cells. Reproduced with permission from ref. 93 Copyright 2024, Wiley. | ||
Fluorescence bioimaging in the near-infrared II (NIR-II) window enables the visualization of deep tissue with ultrahigh resolution. Simultaneously, there is an urgent need for effective biosensing in deep tissue, requiring fluorescent probes that can selectively respond to specific stimuli. NIR-II fluorescent probes facilitate the visualization of biological and pathological processes at greater depths.95 While organic fluorophores offer a wide variety of classes with tunable spectral properties, only a limited number can absorb and emit in the NIR-II range, despite many being available for the NIR-I region (700–1000 nm). These NIR-II organic fluorophores have demonstrated the highest resolution in vivo fluorescence imaging to date. Traditionally, xanthene-type fluorophores, such as fluorescein and rhodamine, are primarily regarded as visible-region fluorophores. However, Yang et al. recently reported a novel bis-benzannulated xanthenoid dye (ECX) with a carbon-based bridging group (xanthene) that holds promise for deep-NIR applications.100 They hypothesize that a silicon analogue of ECX could enhance these properties. By incorporating an atom of larger radius than carbon, they suggested that increased ring strain may suppress the vibrational mode.128 Building on prior work, the researchers reported the rational design, synthesis, spectral analysis, and functionalization of the first tetra-benzannulated xanthene dye in its class, demonstrating its potential for biomedical applications through proof-of-concept two-channel and three-channel models (Fig. 14a).129 The imaging of the entire mouse body revealed the temporal and spatial variations in fluorescence intensity, reflecting the circulation and distribution of ESi5ain vivo. Using a stereomicroscopic imaging system, finer structures, including micro cerebral blood vessels as small as 30 μm in diameter, were visualized. Recognizing the significance of bridged atoms in rhodamines, Zhang et al.52 proposed a dual-bridge strategy to create a new scaffold, termed 2X-rhodamine (2XR). This involved extending the chromophore core with a vinylene moiety to redshift the wavelength and incorporating two atomic bridges, X1 and X2, which formed five- and six-membered rings to enhance the structural rigidity and reduce nonradiative decay. Consequently, the 2XR scaffold, featuring sulfur (S) at X1 and C(CH3)2 at X2, exhibited absorption and emission peaks at approximately 715 nm and 765 nm, respectively, along with a bright emissive tail beyond 1000 nm, high quantum yield (ΦF = 0.11), long fluorescence lifetime (τ = 1.1 ns), and notable log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KL–Z value of −1.8 under aqueous conditions (Fig. 14b).
KL–Z value of −1.8 under aqueous conditions (Fig. 14b).
 | ||
| Fig. 14 (a) Synthesis of EC7 and a PEG-tagged analogue (EC7-PEG5000) and in vivo three-color fluorescence imaging. Reproduced with permission from ref. 129 Copyright 2023, American Chemical Society. (b) Synthetic route of (S, C(CH3)2)-2XR and the fluorescence images in vivo and in vitro. Reproduced with permission from ref. 52 Copyright 2024, American Chemical Society. | ||
Expanding the conjugated structure is an effective approach to redshift the absorption and emission wavelengths of fluorescent probes. One direct method is replacing the 3′,6′-hydroxyls with sp2 carbon. A recent advancement involved synthesizing an NIR-II-emitting xanthene-based fluorophore by substituting indolizine heterocycles for alkyl amine donors, resulting in a ∼400 nm (1.01 eV) bathochromic shift in the absorbance to 930 nm and an SWIR emission maximum at 1092 nm.131 In 2021, Ma et al. para-functionalized styrene-based donors, yielding fluorophores with remarkably low-energy emission maxima at 1210 nm. These compounds were constructed by adding para-substituted styryl groups to the 3′,6′-positions of the xanthene core, thereby enlarging the π-conjugation and enhancing the electron-donating ability, which effectively increased the dye's emission wavelength. Among these, VIX-4 exhibited NIR-II fluorescence at 1210 nm with a large Stokes shift and high brightness, enabling the monitoring of blood circulation in the entire mouse body through high-speed dynamic imaging at frame rates up to 200 fps. Blood flow volumes in femoral vessels were directly measured using high spatiotemporal imaging.138 Following this, J. H. Delcamp et al.137 designed and synthesized a series of SiRos fluorophores, characterizing their photophysical properties. SiRos1300, SiRos1550, and SiRos1700 displayed emission maxima at 1300 nm, 1557 nm, and 1700 nm, with fluorescence quantum yields of 0.0056%, 0.0025%, and 0.0011%, respectively. In vivo NIR-II imaging experiments were conducted using SiRos1300 and SiRos1550 in canola oil-based nanoemulsions, achieving full circulatory distribution and high-resolution imaging of mouse vasculature in the femoral arteries, abdominal cavity, and jugular veins. In 2019, Lei Z et al.183 developed a series of wavelength-tunable, highly stable NIR-II fluorescent dyes (CX-1, CX-2, and CX-3), which demonstrated superior chemical and photostability in aqueous solutions and outperformed ICG in in vivo lymphatic imaging applications.
“Always-on” NIR-II fluorophores for bioimaging often produce unwanted background signals due to accumulation in non-target areas. In contrast, activatable NIR-II probes can alter their fluorescence emission wavelength or intensity in response to specific physiological parameters or clinically relevant analytes, resulting in higher SNR, greater specificity, and lower detection limits compared to traditional “always-on” fluorophores.184–186 NIR-II xanthene fluorophores have been tailored to create off–on probes for biosensing, capitalizing on their flexible modification capabilities and tunable wavelengths. For instance, the groups of Zhang and Lei developed an NIR-II fluorescent probe, PN910, that selectively targets H2O2 and ONOO− at pH levels above 7.4, demonstrating high selectivity and deep tissue penetration in vivo (Fig. 15a).187 In mouse models of cystitis and colitis, PN910 exhibited a significant enhancement in fluorescence compared to control groups, and biochemical analysis confirmed its reliability in detecting these species without false positives. This work provides a simple yet effective tool for monitoring H2O2 and ONOO− associated with various diseases in alkaline environments. To further enhance the versatility of NIR-II activatable small-molecule probes, Yuan's group designed a platform based on the intramolecular charge transfer (ICT) mechanism, creating probes for analytes such as reactive oxygen species (ROS), thiols, and enzymes (Fig. 15b).188 This development not only resulted in multiple NIR-II activatable probes for different diseases in mouse models but also established a paradigm for broader analyte testing within NIR-II biosensing. Similarly, in 2021, Yuan et al.51 introduced the ATP-activatable NIR-II probe NIR-RT4 for imaging drug-induced hepatotoxicity in vivo (Fig. 15c). NIR-RT4, containing a rhodamine spirolactone, exhibited no fluorescence due to the disruption of its π-conjugate system. Upon reacting with ATP, the spirolactone ring opened, restoring the π-conjugate system and resulted in a fluorescence emission increase at 918 nm. The selectivity of NIR-RT4 for ATP was validated by comparing its responses to other biological species, including ADP, AMP, GTP, and biothiols. Given ATP's role as a critical signaling molecule in damaged and stressed cells, NIR-RT4 was utilized to monitor drug-induced hepatotoxicity through ATP fluorescence. Significant increases in NIR-II fluorescence were observed in acetaminophen (APAP)-induced hepatotoxicity, with fluorescence intensifying with longer APAP treatment durations. Additionally, hepatotoxicity induced by CCl4 was also monitored via in vivo NIR-II imaging. In 2023, Zhang's group189 established a reversible NIR-II FRET-based molecular fluorescent probe, CX-RATP, for in vivo ATP detection (Fig. 15d). CX-RATP demonstrated selective and reversible responses to ATP, along with excellent biocompatibility. This probe allows for the real-time recording of fluctuations in ATP levels in response to various drugs, making it a valuable tool for monitoring dynamic physiological changes. Furthermore, as an NIR-II ratiometric probe, CX-RATP possesses the advantages of quantitative detection of ATP in deep tissues and has been successfully applied for in situ imaging. Given its outstanding spectral properties and high performance in vivo, CX-RATP holds promise for various clinical biomedical applications and may inspire the development of additional NIR-II ratiometric fluorescent probes.
 | ||
| Fig. 15 (a) Structures of PN910 and in vivo monitoring of cystitis with PN910. Reproduced with permission from ref. 187 Copyright 2021, Wiley. (b) NIR-II fluorescence imaging of LPS-induced lymphatic inflammation using the NIRII-HD5-ONOO− probe. Reproduced with permission from ref. 188 Copyright 2022, Wiley. (c) In vivo detection of endogenous ATP in the livers of mouse during APAP-induced hepatotoxicity by the NIRII-RT-ATP probe. Reproduced with permission from ref. 51 Copyright 2020, Wiley. (d) Structure of CX-RATP and proposed mechanism for ATP detection and NIR-II imaging in vivo. Reproduced with permission from ref. 189 Copyright 2023, Wiley. | ||
 | ||
| Fig. 16 (a) Images of hindlimbs of nude mice intravenously injected with Rh824-PC. Reproduced with permission from ref. 190 Copyright 2019, American Chemical Society. (b) NIR-II fluorescence images of BALB/c mice with VIX3 (row 1 and 2) and VIX-4 (row 3 and 4) liposomes. Reproduced with permission from ref. 138 Copyright 2021, American Chemical Society. | ||
 | ||
| Fig. 17 (a) Nerve specificity of the potential lead NIR oxazine derivatives. Reproduced with permission from ref. 192 Copyright 2020, American Association for the Advancement of Science. | ||
3.2. Cancer therapy
Xanthene dyes are well known for their high singlet oxygen quantum yield and utility as triplet photosensitizers (PSs) in PTT/PDT. They are mainly planar aromatic compounds with a dibenzofuran (xanthene) structure, and a large number of photosensitisers have been developed for cancer therapy by substituting oxygen atoms and modifying rhodamine with other elements, such as Si, S, P, Ge, Sn, and Te. In 2004, Detty et al. developed a series of rhodamine derivatives with sulfur and selenium atoms, replacing the oxygen atom in the parent nucleus of the xanthene to improve the 1O2 yield (TMR-O, TMR-S, TMR-Se, Fig. 18a).195 Moreover, a (2-thienyl) substituent was introduced at position 9 to facilitate the transport of the molecule through the absorption of P-glycoprotein and expanded the conjugation system to regulate the maximum absorption wavelength of the molecule to >600 nm, which is an ideal wavelength for the photosensitiser. Selenorhodamine photosensitizers are used for the photodynamic therapy of P-glycoprotein-expressing cancer cells (Fig. 18b).196 Two years later, their group197 further extended the wavelength of the previously available selenium-substituted, sulfur-substituted rhodamine molecules to >640 nm. The half-effect concentrations (EC50) of 7-S and 7-Se obtained for Colo-26 cells were 9.0 × 10−8 M and 1.8 × 10−7 M, respectively (Fig. 18c). In March 2022, Kida et al. proposed a design strategy to transform fluorescein into a type I photosensitiser by inducing charge separation (CS) through the self-assembly of fluorescein.198 The CS state of fluorescein may have an energy level lower than the T1 energy level, and the CS state could be generated by symmetry-breaking charge separation (SB-CS) and charge carrier migration in self-assembly.199 It was shown that the CS state energy levels of fluorescein self-assembled supramolecules were lower than those of the triplet states, i.e., FI-C18 formed the S1 state upon illumination and was converted to the CS state either directly or via the SB-CS triplet state. On the contrary, FI-C2 loses the absorbed energy mainly through fluorescence and enters the triplet state through ISC. In October of the same year, based on the previous studies, the group fabricated a type I supramolecular photosensitiser self-assembled from a simple amphiphilic rhodamine Rh9-MA-C18. Rh9-MA-C18 NPs had a high O2− yield and were effective for PDT in human lung cancer cells PC9 node mice.200 In 2022, Zhou et al.201 proposed to introduce phosphate as a strong electron-absorbing group at the meso-position of the xanthene to form a push–pull electron structure with the electron-donating ability of the parent nucleus of the xanthene, which is conducive to electron transfer. Experiments showed that PY-P was a pure type I photosensitizer, and after encapsulation with Pluronic F127 to form nanoparticles, it had strong cytophototoxicity for Hale cells in both normoxia and hypoxia (Fig. 18d). The introduction of a strong electron-absorbing group made the molecule red-shifted for absorption and also had good fluorescence emission (ΦF = 0.35), which had great potential for fluorescence-directed PDT in solid tumours at 626 nm. In 2018, Li et al. developed a novel rhodamine derivative, PS RDM-BDP, utilizing Förster Resonance Energy Transfer (FRET) and SIT.202 At 557 nm, RDM-BDP exhibited a strong absorption peak, and under near-infrared (NIR) irradiation, it generated a linear state of singlet oxygen (1O2) effective for cancer cell apoptosis (Fig. 18e). Similarly, Peng et al. employed this strategy to create a FRET-based photosensitizer, Rh-NBSe, by conjugating rhodamine with benzo[a]phenoselenazinium. Under light, Rh-NBSe produced 1O2 and cleaved in the presence of reactive oxygen species, releasing rhodamine fluorophores and thus restoring the fluorescence inhibited by FRET. This fluorescence signal conversion mechanism can effectively reflect the real-time production of singlet oxygen in photodynamic therapy (PDT) (Fig. 18f).203 However, rhodamine alone does not produce 1O2 upon light exposure, prompting researchers to devise various strategies for its conversion into photosensitizers. Liu et al.204 proposed a versatile approach by complexing different luminescent transition metal systems (M-Rho) with rhodamine, significantly enhancing the generation of the triplet excited state and 1O2 formation under visible light208. Wong et al. further optimized cyclometallic ligands to develop the novel rhodamine-based PS Ir-Rho-G2, which demonstrated increased capacity for singlet oxygen generation and targeting of the endoplasmic reticulum.205 | ||
| Fig. 18 (a) Structures of TMR-O, TMR-S, TMR-Se and their photophysical properties. (b) Structures of 15a-18b and their photophysical properties. (c) Structures of 6-s–7-Se and their photophysical properties. (d) Synthetic routes of PY-P and P2. Reproduced with permission from ref. 201 Copyright 2020, The Royal Society of Chemistry. (e) FRET-based SIT phototheranostic (RDM-BDP) for amplified 1O2 generation, native tumor targeting as well as light-triggered enhanced tumor PDT. Reproduced with permission from ref. 202 Copyright 2018, American Chemical Society. (f) Structure of Rh-NBSe and its application in PDT. Reproduced with permission from ref. 203 Copyright 2021, The Royal Society of Chemistry. | ||
Hypoxia is a common feature of most tumors. Piao et al. constructed a selenium-rhodamine photosensitizer (azoSeR) capable of producing 1O2 even under hypoxic conditions (Fig. 19).206 Additionally, the overexpression of specific enzymes, such as γ-GGT, serves as a hallmark of the tumor microenvironment. In 2017, Urano et al.207 introduced the photosensitizer gGlu-HMSeR specifically responsive to γ-GGT, which can generate 1O2 for tumor therapy. In 2019, Zhang et al.208 developed a π-extended red-absorbing activatable Se-rhodamine (Se-NR-Az) photosensitizer, extending its maximum absorption wavelength to 616 nm, optimizing it for the therapeutic window (600–900 nm) and enhancing its biological applications. That same year, Peng et al. reported the FUCL strategy, employing a one-photon molecular upconversion rhodamine derivative (FUCP-1). In contrast to conventional Stokes emission, FUCP-1 can be excited at longer wavelengths (808 nm), achieving a remarkable upconversion quantum yield (>12%) along with excellent optical stability. In vivo studies indicated that FUCP-1 was selectively concentrated at tumor sites, resulting in a 73.7% tumor growth inhibition rate after PDT.209 To further enhance deep tumor photodynamic therapy (dPDT), Ma et al. introduced a “booster effect” strategy that constructs effective phototherapeutics through hot band absorption. They systematically investigated various rhodamine derivatives containing different heteroatomic aromatic rings, such as FUC-N (no heavy atom), FUC-S (internally weak heavy atom S), FUCP-1 (peripheral strong heavy atom I), and FUC-Se (internally strong heavy atom Se).210 This selenium-based strategy significantly improved the photosensitization performance of anti-Stokes photosensitizers and advanced their clinical application. In 2024, our team synthesized three new photosensitizers (SN-1, SN-2, and SN-3) by introducing phenyl and electron-rich five-membered heterocycles at the meso position of S-rhodamine. In these compounds, the phenyl (SN-1), furyl (SN-2), and thienyl (SN-3) groups acted as electron donors, while the rhodamine backbone served as an electron acceptor. Reactive oxygen species (ROS) tests revealed that SN-1 functioned as a hybrid type I and II photosensitizer, while SN-2 and SN-3 were pure type I photosensitizers. Further in vivo studies demonstrated that SN-3 effectively inhibited solid tumors in hypoxic conditions.57 In another innovative approach, we constructed novel type I photosensitizers by lowering the triplet state (T1) energy level and enhancing donor–acceptor (D–A) interactions. We selected dibenzofurstene (FE) as the backbone for its high conjugation and ability to reduce the T1 level. Amino groups were introduced as donors, while electrophilic groups served as acceptors, resulting in a series of dibenzofurstene-based type I photosensitizers (FE-VSM, FE-MDN, FE-TCF, and FE-TMI). Among them, FE-TMI exhibited the most promising PDT efficacy, validated in a triple-negative breast cancer (TNBC) mouse model, demonstrating significant anti-tumor effects.77
 | ||
| Fig. 19 Structure of azoSeR and inhibited solid tumors under hypoxia. Reproduced with permission from ref. 206 Copyright 2021, The Royal Society of Chemistry. | ||
Over the past decade, organic photothermal therapy (PTT) agents have emerged as promising alternatives or complements to traditional therapies. Xiao et al. introduced a rigid xanthonium moiety in cyanine dyes, resulting in novel near-infrared (NIR)-II xanthonium-cyanine dyes (BHs, Fig. 20a).211 This modification produced a significant 400 nm redshift in maximum absorption compared to traditional iodolium-based cyanine dyes. The xanthonium core enhanced the rigidity of the conjugation system, minimizing internal deactivation and stabilizing the excited state, thus achieving a balance between NIR-II fluorescence, oxygen photosensitization, and photothermal performance. BH1024, in particular, exhibited outstanding performance with high photothermal conversion efficiency and singlet oxygen generation under 1064 nm laser irradiation (ΦF = 0.5%, ΦΔ = 0.07, ηPCE = 41.3%). This deep tissue penetration enabled effective tumor treatment via both NIR-II PDT and PTT in mouse models, marking the first identification of a small-molecule dye capable of directly generating singlet oxygen upon NIR-II laser excitation, providing insights for future photosensitizer designs.
 | ||
| Fig. 20 (a) Schematic of BH 1024 NPs’ preparation, application and the NIR-II fluorescence imaging-guided PTT. Reproduced with permission from ref. 211 Copyright 2021, Wiley. (b) NIR-II fluorescence images of 4T1 tumor-bearing mice at different time points after injecting BHcy-NPs. Reproduced with permission from ref. 212 Copyright 2022, Wiley. (c) Schematic of CN3 NPs’ preparation and application. Reproduced with permission from ref. 213 Copyright 2023, Elsevier. | ||
Building on this, Xiong et al. employed an acceptor engineering strategy to create a near-infrared, heavy-atom-free hemicyanine-based photosensitizer (BHcy) for NIR-II fluorescence-guided PTT and PDT (Fig. 20b).212 A planar, rigid π-conjugated moiety (1-ethyl-benz(c, d)iodolium) was utilized as the acceptor. BHcy displayed redshifted absorption and emission at 770 and 915 nm, respectively, compared to Hcy. Theoretical calculations indicated enhanced planarity and rigidity of BHcy's conjugated system, which reduced the HOMO/LUMO energy gap and improved singlet oxygen generation through enhanced spin–orbit coupling and intersystem crossing (ISC). This increased rigidity facilitated intermolecular π–π interactions, enhancing vibrational coupling and photothermal conversion. Nanoparticles formed by combining BHcy with DSPE-PEG2000 demonstrated excellent photothermal conversion and photosensitizing capabilities (ΦΔ = 0.129, ηPCE = 55.1%), showcasing significant anti-tumor properties both in vitro and in vivo.
Wang et al. introduced a structural bacterial targeting strategy using NIR-II xanthene dyes (CNs) for effective photothermal antibacterial therapy (Fig. 20c).213 The extended π-conjugated structure of CNs resulted in intense absorption bands at about 1180 nm. To improve water solubility and biocompatibility, CNs were encapsulated in Pluronic F127 to form liposomes (CN NPs) via membrane hydration. Due to H-aggregate formation from π–π stacking, CN NPs exhibited blue-shifted absorption peaks and high NIR-II photothermal conversion efficiencies (approximately 40%). CN3 NPs showed a more positive zeta potential than related liposomes, as intermolecular hydrogen bonding within the CN3 dimer embeds the carboxyl group, exposing the positively charged xanthene skeleton. This configuration endowed CN3 NPs with inherent bacterial targeting capability, significantly enhancing their photothermal bactericidal activity against both Gram-positive and Gram-negative bacteria in vitro and in vivo (99.4% and 99.2% effectiveness against S. aureus and E. coli, respectively). Furthermore, the NIR-II photothermal effect of CN3 NPs effectively inhibited infection and promoted wound healing without systemic toxicity, highlighting the potential of this targeting strategy for clinical antimicrobial therapy.
Our team is also investigating xanthene derivatives for tumor PDT/PTT. In 2023, we selected 3,6-diethylamino-fluorenone (FE) as the core for two donor–π–acceptor (D–π–A) photosensitizers (PTAs), FE-BA and FE-IDMN by replacing the carbonyl group with electron-withdrawing groups (EWGs).76 Notably, FE-IDMN in nanoparticle form (FE-IDMN NPs) exhibited a high photothermal conversion efficiency (PCE) of 82.6%. These nanoparticles demonstrated excellent colloidal, pH, and photothermal stability, enabling multimodal imaging (NIR-II FLI, PAI, and PTA)-guided PTT of subcutaneous tumors in mice, successfully ablating the 4T1 tumor under NIR laser irradiation. In the same year, we designed and synthesized a series of PTAs named as ICRs, utilizing rigid Si-xanthene as donors and positively charged benz[e]indole as acceptors.20 Among these, the ICR-Qu nanoparticle formulation exhibited the highest physiological stability, biocompatibility, a high PCE (81.1%), and effective ROS generation, achieving superior anti-tumor efficacy. We successfully conducted NIR-II/photoacoustic imaging-guided tumor ablation and PDT/PTT therapy under NIR laser irradiation.
Although xanthene-based NIR organic phototheranostics agents-mediated PDT/PTT have made great progress in tumor therapy, however, they still have many shortcomings and challenges similar to other photosensitizers. Firstly, the largest problem is the limited depth of light penetration, which may result in the incomplete ablation of tumors. Secondly, the conversion efficiency of some photothermal transduction agents (PTAs) is low, and some strategies are required to improve the efficiency of photothermal transduction to better treat tumors. In addition, the therapeutic effect of solely PDT/PTT is limited, and taking PDT/PTT combined with chemotherapy, radiotherapy, targeted therapy and immunotherapy could improve the therapeutic effect. Furthermore, the biosafety of photosensitizers is particularly important. The current mouse tumor models commonly used in preclinical experiments are insufficient to reflect the physiological conditions of clinical patients. In order to promote the clinical application of PDT/PTT, it is necessary to evaluate the therapeutic efficacy and safety in other large animals such as dogs, pigs and monkeys. In conclusion, the development of new strategies or the optimal combination of existing strategies will surely contribute significantly to the application of xanthene-based NIR organic phototheranostics agents in cancer PTT/PDT, which provides an attractive and challenging opportunity to further enhance the effectiveness of anticancer therapy.
4. Conclusion
This study focuses on reviewing the recent developments in xanthene dyes and their structural modifications, exploring the imaging and sensing technologies based on these fluorophores, as well as summarizing the potential applications of these dyes in diagnosis and therapy. In recent years, the rapid advancement of NIR-II phototherapeutic technologies has driven the development of high-performance xanthene-based phototherapeutic agents. Consequently, researchers have proposed various modification methods to enhance these dyes. These modification strategies have been thoroughly validated as effective and versatile means to significantly improve xanthene dyes exhibiting NIR-I/II emission and large Stokes shifts. Such strategies enrich the toolkit of researchers in chemistry, biology, and medical science, facilitating the development of high-quality NIR-I/II fluorophores for a wide range of applications, ranging from detecting bioactive and environmental species to imaging-guided cancer therapy.However, due to inherent limitations in the xanthene structure, many of these modification strategies struggle to further extend the emission wavelengths of compounds into the NIR II region. There are still many under-explored areas regarding the modification and biological applications of xanthene.
4.1. Enhancing emission efficiency
Strategies to reduce the π–π stacking of these fluorophores and to hinder interactions between water molecules and the fluorophores through bulky alkyl substituents may improve the emission efficiency. Moreover, extending the π-conjugated system and introducing cationic π systems are effective methods to enhance the absorption efficiency.4.2. Absorption wavelength limitations
The absorption capabilities of xanthene dyes often fail to effectively transfer to the near-infrared absorption range. For instance, the emission wavelengths of classic xanthene representatives such as rhodamine and rhodol are centered at about 500–600 nm, which is detrimental to in vivo imaging and phototherapy. Therefore, modifications to the parent structure often need to be combined with other strategies (such as π-extension and substitution methods) to balance NIR-I/II absorption and emission.4.3. Exploring applications
Beyond the modification strategies, the application of xanthene in imaging and therapy requires further exploration. While xanthene dyes have found extensive use in NIR-I fluorescence-based imaging, detection, and therapy, reports on NIR-II imaging and therapeutic methods (such as PDT/PTT) based on xanthene remain limited. We hope that xanthene can be further applied in phototherapy (PDT and PTT), sonodynamic therapy, disease and cancer treatment, as well as in imaging-guided surgeries, thereby promoting the preclinical and clinical applications of xanthene dyes.Xanthene-based NIR organic phototherapeutics represent a revolutionary advancement in the fields of medical imaging and therapy. Through innovative design strategies and a deep understanding of their interactions with biological systems, these agents are laying the groundwork for efficient and minimally invasive medical treatments. As research progresses, it is anticipated that these materials will play an increasingly significant role in the future.
Author contributions
Xiao-Yun Ran: investigation and writing – original draft; Yuan-Feng Wei: writing – original draft; Yan-Lin Wu, Li-Rui Dai and Wen-Li Xia: investigation; Pei-Zhi Zhou and Kun Li: funding acquisition and project administration resources. All authors have approved the final version of the manuscript.Data availability
No primary research results, software or code have been included and no new data were generated or analysed as part of this review.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (U21A20308, 22077088), the Science and Technology Major Project of Tibetan Autonomous Region of China (XZ202201ZD0001G) and the Science and Technology Innovation Seedling Project of Sichuan Province (Grant No:2024JDRC0041).References
- P. J. Keall, C. Brighi, C. Glide-Hurst, G. Liney, P. Z. Y. Liu, S. Lydiard, C. Paganelli, T. Pham, S. Shan, A. C. Tree, U. A. van der Heide, D. E. J. Waddington and B. Whelan, Nat. Rev. Clin. Oncol., 2022, 19, 458–470 CrossRef PubMed.
- X. Xie, J. Zhai, X. Zhou, Z. Guo, P.-C. Lo, G. Zhu, K. W. Y. Chan and M. Yang, Adv. Mater., 2024, 36, 2306450 CrossRef CAS PubMed.
- X. Ou, X. Chen, X. Xu, L. Xie, X. Chen, Z. Hong, H. Bai, X. Liu, Q. Chen, L. Li and H. Yang, Research, 2021, 9892152 CAS.
- M. L. James and S. S. Gambhir, Physiol. Rev., 2012, 92, 897–965 CrossRef CAS PubMed.
- H. Chen, Y. Pang, J. Wu, L. Zhao, B. Hao, J. Wu, J. Wei, S. Wu, L. Zhao, Z. Luo, X. Lin, C. Xie, L. Sun, Q. Lin and H. Wu, Eur. J. Nucl. Med. Mol. Imaging, 2020, 47, 1820–1832 CrossRef PubMed.
- R. Li, G. C. Ravizzini, M. A. Gorin, T. Maurer, M. Eiber, M. R. Cooperberg, M. Alemozzaffar, M. K. Tollefson, S. E. Delacroix and B. F. Chapin, Prostate Cancer Prostatic Dis., 2018, 21, 4–21 CrossRef PubMed.
- Y. Kim, J. Kim, J. Ahn, M. Han, H. G. Lim, K. J. Lee, J. Lee, C. Kim and H. H. Kim, Sci. Rep., 2021, 11, 20416 CrossRef CAS PubMed.
- N. Wang, X. Li, J. Xu, Y. Jiao, Y. Cui and X. Jian, Ultrasonics, 2022, 121, 106669 CrossRef PubMed.
- L. J. Delaney, J. R. Eisenbrey, D. Brown, J. R. Brody, M. Jimbo, B. E. Oeffinger, M. Stanczak, F. Forsberg, J.-B. Liu and M. A. Wheatley, Acta Biomater., 2021, 130, 385–394 CrossRef CAS PubMed.
- C. Wang, X. Chen, L. Wang, M. Makihata, H.-C. Liu, T. Zhou and X. Zhao, Science, 2022, 377, 517–523 CrossRef CAS PubMed.
- A. R. Rezai, P.-F. D’Haese, V. Finomore, J. Carpenter, M. Ranjan, K. Wilhelmsen, R. I. Mehta, P. Wang, U. Najib, C. V. L. Teixeira, T. Arsiwala, A. Tarabishy, P. Tirumalai, D. O. Claassen, S. Hodder and M. W. Haut, N. Engl. J. Med., 2024, 390, 55–62 CrossRef CAS PubMed.
- G. Song, L. Cheng, Y. Chao, K. Yang and Z. Liu, Adv. Mater., 2017, 29, 1700996 CrossRef PubMed.
- Y. Zhang, Z. Xing, T. Liu, M. Tang, L. Mi, J. Zhu, W. Wu and T. Wei, Eur. J. Med. Chem., 2022, 238, 114500 CrossRef CAS PubMed.
- J. Shi, P. W. Kantoff, R. Wooster and O. C. Farokhzad, Nat. Rev. Cancer, 2017, 17, 20–37 CrossRef CAS PubMed.
- R. H. Fang, W. Gao and L. Zhang, Nat. Rev. Clin. Oncol., 2023, 20, 33–48 CrossRef PubMed.
- G. Feng, G.-Q. Zhang and D. Ding, Chem. Soc. Rev., 2020, 49, 8179–8234 RSC.
- Y. Xu, J. Zhang, Z. Wang, P. Zhang, Z. Zhang, Z. Yang, J. W. Y. Lam, R. T. K. Kwok, L. Meng, D. Dang and B. Z. Tang, Biomaterials, 2025, 314, 122847 CrossRef CAS PubMed.
- Z. Mao, J. H. Kim, J. Lee, H. Xiong, F. Zhang and J. S. Kim, Coord. Chem. Rev., 2023, 476, 214908 CrossRef CAS.
- K. Liu, Z. Jiang, R. A. Lalancette, X. Tang and F. Jäkle, J. Am. Chem. Soc., 2022, 144, 18908–18917 CrossRef CAS PubMed.
- X.-Y. Ran, P. Chen, Y.-Z. Liu, L. Shi, X. Chen, Y.-H. Liu, H. Zhang, L.-N. Zhang, K. Li and X.-Q. Yu, Adv. Mater., 2023, 35, 2210179 CrossRef CAS PubMed.
- P. Cheng and K. Pu, Nat. Rev. Mater., 2021, 6, 1095–1113 CrossRef CAS.
- K. Wang, Y. Du, Z. Zhang, K. He, Z. Cheng, L. Yin, D. Dong, C. Li, W. Li, Z. Hu, C. Zhang, H. Hui, C. Chi and J. Tian, Nat. Rev. Bioeng., 2023, 1, 161–179 CrossRef CAS.
- F. Ge, Y. Sun, Y. Wang, D. Yu, Z. Wang, F. Yu, B. Yu and H. Fu, J. Mater. Chem. B, 2024, 12, 11165–11171 RSC.
- Z. Zhang, Y. Du, X. Shi, K. Wang, Q. Qu, Q. Liang, X. Ma, K. He, C. Chi, J. Tang, B. Liu, J. Ji, J. Wang, J. Dong, Z. Hu and J. Tian, Nat. Rev. Clin. Oncol., 2024, 21, 449–467 CrossRef PubMed.
- A. M. Smith, M. C. Mancini and S. Nie, Nat. Nanotechnol., 2009, 4, 710–711 CrossRef CAS PubMed.
- K. Welsher, Z. Liu, S. P. Sherlock, J. T. Robinson, Z. Chen, D. Daranciang and H. Dai, Nat. Nanotechnol., 2009, 4, 773–780 CrossRef CAS PubMed.
- Z. Zhang, K. He, C. Chi, Z. Hu and J. Tian, Eur. J. Nucl. Med. Mol. Imaging, 2022, 49, 2531–2543 CrossRef PubMed.
- C. Li, G. Chen, Y. Zhang, F. Wu and Q. Wang, J. Am. Chem. Soc., 2020, 142, 14789–14804 CrossRef CAS PubMed.
- S. E. Miller, W. S. Tummers, N. Teraphongphom, N. S. van den Berg, A. Hasan, R. D. Ertsey, S. Nagpal, L. D. Recht, E. D. Plowey, H. Vogel, G. R. Harsh, G. A. Grant, G. H. Li and E. L. Rosenthal, J. Neuro-Oncol., 2018, 139, 135–143 CrossRef CAS PubMed.
- X. Ran, Q. Zhou, J. Zhang, S. Wang, G. Wang, H. Yang, X. Liu, Z. Wang and X. Yu, Org. Chem. Front., 2021, 8, 3631–3638 RSC.
- G. Hong, J. C. Lee, J. T. Robinson, U. Raaz, L. Xie, N. F. Huang, J. P. Cooke and H. Dai, Nat. Med., 2012, 18, 1841–1846 CrossRef CAS PubMed.
- O. T. Bruns, T. S. Bischof, D. K. Harris, D. Franke, Y. Shi, L. Riedemann, A. Bartelt, F. B. Jaworski, J. A. Carr, C. J. Rowlands, M. W. B. Wilson, O. Chen, H. Wei, G. W. Hwang, D. M. Montana, I. Coropceanu, O. B. Achorn, J. Kloepper, J. Heeren, P. T. C. So, D. Fukumura, K. F. Jensen, R. K. Jain and M. G. Bawendi, Nat. Biomed. Eng., 2017, 1, 0056 CrossRef CAS PubMed.
- S. Li, J. Wei, Q. Yao, X. Song, J. Xie and H. Yang, Chem. Soc. Rev., 2023, 52, 1672–1696 RSC.
- Y. Liu, Y. Li, S. Koo, Y. Sun, Y. Liu, X. Liu, Y. Pan, Z. Zhang, M. Du, S. Lu, X. Qiao, J. Gao, X. Wang, Z. Deng, X. Meng, Y. Xiao, J. S. Kim and X. Hong, Chem. Soc. Rev., 2022, 122, 209–268 CrossRef CAS PubMed.
- Y. Chen, S. Wang and F. Zhang, Nat. Biomed. Eng., 2023, 1, 60–78 CAS.
- Z. Feng, T. Tang, T. Wu, X. Yu, Y. Zhang, M. Wang, J. Zheng, Y. Ying, S. Chen, J. Zhou, X. Fan, D. Zhang, S. Li, M. Zhang and J. Qian, Light: Sci. Appl., 2021, 10, 197 CrossRef CAS PubMed.
- Z. Fang, C. Wang, J. Yang, Z. Song, C. Xie, Y. Ji, Z. Wang, X. Du, Q. Zheng, C. Chen, Z. Hu and Y. Zhong, Nat. Nanotechnol., 2024, 19, 124–130 CrossRef CAS PubMed.
- J. Mu, M. Xiao, Y. Shi, X. Geng, H. Li, Y. Yin and X. Chen, Angew. Chem., Int. Ed., 2022, 61, e202114722 CrossRef CAS PubMed.
- Y. Su, B. Yu, S. Wang, H. Cong and Y. Shen, Biomaterials, 2021, 271, 120717 CrossRef CAS PubMed.
- H. Li, Y. Kim, H. Jung, J. Y. Hyun and I. Shin, Chem. Soc. Rev., 2022, 51, 8957–9008 RSC.
- X. Wang, X. Wang, Z. Zhu, W. Li, G. Yu, Z. Jia and X. Wang, Biomed. Pharmacother., 2019, 117, 109109 CrossRef CAS PubMed.
- S. Zeng, X. Liu, Y. S. Kafuti, H. Kim, J. Wang, X. Peng, H. Li and J. Yoon, Chem. Soc. Rev., 2023, 52, 5607–5651 RSC.
- A. Turksoy, D. Yildiz and E. U. Akkaya, Coord. Chem. Rev., 2019, 379, 47–64 CrossRef CAS.
- S. Wang, L. Gai, Y. Chen, X. Ji, H. Lu and Z. Guo, Chem. Soc. Rev., 2024, 53, 3976–4019 RSC.
- A. N. Bismillah and I. Aprahamian, Chem. Soc. Rev., 2021, 50, 5631–5649 RSC.
- A. L. Antaris, H. Chen, K. Cheng, Y. Sun, G. Hong, C. Qu, S. Diao, Z. Deng, X. Hu, B. Zhang, X. Zhang, O. K. Yaghi, Z. R. Alamparambil, X. Hong, Z. Cheng and H. Dai, Nat. Mater., 2016, 15, 235–242 CrossRef CAS PubMed.
- X. Liu, B. Yu, Y. Shen and H. Cong, Coord. Chem. Rev., 2022, 468, 214609 CrossRef CAS.
- X. Hu, C. Zhu, F. Sun, Z. Chen, J. Zou, X. Chen and Z. Yang, Adv. Mater., 2024, 36, 2304848 CrossRef CAS PubMed.
- X. Chen, T. Pradhan, F. Wang, J. S. Kim and J. Yoon, Chem. Rev., 2012, 112, 1910–1956 CrossRef CAS PubMed.
- J. H. Choi, S. Kim, O.-Y. Kang, S. Y. Choi, J. Y. Hyun, H. S. Lee and I. Shin, Chem. Soc. Rev., 2024, 53, 9446–9489 RSC.
- T.-B. Ren, Z.-Y. Wang, Z. Xiang, P. Lu, H.-H. Lai, L. Yuan, X.-B. Zhang and W. Tan, Angew. Chem., Int. Ed., 2021, 60, 800–805 CrossRef CAS PubMed.
- W. Wu, K. Yan, Z. He, L. Zhang, Y. Dong, B. Wu, H. Liu, S. Wang and F. Zhang, J. Am. Chem. Soc., 2024, 146, 11570–11576 CAS.
- J. B. Grimm, A. N. Tkachuk, L. Xie, H. Choi, B. Mohar, N. Falco, K. Schaefer, R. Patel, Q. Zheng, Z. Liu, J. Lippincott-Schwartz, T. A. Brown and L. D. Lavis, Nat. Methods, 2020, 17, 815–821 CrossRef CAS PubMed.
- N. Lardon, L. Wang, A. Tschanz, P. Hoess, M. Tran, E. D’Este, J. Ries and K. Johnsson, J. Am. Chem. Soc., 2021, 143, 14592–14600 CrossRef CAS PubMed.
- G. Lukinavičius, K. Umezawa, N. Olivier, A. Honigmann, G. Yang, T. Plass, V. Mueller, L. Reymond, I. R. Corrêa Jr, Z.-G. Luo, C. Schultz, E. A. Lemke, P. Heppenstall, C. Eggeling, S. Manley and K. Johnsson, Nat. Chem., 2013, 5, 132–139 CrossRef PubMed.
- K. Fujita and Y. Urano, Chem. Rev., 2024, 124, 4021–4078 CrossRef CAS PubMed.
- J. Liang, X. Ran, Y. Liu, X. Yu, S. Chen and K. Li, J. Mater. Chem. B, 2024, 12, 3686–3693 RSC.
- A. Baeyer, Ber. Dtsch. Chem. Ges., 1871, 4, 555–558 CrossRef.
- S. G. Keller, M. Kamiya and Y. Urano, Molecules, 2020, 25, 5964 CrossRef CAS PubMed.
- L. Qian, L. Li and S. Q. Yao, Acc. Chem. Res., 2016, 49, 626–634 CrossRef CAS PubMed.
- L. Wang, W. Du, Z. Hu, K. Uvdal, L. Li and W. Huang, Angew. Chem., Int. Ed., 2019, 58, 14026–14043 CrossRef CAS PubMed.
- W. Zhou, X. Fang, Q. Qiao, W. Jiang, Y. Zhang and Z. Xu, Chin. Chem. Lett., 2021, 32, 943–946 CrossRef CAS.
- L. Yin, B. Zhao, J. Zhou, Y. Huang, H. Ma, T. Zhou, J. Mou, P. Min, J. Chen, G. Ge, X. Qian, X. Luo and Y. Yang, Angew. Chem., Int. Ed., 2024, 63, e202402949 CrossRef CAS PubMed.
- M. S. Frei, P. Hoess, M. Lampe, B. Nijmeijer, M. Kueblbeck, J. Ellenberg, H. Wadepohl, J. Ries, S. Pitsch, L. Reymond and K. Johnsson, Nat. Commun., 2019, 10, 4580 CrossRef PubMed.
- F. Wang, Y. Zhong, O. Bruns, Y. Liang and H. Dai, Nat. Photonics, 2024, 18, 535–547 CrossRef CAS.
- F. Ren, F. Wang, A. Baghdasaryan, Y. Li, H. Liu, R. Hsu, C. Wang, J. Li, Y. Zhong, F. Salazar, C. Xu, Y. Jiang, Z. Ma, G. Zhu, X. Zhao, K. K. Wong, R. Willis, K. Christopher Garcia, A. Wu, E. Mellins and H. Dai, Nat. Biomed. Eng., 2024, 8, 726–739 CrossRef CAS PubMed.
- Y. Zeng, J. Qu, G. Wu, Y. Zhao, J. Hao, Y. Dong, Z. Li, J. Shi, J. S. Francisco and X. Zheng, J. Am. Chem. Soc., 2024, 146, 9888–9896 CrossRef CAS PubMed.
- L. Wu and K. Burgess, Org. Lett., 2008, 10, 1779–1782 CrossRef CAS PubMed.
- G. Jiang, H. Liu, H. Liu, G. Ke, T.-B. Ren, B. Xiong, X.-B. Zhang and L. Yuan, Angew. Chem., Int. Ed., 2024, 63, e202315217 CrossRef CAS PubMed.
- P. Horváth, P. Šebej, T. Šolomek and P. Klán, J. Org. Chem., 2015, 80, 1299–1311 CrossRef PubMed.
- A. N. Butkevich, G. Lukinavičius, E. D’Este and S. W. Hell, J. Am. Chem. Soc., 2017, 139, 12378–12381 CrossRef CAS PubMed.
- H. Zhang, J. Liu, L. Wang, M. Sun, X. Yan, J. Wang, J.-P. Guo and W. Guo, Biomaterials, 2018, 158, 10–22 CrossRef CAS PubMed.
- H. Zhang, J. Liu, C. Liu, P. Yu, M. Sun, X. Yan, J.-P. Guo and W. Guo, Biomaterials, 2017, 133, 60–69 CrossRef CAS PubMed.
- T. Pastierik, P. Šebej, J. Medalová, P. Štacko and P. Klán, J. Org. Chem., 2014, 79, 3374–3382 CrossRef CAS PubMed.
- L. Wei, Z. Chen, L. Shi, R. Long, A. V. Anzalone, L. Zhang, F. Hu, R. Yuste, V. W. Cornish and W. Min, Nature, 2017, 544, 465–470 CrossRef CAS PubMed.
- Y.-Z. Liu, X.-Y. Ran, D.-H. Zhou, H. Zhang, Y.-J. Chen, J.-X. Xu, S.-Y. Chen, Q.-Q. Kong, X.-Q. Yu and K. Li, Adv. Funct. Mater., 2024, 34, 2311365 CrossRef CAS.
- X.-Y. Ran, W.-L. Xia, L.-N. Zhang, X.-Q. Yu, P. Chen, K.-P. Xie, Y. Zhao, C. Yi and K. Li, Mater. Horiz., 2024, 11, 5589–5599 RSC.
- L.-N. Zhang, X.-Y. Ran, H. Zhang, Y. Zhao, Q. Zhou, S.-Y. Chen, C. Yang, X.-Q. Yu and K. Li, Adv. Healthcare Mater., 2024, 14, 2402295 CrossRef PubMed.
- D. He, L. Zhang and Y. Sun, Coord. Chem. Rev., 2022, 461, 214507 CrossRef CAS.
- Y.-F. Kang, L.-Y. Niu and Q.-Z. Yang, Chin. Chem. Lett., 2019, 30, 1791–1798 CrossRef CAS.
- N. Kaur, S. Chopra, G. Singh, P. Raj, A. Bhasin, S. K. Sahoo, A. Kuwar and N. Singh, J. Mater. Chem. B, 2018, 6, 4872–4902 RSC.
- W. Pervez, Laraib, C. Yin and F. Huo, Coord. Chem. Rev., 2025, 522, 216202 CrossRef CAS.
- H. Yan, F. Huo, Y. Yue, J. Chao and C. Yin, J. Am. Chem. Soc., 2021, 143, 318–325 CrossRef CAS PubMed.
- R. Kaushik, N. Nehra, V. Novakova and P. Zimcik, ACS Omega, 2023, 8, 98–126 CrossRef CAS PubMed.
- Y.-J. Chen, H. Zhang, Y.-Z. Liu, L. Shi, F.-F. Xiang, R.-D. Lin, Y.-H. Liu, S.-Y. Chen, X.-Q. Yu and K. Li, ACS Sens., 2023, 8, 2359–2367 CrossRef CAS PubMed.
- L.-N. Zhang, H. Zhang, S.-Y. Chen, Y.-Z. Liu, X.-H. Yang, F.-F. Xiang, Y.-H. Liu, K. Li and X.-Q. Yu, Chem. Commun., 2023, 59, 2795–2798 RSC.
- J. Liu, M. Liu, H. Zhang, X. Wei, J. Wang, M. Xian and W. Guo, Chem. Sci., 2019, 10, 10065–10071 RSC.
- X. B. Zhou, Y. W. Liu, Q. Y. Liu, L. Z. Yan, M. Xue, W. Yuan, M. Shi, W. Feng, C. J. Xu and F. Y. Li, Theranostics, 2019, 9, 4597–4607 CrossRef CAS PubMed.
- H. Zhang, J. Liu, B. Hu, L. Wang, Z. Yang, X. Han, J. Wang, W. Bai and W. Guo, Chem. Sci., 2018, 9, 3209–3214 RSC.
- Q. Zhou, S. Wang, X. Ran, L. Shen, X. Luo, G. Wang, H. Yang, Z. Wang and X. Yu, Chin. Chem. Lett., 2023, 34, 107922 CrossRef CAS.
- C. S. L. Rathnamalala, S. Hernandez, M. Y. Lucero, C. B. Swartchick, A. Kalam Shaik, N. I. Hammer, A. K. East, S. R. Gwaltney, J. Chan and C. N. Scott, Angew. Chem., Int. Ed., 2023, 62, e202214855 CrossRef CAS PubMed.
- H. Zhang, G.-N. Zhu, F.-F. Xiang, Y.-J. Chen, S.-Y. Chen, M. Wu and K. Li, J. Med. Chem., 2024, 67, 17855–17865 CrossRef CAS PubMed.
- L.-N. Zhang, S.-Y. Chen, L. Shi, X.-Y. Ran, H. Zhang, H. Moon, Y. Lee, X.-Q. Yu, J. S. Kim and K. Li, Adv. Funct. Mater., 2025, 35, 2412595 CrossRef CAS.
- J.-M. Li, F.-F. Xiang, D.-H. Zhou, J.-X. Xu, H. Zhang, Y.-Z. Liu, Q.-Q. Kong, X.-Q. Yu and K. Li, Chem. Biomed. Imaging, 2024, 2, 126–134 CrossRef CAS PubMed.
- Z. Khan and N. Sekar, Dyes Pigm., 2023, 208, 110735 CrossRef.
- Z. Mao, H. Rha, J. Kim, X. You, F. Zhang, W. Tao and J. S. Kim, Adv. Sci., 2023, 10, 2301177 CrossRef CAS PubMed.
- J. B. Grimm, A. N. Tkachuk, R. Patel, S. T. Hennigan, A. Gutu, P. Dong, V. Gandin, A. M. Osowski, K. L. Holland, Z. J. Liu, T. A. Brown and L. D. Lavis, J. Am. Chem. Soc., 2023, 145, 23000–23013 CrossRef CAS PubMed.
- J. Arden-Jacob, J. Frantzeskos, N. U. Kemnitzer, A. Zilles and K. H. Drexhage, Spectrochim. Acta, Part A, 2001, 57, 2271–2283 CrossRef CAS PubMed.
- K. Kolmakov, V. N. Belov, C. A. Wurm, B. Harke, M. Leutenegger, C. Eggeling and S. W. Hell, Eur. J. Org. Chem., 2010, 3593–3610 CrossRef CAS.
- Z. Lei, X. Li, X. Luo, H. He, J. Zheng, X. Qian and Y. Yang, Angew. Chem., Int. Ed., 2017, 56, 2979–2983 CrossRef CAS PubMed.
- T. Y. Ohulchanskyy, D. J. Donnelly, M. R. Detty and P. N. Prasad, J. Phys. Chem. B, 2004, 108, 8668–8672 CrossRef CAS.
- B. Calitree, D. J. Donnelly, J. J. Holt, M. K. Gannon, C. L. Nygren, D. K. Sukumaran, J. Autschbach and M. R. Detty, Organometallics, 2007, 26, 6248–6257 CrossRef CAS.
- Y. Koide, M. Kawaguchi, Y. Urano, K. Hanaoka, T. Komatsu, M. Abo, T. Terai and T. Nagano, Chem. Commun., 2012, 48, 3091–3093 RSC.
- M. Fu, Y. Xiao, X. Qian, D. Zhao and Y. Xu, Chem. Commun., 2008, 1780–1782 RSC.
- Y. Koide, Y. Urano, K. Hanaoka, T. Terai and T. Nagano, ACS Chem. Biol., 2011, 6, 600–608 CrossRef CAS PubMed.
- Y. Koide, Y. Urano, K. Hanaoka, W. Piao, M. Kusakabe, N. Saito, T. Terai, T. Okabe and T. Nagano, J. Am. Chem. Soc., 2012, 134, 5029–5031 CrossRef CAS PubMed.
- L. Lesiak, N. Dadina, S. Zheng, M. Schelvis and A. Schepartz, ACS Cent. Sci., 2024, 10, 19–27 CrossRef CAS PubMed.
- A. Jemas, Y. Xie, J. E. Pigga, J. L. Caplan, C. W. Am Ende and J. M. Fox, J. Am. Chem. Soc., 2022, 144, 1647–1662 CrossRef CAS PubMed.
- C. Wang, H. Zhang, T. Zhang, X. Zou, H. Wang, J. E. Rosenberger, R. Vannam, W. S. Trout, J. B. Grimm, L. D. Lavis, C. Thorpe, X. Jia, Z. Li and J. M. Fox, J. Am. Chem. Soc., 2021, 143, 10793–10803 CrossRef CAS PubMed.
- G. Lukinavičius, L. Reymond, K. Umezawa, O. Sallin, E. D’Este, F. Göttfert, H. Ta, S. W. Hell, Y. Urano and K. Johnsson, J. Am. Chem. Soc., 2016, 138, 9365–9368 CrossRef PubMed.
- J. Liu, X. Yang, S. Wu, P. Gong, F. Pan, P. Zhang, C.-S. Lee, C. Liu and K. M.-C. Wong, J. Mater. Chem. B, 2024, 12, 3710–3718 RSC.
- X. Chai, X. Cui, B. Wang, F. Yang, Y. Cai, Q. Wu and T. Wang, Chem. – Eur. J., 2015, 21, 16754–16758 CrossRef CAS PubMed.
- J. Liu, Y.-Q. Sun, H. Zhang, H. Shi, Y. Shi and W. Guo, ACS Appl. Mater. Interfaces, 2016, 8, 22953–22962 CrossRef CAS PubMed.
- X. Zhou, L. Lesiak, R. Lai, J. R. Beck, J. Zhao, C. G. Elowsky, H. Li and C. I. Stains, Angew. Chem., Int. Ed., 2017, 56, 4197–4200 CrossRef CAS PubMed.
- H. C. Daly, S. S. Matikonda, H. C. Steffens, B. Ruehle, U. Resch-Genger, J. Ivanic and M. J. Schnermann, Photochem. Photobiol., 2022, 98, 325–333 CrossRef CAS PubMed.
- X. Zhou, Y. Fang, V. Wimalasiri, C. I. Stains and E. W. Miller, Chem. Commun., 2022, 58, 11941–11944 RSC.
- A. Barker and C. C. Barker, J. Chem. Soc., 1954, 1307–1309 RSC.
- S. i Nakatsuji, H. Nakazumi, H. Fukuma, T. Yahiro, K. Nakashima, M. Iyoda and S. Akiyama, J. Chem. Soc., Perkin Trans. 1, 1991, 1881–1886 RSC.
- S. G. R. Guinot, J. D. Hepworth and M. Wainwright, Dyes Pigm., 1999, 40, 151–156 CrossRef CAS.
- M. Grzybowski, O. Morawski, K. Nowak and P. Garbacz, Chem. Commun., 2022, 58, 5455–5458 RSC.
- K. Yan, Z. Hu, P. Yu, Z. He, Y. Chen, J. Chen, H. Sun, S. Wang and F. Zhang, Nat. Commun., 2024, 15, 2593 CrossRef CAS PubMed.
- X.-X. Zhang, F. Yang, X. Zhao, Q. Wu, L. He, Z. Li, Z. Zhou, T.-B. Ren, X.-B. Zhang and L. Yuan, Angew. Chem., Int. Ed., 2024, 63, e202410666 CAS.
- S. Kamino, Y. Horio, S. Komeda, K. Minoura, H. Ichikawa, J. Horigome, A. Tatsumi, S. Kaji, T. Yamaguchi, Y. Usami, S. Hirota, S. Enomoto and Y. Fujita, Chem. Commun., 2010, 46, 9013–9015 RSC.
- J. B. Grimm and L. D. Lavis, Org. Lett., 2011, 13, 6354–6357 CrossRef CAS PubMed.
- M. Sibrian-Vazquez, J. O. Escobedo, M. Lowry and R. M. Strongin, Pure Appl. Chem., 2012, 84, 2443–2456 CrossRef CAS PubMed.
- Y.-J. Gong, X.-B. Zhang, G.-J. Mao, L. Su, H.-M. Meng, W. Tan, S. Feng and G. Zhang, Chem. Sci., 2016, 7, 2275–2285 RSC.
- H. Bian, D. Ma, F. Pan, X. Zhang, K. Xin, X. Zhang, Y. Yang, X. Peng and Y. Xiao, J. Am. Chem. Soc., 2022, 144, 22562–22573 CrossRef CAS PubMed.
- J. Li, Y. Dong, R. Wei, G. Jiang, C. Yao, M. Lv, Y. Wu, S. H. Gardner, F. Zhang, M. Y. Lucero, J. Huang, H. Chen, G. Ge, J. Chan, J. Chen, H. Sun, X. Luo, X. Qian and Y. Yang, J. Am. Chem. Soc., 2022, 144, 14351–14362 CrossRef CAS PubMed.
- R. Wei, Y. Dong, X. Wang, J. Li, Z. Lei, Z. Hu, J. Chen, H. Sun, H. Chen, X. Luo, X. Qian and Y. Yang, J. Am. Chem. Soc., 2023, 145, 12013–12022 CrossRef CAS PubMed.
- I. Rajapaksha, H. Chang, Y. Xiong, S. Marder, S. R. Gwaltney and C. N. Scott, J. Org. Chem., 2020, 85, 12108–12116 CrossRef CAS PubMed.
- C. S. L. Rathnamalala, J. N. Gayton, A. L. Dorris, S. A. Autry, W. Meador, N. I. Hammer, J. H. Delcamp and C. N. Scott, J. Org. Chem., 2019, 84, 13186–13193 CrossRef CAS PubMed.
- C. S. L. Rathnamalala, N. W. Pino, B. S. Herring, M. Hooper, S. R. Gwaltney, J. Chan and C. N. Scott, Org. Lett., 2021, 23, 7640–7644 CrossRef CAS PubMed.
- S. Chatterjee, W. E. Meador, C. Smith, I. Chandrasiri, M. F. Zia, J. Nguyen, A. Dorris, A. Flynt, D. L. Watkins, N. I. Hammer and J. H. Delcamp, RSC Adv., 2021, 11, 27832–27836 RSC.
- S. Chatterjee, A. K. Shaik, K. H. Wijesinghe, D. Ndaleh, A. Dass, N. I. Hammer and J. H. Delcamp, J. Org. Chem., 2022, 87, 11319–11328 CrossRef CAS PubMed.
- M. A. Saucier, N. A. Kruse, B. E. Seidel, N. I. Hammer, G. S. Tschumper and J. H. Delcamp, J. Org. Chem., 2024, 89, 9092–9097 CrossRef CAS PubMed.
- W. E. Meador, M. A. Saucier, M. R. Tucker, N. A. Kruse, A. J. Mobley, C. R. Brower, S. R. Parkin, K. M. Clark, N. I. Hammer, G. S. Tschumper and J. H. Delcamp, Chem. Sci., 2024, 15, 12349–12360 RSC.
- W. E. Meador, E. Y. Lin, I. Lim, H. C. Friedman, D. Ndaleh, A. K. Shaik, N. I. Hammer, B. Yang, J. R. Caram, E. M. Sletten and J. H. Delcamp, Nat. Chem., 2024, 16, 970–978 CrossRef CAS PubMed.
- D. Liu, Z. He, Y. Zhao, Y. Yang, W. Shi, X. Li and H. Ma, J. Am. Chem. Soc., 2021, 143, 17136–17143 CrossRef CAS PubMed.
- R. Kaur, N. A. Kruse, C. Smith, N. I. Hammer and J. H. Delcamp, ChemPhotoChem, 2024, 8, e202400023 CrossRef CAS.
- R. Z. Du, Y. Zhang, Y. Bian, C. Y. Yang, X. S. Feng and Z. W. He, Food Chem., 2024, 459, 140384 CrossRef CAS PubMed.
- L. Kong, Z. Deng and D. You, Nat. Prod. Rep., 2022, 39, 2057–2095 RSC.
- A. Haque, K. M. Alenezi, A. K. D. Alsukaibi, A. A. Al-Otaibi and W. Y. Wong, Top. Curr. Chem., 2024, 382, 14 CrossRef CAS PubMed.
- S. Keller, M. Kamiya and Y. Urano, Molecules, 2020, 25, 5964 CrossRef CAS PubMed.
- Z. Mao, H. Rha, J. Kim, X. You, F. Zhang, W. Tao and J. S. Kim, Adv. Sci., 2023, 10, e2301177 CrossRef PubMed.
- A. O. Oriola and P. Kar, Molecules, 2024, 29, 4241 CrossRef CAS PubMed.
- C. F. M. Silva, D. Pinto, P. A. Fernandes and A. M. S. Silva, Pharmaceuticals, 2022, 15, 148 CrossRef CAS PubMed.
- M. Maia, D. Resende, F. Durães, M. M. M. Pinto and E. Sousa, Eur. J. Med. Chem., 2021, 210, 113085 CrossRef CAS PubMed.
- M. Dai, Y. J. Yang, S. Sarkar and K. H. Ahn, Chem. Soc. Rev., 2023, 52, 6344–6358 RSC.
- S. Samanta, K. Lai, F. Wu, Y. Liu, S. Cai, X. Yang, J. Qu and Z. Yang, Chem. Soc. Rev., 2023, 52, 7197–7261 RSC.
- D. Si, Q. Li, Y. Bao, J. Zhang and L. Wang, Angew. Chem., Int. Ed., 2023, 62, e202307641 CrossRef CAS PubMed.
- Z. Lei and F. Zhang, Angew. Chem., Int. Ed., 2021, 60, 16294–16308 CrossRef CAS PubMed.
- C. Chen, C. Wu, J. Yu, X. Zhu, Y. Wu, J. Liu and Y. Zhang, Coord. Chem. Rev., 2022, 461, 214495 CrossRef CAS.
- J. Zhang, W. Wang, J. Shao, J. Chen and X. Dong, Coord. Chem. Rev., 2024, 516, 215986 CrossRef CAS.
- S. L. Troyan, V. Kianzad, S. L. Gibbs-Strauss, S. Gioux, A. Matsui, R. Oketokoun, L. Ngo, A. Khamene, F. Azar and J. V. Frangioni, Ann. Surg. Oncol., 2009, 16, 2943–2952 CrossRef PubMed.
- Y. Cai, Z. Wei, C. Song, C. Tang, W. Han and X. Dong, Chem. Soc. Rev., 2019, 48, 22–37 RSC.
- S. He, J. Song, J. Qu and Z. Cheng, Chem. Soc. Rev., 2018, 47, 4258–4278 RSC.
- Z. Lei and F. Zhang, Angew. Chem., Int. Ed., 2021, 60, 16294–16308 CrossRef CAS PubMed.
- Y. Sun, F. Ding, Z. Zhou, C. Li, M. Pu, Y. Xu, Y. Zhan, X. Lu, H. Li, G. Yang, Y. Sun and P. J. Stang, Proc. Natl. Acad. Sci. U. S. A., 2019, 116, 1968–1973 CrossRef CAS PubMed.
- C. Mi, X. Zhang, C. Yang, J. Wu, X. Chen, C. Ma, S. Wu, Z. Yang, P. Qiao, Y. Liu, W. Wu, Z. Guo, J. Liao, J. Zhou, M. Guan, C. Liang, C. Liu and D. Jin, Nat. Commun., 2023, 14, 6287 CrossRef CAS PubMed.
- M. Zhang, J. Yue, R. Cui, Z. Ma, H. Wan, F. Wang, S. Zhu, Y. Zhou, Y. Kuang, Y. Zhong, D.-W. Pang and H. Dai, Proc. Natl. Acad. Sci. U. S. A., 2018, 115, 6590–6595 CrossRef CAS PubMed.
- R. W. Ramette and E. B. Sandell, J. Am. Chem. Soc., 1956, 78, 4872–4878 CrossRef CAS.
- X. Lv, C. Gao, T. Han, H. Shi and W. Guo, Chem. Commun., 2020, 56, 715–718 RSC.
- W. Liangxing and B. Kevin, Org. Lett., 2008, 10, 1779–1782 CrossRef PubMed.
- A. N. Butkevich, G. Lukinavičius, E. D’Este and S. W. Hell, J. Am. Chem. Soc., 2017, 139, 12378–12381 CrossRef CAS PubMed.
- H. Peter, Š. Peter, Š. Tomáš and K. Petr, J. Org. Chem., 2014, 80, 1299–1311 Search PubMed.
- Z. Hong, S. Lei, L. Kun, L. Xin, W. Miae, L. Yan-Zhao, C. Youmi, L. Xin-Yao, L. Yan-Hong, C. Shan-Yong, Y. Kang-Kang, K. Jong Seung and Y. Xiao-Qi, Angew. Chem., Int. Ed., 2021, 61, e202116439 Search PubMed.
- M. R. Detty, P. N. Prasad, D. J. Donnelly, T. Ohulchanskyy, S. L. Gibson and R. Hilf, Bioorg. Med. Chem., 2004, 12, 2537–2544 CrossRef CAS PubMed.
- K. Yuichiro, U. Yasuteru, H. Kenjiro, T. Takuya and N. Tetsuo, ACS Chem. Biol., 2011, 6, 600–608 CrossRef PubMed.
- C. Xiaoyun, C. Xiaoyan, W. Baogang, Y. Fan, C. Yi, W. Qiuye and W. Ting, Chem. – Eur. J., 2015, 21, 16754–16758 Search PubMed.
- L. Jing, S. Yuan-Qiang, Z. Hongxing, S. Heping, S. Yawei and G. Wei, ACS Appl. Mater. Interfaces, 2016, 8, 22953–22962 CrossRef PubMed.
- X. Zhang, T. Ren, F. Yang and L. Yuan, Chin. Chem. Lett., 2021, 32, 3890–3894 CrossRef CAS.
- T. B. Ren, W. Xu, W. Zhang, X. X. Zhang, Z. Y. Wang, Z. Xiang, L. Yuan and X. B. Zhang, J. Am. Chem. Soc., 2018, 140, 7716–7722 CrossRef CAS PubMed.
- X. L. Shi, G. J. Mao, X. B. Zhang, H. W. Liu, Y. J. Gong, Y. X. Wu, L. Y. Zhou, J. Zhang and W. Tan, Talanta, 2014, 130, 356–362 CrossRef CAS PubMed.
- J. Qiu, C. Zhong, M. Liu, X. Xiong, Y. Gao and H. Zhu, Sens. Actuators, B, 2022, 371, 132606 CrossRef CAS.
- S. Shi-Li, C. Xin-Peng, Z. Xiao-Fan, M. Jun-Ying and Z. Bao-Xiang, J. Mater. Chem. B, 2015, 3, 919–925 RSC.
- K. Numasawa, K. Hanaoka, N. Saito, Y. Yamaguchi, T. Ikeno, H. Echizen, M. Yasunaga, T. Komatsu, T. Ueno, M. Miura, T. Nagano and Y. Urano, Angew. Chem., Int. Ed., 2020, 59, 6015–6020 CrossRef CAS PubMed.
- A. K. Kanduluru, M. Srinivasarao and P. S. Low, Bioconjugate Chem., 2016, 27, 2157–2165 CrossRef CAS PubMed.
- W. Wang, J. Xiong, X. Song, Z. Wang, F. Zhang and Z. Mao, Anal. Chem., 2020, 92, 13305–13312 CrossRef CAS PubMed.
- G. Jiang, X.-F. Lou, S. Zuo, X. Liu, T.-B. Ren, L. Wang, X.-B. Zhang and L. Yuan, Angew. Chem., Int. Ed., 2023, 62, e202218613 CrossRef CAS PubMed.
- H. Zhang, K. Li, L.-L. Li, K.-K. Yu, X.-Y. Liu, M.-Y. Li, N. Wang, Y.-H. Liu and X.-Q. Yu, Chin. Chem. Lett., 2019, 30, 1063–1066 CrossRef CAS.
- H. Zhang, F.-F. Xiang, Y.-Z. Liu, Y.-J. Chen, D.-H. Zhou, Y.-H. Liu, S.-Y. Chen, X.-Q. Yu and K. Li, JACS Au, 2023, 3, 3462–3472 CrossRef CAS PubMed.
- F. F. Xiang, H. Zhang, Y. L. Wu, Y. J. Chen, Y. Z. Liu, S. Y. Chen, Y. Z. Guo, X. Q. Yu and K. Li, Adv. Mater., 2024, 36, e2404828 CrossRef PubMed.
- Z. Lei, C. Sun, P. Pei, S. Wang, D. Li, X. Zhang and F. Zhang, Angew. Chem., Int. Ed., 2019, 58, 8166–8171 CrossRef CAS PubMed.
- S. He, P. Cheng and K. Pu, Nat. Biomed. Eng., 2023, 7, 281–297 CrossRef CAS PubMed.
- K. Wang, K.-F. Gu, J. Cao, Y.-S. Yang, H.-L. Zhu, J.-H. Shang and J.-L. Zhou, ACS Sens., 2024, 9, 4898–4905 CrossRef CAS PubMed.
- L. Shen, J. Li, C. Wen, H. Wang, N. Liu, X. Su, J. Chen and X. Li, Sci. Adv., 2024, 10, eado2037 CrossRef CAS PubMed.
- X. Zhang, Y. Chen, H. He, S. Wang, Z. Lei and F. Zhang, Angew. Chem., Int. Ed., 2021, 60, 26337–26341 CrossRef CAS PubMed.
- Z. Qin, T.-B. Ren, H. Zhou, X. Zhang, L. He, Z. Li, X.-B. Zhang and L. Yuan, Angew. Chem., Int. Ed., 2022, 61, e202201541 CrossRef CAS PubMed.
- Y. Liu, L. Zhang, Y. Chen, H. Sun, J. Chen, A. M. El-Toni, A. Khan, Z. Lei and F. Zhang, Adv. Opt. Mater., 2023, 11, 2301144 CrossRef CAS.
- Y. Shi, W. Yuan, Q. Liu, M. Kong, Z. Li, W. Feng, K. Hu and F. Li, ACS Mater. Lett., 2019, 1, 418–424 CrossRef CAS.
- M. H. Park, H. Hyun, Y. Ashitate, H. Wada, G. Park, J. H. Lee, C. Njiojob, M. Henary, J. V. Frangioni and H. S. Choi, Theranostics, 2014, 4, 823–833 CrossRef PubMed.
- L. G. Wang, C. W. Barth, C. H. Kitts, M. D. Mebrat, A. R. Montaño, B. J. House, M. E. McCoy, A. L. Antaris, S. N. Galvis, I. McDowall, J. M. Sorger and S. L. Gibbs, Sci. Transl. Med., 2020, 12, eaay0712 CrossRef CAS PubMed.
- C. W. Barth and S. L. Gibbs, Theranostics, 2017, 7, 573–593 CrossRef CAS PubMed.
- L. Wang, C. Barth, J. Combs, A. Montaño and S. Gibbs, Proc. SPIE Int. Soc. Opt. Eng., 2019, 108620H, DOI:10.1117/12.2507296.
- M. R. Detty, P. N. Prasad, D. J. Donnelly, T. Ohulchanskyy, S. L. Gibson and R. Hilf, Bioorg. Med. Chem., 2004, 12, 2537–2544 CrossRef CAS PubMed.
- J. E. Hill, M. K. Linder, K. S. Davies, G. A. Sawada, J. Morgan, T. Y. Ohulchanskyy and M. R. Detty, J. Med. Chem., 2014, 57, 8622–8634 CrossRef CAS PubMed.
- K. S. Davies, M. K. Linder, M. W. Kryman and M. R. Detty, Bioorg. Med. Chem., 2016, 24, 3908–3917 CrossRef CAS PubMed.
- H. Shigemitsu, K. Ohkubo, K. Sato, A. Bunno, T. Mori, Y. Osakada, M. Fujitsuka and T. Kida, JACS Au, 2022, 2, 1472–1478 CrossRef CAS PubMed.
- A. Paziewska-Nowak, M. Dawgul and D. G. Pijanowska, Int. J. Electrochem. Sci., 2019, 14, 3764–3776 CrossRef CAS PubMed.
- H. Shigemitsu, K. Sato, S. Hagio, Y. Tani, T. Mori, K. Ohkubo, Y. Osakada, M. Fujitsuka and T. Kida, ACS Appl. Nano Mater., 2022, 5, 14954–14960 CrossRef CAS.
- Y. Wang, J. Li, Y. Zhang, Y. Nan and X. Zhou, Chem. Commun., 2022, 58, 7797–7800 RSC.
- M. Li, S. Long, Y. Kang, L. Guo, J. Wang, J. Fan, J. Du and X. Peng, J. Am. Chem. Soc., 2018, 140, 15820–15826 CrossRef CAS PubMed.
- T. Xiong, M. Li, Y. Chen, J. Du, J. Fan and X. Peng, Chem. Sci., 2021, 12, 2515–2520 RSC.
- C. Liu, L. Zhou, F. Wei, L. Li, S. Zhao, P. Gong, L. Cai and K. M.-C. Wong, ACS Appl. Mater. Interfaces, 2019, 11, 8797–8806 CrossRef CAS PubMed.
- K. Reynolds, D. Schlessinger, A. Yanes, V. Godinez-Puig, B. Chen, A. Kurta, J. Cotseones, S. Chiren, S. Iyengar, S. Ibrahim, B. Kang, B. Worley, R. Behshad, D. DeHoratius, P. Denes, A. Drucker, L. Dzubow, J. Etzkorn, C. Harwood, J. Kim, N. Lawrence, E. Lee, G. Lissner, A. Marghoob, R. Matin, A. Mattox, B. Mittal, J. Thomas, X. Zhou, D. Zloty, B. Hughes, M. Nottage, A. Green, A. Testori, G. Argenziano, C. Longo, I. Zalaudek, C. Lebbe, J. Malvehy, P. Saiag, S. Cernea, J. Schmitt, J. Kirkham, E. Poon, J. Sobanko, T. Cartee, I. Maher and M. Alam, Brit. J. Dermatol., 2021, 184, 1113–1122 CrossRef CAS PubMed.
- W. Piao, K. Hanaoka, T. Fujisawa, S. Takeuchi, T. Komatsu, T. Ueno, T. Terai, T. Tahara, T. Nagano and Y. Urano, J. Am. Chem. Soc., 2017, 139, 13713–13719 CrossRef CAS PubMed.
- M. Chiba, Y. Ichikawa, M. Kamiya, T. Komatsu, T. Ueno, K. Hanaoka, T. Nagano, N. Lange and Y. Urano, Angew. Chem., Int. Ed., 2017, 56, 10418–10422 CrossRef CAS PubMed.
- W. Lv, S. Chi, W. Feng, T. Liang, D. Song and Z. Liu, Chem. Commun., 2019, 55, 7037–7040 RSC.
- R. Tian, W. Sun, M. Li, S. Long, M. Li, J. Fan, L. Guo and X. Peng, Chem. Sci., 2019, 10, 10106–10112 RSC.
- D. Ma, H. Bian, S. Long, P. Zhou, R. Tian, Y. Wu, H. Ge, M. Li, J. Du, J. Fan, Y. Zhang and X. Peng, Sci. China: Chem., 2022, 65, 563–573 CrossRef CAS.
- H. Bian, D. Ma, X. Zhang, K. Xin, Y. Yang, X. Peng and Y. Xiao, Small, 2021, 17, 2100398 CrossRef CAS PubMed.
- W. Cao, Y. Zhu, F. Wu, Y. Tian, Z. Chen, W. Xu, S. Liu, T. Liu and H. Xiong, Small, 2022, 18, 2204851 CrossRef CAS PubMed.
- C. Zhang, J. Wu, W. Liu, W. Zhang, C.-S. Lee and P. Wang, Acta Biomater., 2023, 159, 247–258 CrossRef CAS PubMed.
Footnote |
| † These authors contributed equally to the work. |
| This journal is © The Royal Society of Chemistry 2025 |