Centimeter-scale Gua3SbBr6 single crystals for white light-emitting diodes enabled by inhibition of multi-site nucleation†
Yongjiang
Dou‡
a,
Zirui
Liu‡
b,
Quanzhen
Huang
a,
Tiantian
Shi
a,
Sheng
Wang
 *b and
Xuyong
Yang
*b and
Xuyong
Yang
 *b
*b
aSchool of Electrical and Information Engineering, Henan University of Engineering, 001 Xianghe Road, Zhengzhou 451191, China. E-mail: douyongjiang@126.com
bKey Laboratory of Advanced Display and System Applications of Ministry of Education, Shanghai University, 149 Yanchang Road, Shanghai 200072, China. E-mail: nanocrystal@shu.edu.cn; yangxy@shu.edu.cn
First published on 10th December 2024
Abstract
High-quality single crystals (SCs) are crucial for advanced photoelectronic devices like light-emitting diodes (LEDs), lasers, and photodetectors. Zero-dimensional organic antimony-based metal halides, such as Gua3SbX6, offer great promise due to their unique structure and high photoluminescence quantum yield (PLQY). However, producing large-sized SCs remains challenging, because the multi-site nucleation leads to parasitic crystal formation, which consumes the abundant precursors. In this study, we utilized zinc acetate as an additive to cultivate centimeter-scale Gua3SbBr6 SCs. Zn2+ ions robustly coordinate with Br− ions, effectively retarding their participation in the SC seed formation and suppressing multi-site nucleation. These optimized SCs were used to fabricate a white light-emitting diode (WLED) with a high color rendering index (CRI) of 89 and a maximum power efficiency of 48.6 lm/W, significantly outperforming conventional WLEDs. This study not only deepens our understanding of crystal growth dynamics but also addresses a key challenge, paving the way for high-performance, eco-friendly photoelectronic devices using Gua3SbBr6 SCs.
Introduction
Single perovskite crystals (SCs) have been garnering significant attention in photoelectronic devices, including light-emitting diodes (LEDs), lasers, and photodetectors.1–9 Recently, zero-dimensional (0D) organic antimony-based metal halides, such as Gua3SbX6, have attracted interest due to their non-toxic nature, large Stokes shift, high photoluminescence quantum yield (PLQY), and stable crystal structure.10,11 Typically, SCs are synthesized via the solvent evaporation method, where Gua3SbX6 precipitates from a supersaturated solution as the solvent evaporates. However, this process often suffers from multi-site nucleation and parasitic crystallization due to the ultrafast nucleation and growth rate, which significantly consumes the precursors, thereby suppressing the emergence of large-sized and defect-free SCs.12–14 In response, the polymer-controlled nucleation process emerged as a means to manage nucleation via organic polymers, thereby facilitating more efficient growth of large-sized perovskite SCs.15 However, in the case of 0D perovskite, the diffusion of Gua ions, hindered by their intrinsic spatial bulkiness, is constrained by the presence of polymers, thereby impeding the growth of large-sized 0D SCs.In this study, we introduce Zn ions as a negative catalyst to limit the burst formation of crystal seeds. By strongly coordinating with halide ions, Zn ions retard the participation of halide ions in the nucleation and growth process of SCs. This approach enabled us to achieve centimeter-scale SCs with a nearly 100% PLQY and single-exponential lifetime decay. Utilizing the high-quality Gua3SbBr6 SCs, we fabricated white light-emitting diodes (WLEDs) with a high color rendering index (CRI) of 89 and a maximum power efficiency of 48.6 lm/W. This work paves a new way for the synthesis of large-sized and high-quality perovskite SCs for photoelectronic devices.
Results and discussion
The solvent evaporation technique was employed to control the nucleation and growth of single crystals (SCs). In a typical procedure, a mixture of N,N-diphenylguanidine hydrobromate (Gua⋅HBr), SbBr3, and methanol was introduced into a perforated glass bottle (Fig. S1, ESI†). We maintained a growth period of 24 hours for the SCs and subsequently evaluated the effect of Zn ion concentration on the crystallization process of Gua3SbBr6 SCs (Fig. S2, ESI†). In the control experiment, numerous small particles were precipitated. In contrast, with the increase in Zn concentration, the size of SCs are gradually larger and the unwanted parasitic crystals are eliminated. Notably, when the Zn(Ac)2 concentration was increased to 16%, white precipitates were formed, likely due to the generation of an impurity phase. Specifically, we measured the PL spectra, including untreated Gua3SbBr6 SC and samples with 2%, 4%, 8%, and 16% Zn(Ac)2, respectively. The results indicate that Gua3SbBr6 SC with 8% Zn(Ac)2 exhibit the highest PL intensity (Fig. S3, ESI†). We speculate that this may be due to the appropriate amount of Zn(Ac)2 promoting a more suitable growth rate, leading to better crystallinity. However, when the amount of Zn(Ac)2 increases to 16%, due to the excessive introduction of Zn ions causing more defects, the crystal quality and PL performance are affected.Consequently, 8% Zn concentration was selected as the optimal parameter. Fig. 1a demonstrated the schematic illustration of the Gua3SbBr6 SC growth process without Zn(Ac)2 and with 8% Zn(Ac)2. With further extending the SC growth time, we obtained a Gua3SbBr6 SC with dimensions of approximately 10 mm × 6.8 mm × 4.5 mm. As shown in Fig. 1b, the SC sample exhibited bright yellow emission under ultraviolet excitation. The crystal structure of Gua3SbBr6 was determined through powder X-ray diffraction (XRD) analysis (Fig. 1c), revealing that it belongs to the trigonal space group R3, with [SbBr6]3− anions surrounded by six large organic cations of Gua. The antimony center adopts a typical octahedral geometry, bonded to bromide ions, similar to previously reported SbBr6 complexes.16,17 The powder XRD pattern of Gua3SbBr6 closely matches the simulated XRD data, indicating the high phase purity of the as-prepared SCs. In Fig. 1d, high-resolution transmission electron microscopy (HRTEM) image reveals a defect-free crystal with continuous lattice fringes.18 The accompanying fast Fourier transform (FFT) pattern further confirms the trigonal crystal system of Gua3SbBr6.
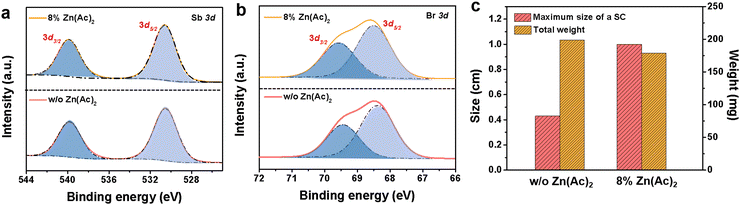
To gain deeper insights into the role of Zn2+ in the formation of Gua3SbBr6 SC, we employed X-ray photoelectron spectroscopy (XPS) measurements. The XPS signals were calibrated using the C 1s peak with a reference value of 284.8 eV (Fig. S4, ESI†). For the Gua3SbBr6 sample with 8% Zn(Ac)2, the Sb 3d spectrum (Fig. 2a) reveals distinct characteristic peaks at 530.6 eV (Sb 3d5/2) and 539.9 eV (Sb 3d3/2). The Br 3d spectrum (Fig. 2b) shows binding energy peaks at 68.5 eV (Br 3d5/2) and 69.5 eV (Br 3d3/2). Notably, the XPS signals for both Sb and Br in the sample with 8% Zn(Ac)2 exhibit a significant shift towards higher binding energies compared to the Gua3SbBr6 crystal without Zn(Ac)2. This shift is attributed to the strengthened Sb–Br bond interactions, which are indicative of improved crystal quality facilitated by the addition of Zn(Ac)2.6,19 However, no Zn 2p signal was detected in either sample (Fig. S5, ESI†), suggesting that Zn2+ ions did not incorporate into the Gua3SbBr6 lattice or form a substantial enrichment layer on the surface of the SC. Therefore, we consider Zn ions as catalysts that significantly influence the SC growth process.
As shown in Fig. 2c, we further collected the crystals from both the control and 8% Zn(Ac)2-treated samples. Subsequently, we weighed their total mass and measured the size of their largest SC. The results indicate that the total mass of both samples is similar, but the size of the 8% Zn(Ac)2-treated sample (1 cm) is much larger than that of the control sample (0.4 cm) because of no parasitic crystallization. We hypothesize that Zn2+ competes with Sb3+ in the precursor, interacting with Br− and inhibiting further crystal growth upon reaching a certain saturation level, thereby acting as a crystal growth modulator.
Furthermore, we conducted density functional theory (DFT) calculations to investigate the interactions between DGP and various components (Br−, Sb3+, and Zn2+) in the perovskite precursor solution, as well as the binding energies of Zn–Br and Sb–Br (Fig. S6a and b, ESI†). The results indicate that the formation energies of DGP with Br− and Sb3+ are 4.19 eV and 5.28 eV, respectively, while the formation energy with Zn2+ is significantly lower at 0.0728 eV (Fig. S6a, ESI†). This suggests that during the crystallization process, DGP preferentially forms an unstable intermediate with Zn2+, which then transitions to stable crystals by interacting with Br− and Sb3+. Additionally, the binding energies of Zn–Br and Sb–Br are 3.76 eV and 3.51 eV, respectively (Fig. S6b, ESI†). Zn2+ ions robustly coordinate with Br− ions, effectively retarding their involvement in the SC seed formation and suppressing multi-site nucleation. It is noted that when the addition of Zn(Ac)2 reached 16%, white precipitates would form at the bottom of the reaction vessel as the growth time increased. To clarify the composition of these precipitates, we conducted XRD analysis on them, as shown in Fig. S7 (ESI†). The analysis results showed that these white precipitates were ZnBr2. This finding indicates that when the concentration of Zn ions in the precursor solution increases significantly, the interaction between Zn ions and Br ions will significantly increase, resulting in the formation of ZnBr2.
Fig. 3a depicts the UV/Vis absorption and PL spectrum of Gua3SbBr6 SCs, exhibiting a sharp absorption onset at 420 nm and a faint tail spanning 400–760 nm, attributed to the absorption of [SbBr6] octahedron units.20–22 Upon 300–400 nm excitation, bright yellow emission peaks at 611 nm with a 126 nm FWHM and ∼100% PLQY. The large >200 nm Stokes shift implies negligible self-absorption, favorable for luminescence applications.22,23 The PLE spectrum (Fig. S8a, ESI†) mirrors the absorption, with a 420 nm edge, confirming low visible absorbance. The emission's CIE coordinates (0.515, 0.450) and 2334 K CCT are presented in Fig. S8b (ESI†). Preliminary time-resolved photo-luminescence (TRPL) measurements (Fig. 3b) reveal a long 3.2 μs single-exponential decay lifetime (R = 0.998), alongside the large Stokes shift, indicating intrinsic self-trapping excitons (STEs) as the source of the exceptional broad emission.
Beyond the deformable lattice, robust electron–phonon coupling plays a pivotal role in enhancing emission characteristics of STEs.24,25 To elucidate this interaction in Gua3SbBr6, Raman spectroscopy using a 633 nm laser (Fig. S9, ESI†) and temperature-dependent PL under 365 nm UV light (Fig. 3c) were conducted. As a control, no Raman signals were observed for the organic Gua component (Fig. S9b, ESI†), confirming that the Raman bands in Gua3SbBr6 stem from the inorganic polyhedron. Notably, three distinct bands at 65, 127, and 151 cm−1 align perfectly with the octahedral vibrational modes in eqn (1):
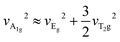 | (1) |
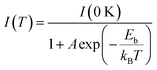 | (2) |
Furthermore, we leveraged fs-TA spectroscopy to delve into the ultrafast charge carrier dynamics. Fig. 3f depicts the wavelength and time-resolved fs-TA map of Gua3SbBr6 thin films, revealing a broad PIA signal spanning 475–700 nm upon 360 nm pulsed excitation, confirming the presence of STEs.32,33Fig. 3j illustrates the STE formation dynamics through PIA rise times at various wavelengths. The TA spectra exhibit distinct GSB (around 410 nm) and PIA (around 620 nm) features, attributable to light absorption saturation34,35 and nonlinear exciton annihilation,36,37 respectively. Fig. 3h presents the fitted TA decay curves at 620 nm, unraveling three components: τ1 (0.1 ps) attributed to hot STE cooling,19,38τ2 (25.2 ps) as the intersystem crossing time from spin-singlet to spin-triplet STEs, and τ3 (>2 ns) corresponding to the STE lifetime.22 This analysis sheds light on the intricate dynamics governing the broadband emission of Gua3SbBr6. Fig. 3i illustrates the excitation and recombination processes of Gua3SbBr6, which can be described by the following photophysical model: upon absorption of incident light, electrons in the valence band of Gua3SbBr6 are promoted to an excited state manifold. During the relaxation process, these free electrons rapidly transition into low-energy self-trapped excited states due to ultrafast structural reorganization. As a result, the trapped electrons recombine with holes, leading to a broadband emission characterized by a large Stokes shift.24,39
Fig. S10 (ESI†) presents the temperature, humidity stability, and photostability of the Gua3SbBr6 SC. This figure elucidates the fluctuations in relative PL intensities under various conditions: when the SC is positioned on a hotplate, subjected to UV light exposure, and following prolonged exposure to an environment with 70% humidity. The results demonstrate the SC's impressive temperature stability, resistance to photodegradation, and robust stability against humidity. These observations unequivocally affirm that the Gua3SbBr6 SC exhibits outstanding stability across diverse environmental conditions, offering a thorough evaluation of the crystal's stability and reproducibility characteristics.
White light-emitting diodes (WLEDs) based on Pb-based perovskites have demonstrated exceptional performance. For instance, Zhu et al. doped Zn2+ into CsPbBr3 nanocrystals (NCs) achieving a luminous efficacy of radiation of 312 lm/W and photoluminescence stability for 156 days.40 However, the performance of white LEDs fabricated using lead-free perovskites still lags behind and the date is shown in Table 1. To demonstrate the potential application of the Gua3SbBr6 SC, a WLED was fabricated by combining blue-emissive BaMgAl10O17:Eu2+ phosphors, single yellow-emitting Gua3SbBr6 SC, and green CsPbBr3 NCs on a violet-emissive GaN chip. The emission spectrum of the resulting WLED is shown in Fig. 4a. The blue emission, centered around 453 nm, is attributed to the BaMgAl10O17:Eu2+ phosphors; the green emission, centered at approximately 512 nm, originates from the CsPbBr3 NCs; and the red emission, centered at about 611 nm, is from Gua3SbBr6 SC. Fig. 4b displays the CIE color coordinates and correlated color temperatures (CCTs) of the light emissions on the CIE1931 chromaticity diagram. By optimizing the blue-to-yellow phosphor weight ratio to 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, the WLED exhibits a near-ideal white emission with CIE coordinates of (0.323, 0.344), a CCT of 5851 K and a color rendering index (CRI) of 89. The high CRI is attributed to the broader emission spectrum of Gua3SbBr6, which ensures improved coverage in the green and red spectral regions. Fig. 4c illustrates the luminance and power efficiency (PE) as a function of forward-bias current. The WLED luminance increases with higher injection currents, reaching a maximum of over 5
1, the WLED exhibits a near-ideal white emission with CIE coordinates of (0.323, 0.344), a CCT of 5851 K and a color rendering index (CRI) of 89. The high CRI is attributed to the broader emission spectrum of Gua3SbBr6, which ensures improved coverage in the green and red spectral regions. Fig. 4c illustrates the luminance and power efficiency (PE) as a function of forward-bias current. The WLED luminance increases with higher injection currents, reaching a maximum of over 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000
000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cd m−2. The maximum PE is 48.6 lm/W, nearly three times that of a typical incandescent lamp.41Fig. 4d shows a photograph of the WLED output at 10 mA, revealing a bright and uniform white emission. Given their inherent nontoxic nature, the Gua3SbBr6 SC represents a superior choice for down-conversion fluorescent materials in WLED applications and holds promise for future photoelectronic systems.
000 cd m−2. The maximum PE is 48.6 lm/W, nearly three times that of a typical incandescent lamp.41Fig. 4d shows a photograph of the WLED output at 10 mA, revealing a bright and uniform white emission. Given their inherent nontoxic nature, the Gua3SbBr6 SC represents a superior choice for down-conversion fluorescent materials in WLED applications and holds promise for future photoelectronic systems.
| Emitter | PL peak (nm) | PLQY (%) | Power efficiency (lm W−1) | CRI | Ref. |
|---|---|---|---|---|---|
| (Gua-DPG)3SbCl6 | 584 | 100 | — | 86.5 | 42 |
| Cs2NaInCl6:Sb3+ | 605 | 92.4 | — | 95.4 | 43 |
| Cs2Cu2Cl5:Zn2+ | 522 | 70.19 | — | 95.8 | 44 |
| Cs3InCl6:Sb3+/Mn2+ | 620 | 51.38 | — | 85.5 | 45 |
| (OTA)2+xSnI4+x | 617 | 92 | — | 92 | 46 |
| Cs2NaInCl6:Sb3+ NCs | 460 | 80.1 | 37.5 | 80 | 47 |
| CsPbBr3 NCs | 517 | 92 | — | 86 | 40 |
| (DPG) 3 SbBr 6 | 611 | 100 | 48.6 | 89 | This work |
Conclusions
In this work, we successfully synthesized high-quality Gua3SbBr6 SCs using Zn(Ac)2 as a crystal growth modulator. Zn(Ac)2 effectively suppresses multi-site nucleation of SC, enabling the growth of centimeter-scale Gua3SbBr6 SCs. This is achieved through the strong coordination of Zn2+ ions with Br− ions, which retard their participation in the seed formation process, thereby preventing the formation of parasitic crystals. The resulting SCs exhibit a high PLQY of 100% and single-exponential lifetime decay. The WLED based on Gua3SbBr6 SCs presented a maximum PE is 48.6 lm/W, three-fold that of commercial incandescent lamps. Our work provides a new strategy to prepare large-sized perovskite SCs for photoelectronic devices.Experimental section
Materials and methods
Gua⋅HBr (N,N-diphenylguanidine hydrobromate, >98%, Toronto Research Chemicals), SbBr3 (antimony(III) bromide, 99.995%, Alfa), anhydrous methanol (99.9%, Alfa), zinc acetate dihydrate (99.999%, Sigma-Aldrich). All manipulations of reactants and products were performed in a nitrogen-filled glovebox. Unless otherwise indicated, all reagents and solvents were used without further purification.Gua3SbBr6 SC synthesis
The synthesis of Gua3SbBr6 SC was based on the solvent evaporation method. Gua⋅HBr and SbBr3 with the molar ratio of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, such as 3 mmol Gua⋅HBr (0.876 g), 1 mmol SbBr3 (0.361 g) and different molar ratios of Zn(Ac)2 were dissolved in 10 ml anhydrous methanol and stirred for 30 min at 30 °C in a fully sealed glass container. The precursor concentration is maintained at 0.1 mmol ml−1. After complete dissolution, the transparent solution was setting and cooling down to room temperature (25 °C). Some irregular crystals will appear at the bottom of the glass container. The saturated solution was filtered with a polytetrafluoroethylene (0.45 μm) syringe, and the filtered solution was injected into vials and sealed with a paraffin film with a small hole. The vial was placed without shaking in a dark environment for 24 h in the room temperature. High-quality SCs with a size of about 5 mm × 3 mm × 2 mm were obtained.
1, such as 3 mmol Gua⋅HBr (0.876 g), 1 mmol SbBr3 (0.361 g) and different molar ratios of Zn(Ac)2 were dissolved in 10 ml anhydrous methanol and stirred for 30 min at 30 °C in a fully sealed glass container. The precursor concentration is maintained at 0.1 mmol ml−1. After complete dissolution, the transparent solution was setting and cooling down to room temperature (25 °C). Some irregular crystals will appear at the bottom of the glass container. The saturated solution was filtered with a polytetrafluoroethylene (0.45 μm) syringe, and the filtered solution was injected into vials and sealed with a paraffin film with a small hole. The vial was placed without shaking in a dark environment for 24 h in the room temperature. High-quality SCs with a size of about 5 mm × 3 mm × 2 mm were obtained.
Centimeter-scale Gua3SbBr6 SCs synthesis
We used the obtained SCs as seed SCs. By placing the seed into fresh solution, keeping it in the vial with a small hole at 0 °C for three days, the original ∼5 mm seed was found to grow into a larger (∼10 mm) one.Fabrication of WLEDs
A BaMgAl10O17:Eu2+ phosphor blue-emitting powder (2 mg) was blended with ET-821A and ET-821B silicone resin under stirring, and cured under 150 °C. Then the composite layer was coated on the surface of the violet-emissive GaN chip (1 cm × 1 cm), followed by placing single yellow-emitting Gua3SbBr6 SC and deposited green CsPbBr3 nanocrystals (NCs) (50 mg mL−1).Crystal structure characterization
The determination of unit-cell parameters and data collections were performed on XtaLAB Synergy-i using a scanning technique with Mo Kα radiation. XPREP was used to obtain an indication of the space group and the structure was typically solved using direct methods and refined by SHELXTL and OLEX2. Powder X-ray diffraction (PXRD) data were obtained using a Bruker D8 Advance diffractometer with a Cu Kα source. TGA was performed under a N2 atmosphere on a PerkinElmer TGA instrument, using a heating rate of 5 °C min−1 up to a temperature of 500 °C. FTIR micro-spectroscopy was carried out using a PerkinElmer, Spotlight 400 FT-IR Imaging System model Spectrum Frontier mid-IR spectral range, in the reflectance mode. TEM measurements were performed on a FEI Tecnai G20 transmission electron microscope.Spectrum measurements
Excitation spectra were collected by using an Edinburgh FLS1000 spectrofluorometer at room temperature. PL spectra, time-resolved PL measurements and absolute PL QYs were obtained using an Edinburgh FLS920 PL spectrometer. X-ray photoelectron spectroscopy was tested by using a Thermo Scientific ESCALAB 250 instrument.Temperature-dependence photoluminescence
The sample was kept in a thermostat modifying low temperature with liquid nitrogen. The emission spectra were collected by spectrophotometer with a photomultiplier tube detection system. The excitation light source was a He–Ne 325 nm laser, and the temperature was changed via an automatic tuning temperature controller (Linkon) from 80 to 450 K, increasing 10 K with each spectrum being recorded.Calculation methods
All the density functional theory (DFT) calculations are carried out using a Vienna ab initio simulation package (VASP5.4).1 The exchange–correlation energy is treated by the projector augmented wave (PAW)2 approach in conjunction with a generalized gradient approximation (GGA)3 in the form of Perdew–Burke–Ernzerhof (PBE)4 is used to treat the exchange–correlation energy. The DFT-D35 correction is adopted to treat the van der Waals (vdW) interactions. The virtual crystal approximation (VCA)6 is adopted, which is based on building a fictitious “virtual atom” potential by averaging the ionic potentials of the atoms that alternate at the same position in the structure. Considering the large number of atoms of the systems, we directly use the crystal structure obtained from the experiment to calculate the electronic structures. The energy cutoff and the energy convergence criterion are respectively set as 500 eV and 10−4 eV, and a 3 × 3 × 3 k-point is applied. Besides, the valence configurations of Sb (5s22p3), Br (4s24p5), Cl (3s23p5), N (2s22p3), C (2s22p4), and H (1s1) are adopted to construct the PAW potentials.Data availability
The authors confirm that the data supporting the findings of this study are available within the article and its ESI.†Conflicts of interest
The authors declare no competing interests.Acknowledgements
The authors acknowledge financial support from the Henan Provincial Natural Science Foundation (242300420681), Key Scientific Research Project of Higher Education Institutions in Henan Province (24A510002), and National College Students' Innovation and Entrepreneurship Training Program in Henan Province, 2024 (202411517018).References
- H. Wei, D. Desantis, W. Wei, Y. Deng, D. Guo, T. J. Savenije, L. Cao and J. Huang, Nat. Mater., 2017, 16, 826–833 CrossRef CAS
.
- L. Zhou, J.-F. Liao, Z.-G. Huang, J.-H. Wei, X.-D. Wang, W.-G. Li, H.-Y. Chen, D.-B. Kuang and C.-Y. Su, Angew. Chem., Int. Ed., 2019, 58, 5277–5281 CrossRef CAS
.
- W. Chen, Z. Huang, H. Yao, Y. Liu, Y. Zhang, Z. Li, H. Zhou, P. Xiao, T. Chen, H. Sun, J. Huang and Z. Xiao, Nat. Photonics, 2023, 17, 401–407 CrossRef CAS
.
- M. I. Saidaminov, V. Adinolfi, R. Comin, A. L. Abdelhady, W. Peng, I. Dursun, M. Yuan, S. Hoogland, E. H. Sargent and O. M. Bakr, Nat. Commun., 2015, 6, 8724 CrossRef CAS
.
- L. Kong, X. Zhang, Y. Li, H. Wang, Y. Jiang, S. Wang, M. You, C. Zhang, T. Zhang, S. V. Kershaw, W. Zheng, Y. Yang, Q. Lin, M. Yuan, A. L. Rogach and X. Yang, Nat. Commun., 2021, 12, 1246 CrossRef CAS PubMed
.
- Y. Wang, S. Wang, R. Li, W. Li, T. Long, L. Wang, L. Kong, F. Cao, Q. Wu, G. Jia and X. Yang, Small, 2024, 20, 2402825 CrossRef CAS
.
- L. Kong, Y. Sun, B. Zhao, K. Ji, J. Feng, J. Dong, Y. Wang, Z. Liu, S. Maqbool, Y. Li, Y. Yang, L. Dai, W. Lee, C. Cho, S. D. Stranks, R. H. Friend, N. Wang, N. C. Greenham and X. Yang, Nature, 2024, 631, 73–79 CrossRef CAS
.
- T. Zhang, L. Wang, L. Kong, C. Zhang, H. He, B. Wei and X. Yang, J. Mater. Chem. C, 2021, 9, 7532–7538 RSC
.
- Y. Sun, L. Ge, L. Dai, C. Cho, J. Ferrer Orri, K. Ji, S. J. Zelewski, Y. Liu, A. J. Mirabelli, Y. Zhang, J. Y. Huang, Y. Wang, K. Gong, M. C. Lai, L. Zhang, D. Yang, J. Lin, E. M. Tennyson, C. Ducati, S. D. Stranks, L. S. Cui and N. C. Greenham, Nature, 2023, 615, 830–835 CrossRef CAS PubMed
.
- M. L. Zaffalon, Y. Wu, F. Cova, L. Gironi, X. Li, V. Pinchetti, Y. Liu, M. Imran, A. Cemmi, I. Di Sarcina, L. Manna, H. Zeng and S. Brovelli, Adv. Funct. Mater., 2023, 33, 2305564 CrossRef CAS
.
- C. Hou, X. Liu, C. Wan, B. Li, T. Lu, C. Ge, Y. Song, A. Wang, Y. Kang and Q. Dong, Chem. Mater., 2023, 35, 10635–10644 CrossRef CAS
.
- F. Zhang, Z. Zhao, B. Chen, H. Zheng, L. Huang, Y. Liu, Y. Wang and A. L. Rogach, Adv. Opt. Mater., 2020, 8, 1901723 CrossRef CAS
.
- G. Saravana Kumar and P. Murugakoothan, Spectrochim. Acta, Part A, 2014, 131, 17–21 CrossRef CAS PubMed
.
- F. Palazon, S. Dogan, S. Marras, F. Locardi, I. Nelli, P. Rastogi, M. Ferretti, M. Prato, R. Krahne and L. Manna, J. Phys. Chem. C, 2017, 121, 11956–11961 CrossRef CAS PubMed
.
- L. Ma, Z. Yan, X. Zhou, Y. Pi, Y. Du, J. Huang, K. Wang, K. Wu, C. Zhuang and X. Han, Nat. Commun., 2021, 12, 2023 CrossRef CAS PubMed
.
- V. Morad, S. Yakunin and M. V. Kovalenko, ACS Mater. Lett., 2020, 2, 845–852 CrossRef CAS
.
- F. Lin, H. Wang, W. Liu and J. Li, J. Mater. Chem. C, 2020, 8, 7300–7303 RSC
.
- R. Lin, Q. Zhu, Q. Guo, Y. Zhu, W. Zheng and F. Huang, J. Phys. Chem. C, 2020, 124, 20469–20476 CrossRef CAS
.
- D. A. Wheeler and J. Z. Zhang, Adv. Mater., 2013, 25, 2878–2896 CrossRef CAS PubMed
.
- J. Zhang, Y. Yang, H. Deng, U. Farooq, X. Yang, J. Khan, J. Tang and H. Song, ACS Nano, 2017, 11, 9294–9302 CrossRef CAS PubMed
.
- Y. Jing, Y. Liu, X. Jiang, M. S. Molokeev, M. S. Molokeev, M. S. Molokeev, Z. Lin, Z. Lin, Z. Xia and Z. Xia, Chem. Mater., 2020, 32, 5327–5334 CrossRef CAS
.
- L. Zhou, J. F. Liao, Z. G. Huang, J. H. Wei, X. D. Wang, H. Y. Chen and D. Bin Kuang, Angew. Chem., Int. Ed., 2019, 58, 15435–15440 CrossRef CAS PubMed
.
- Z. Wen, F. Fang, C. Zhang, S. Ding, J. Sun, H. Tang, B. Xu, K. Wang, K. L. Teo and X. W. Sun, J. Soc. Inf. Disp., 2019, 27, 587–596 CrossRef CAS
.
- J. Luo, X. Wang, S. Li, J. Liu, Y. Guo, G. Niu, L. Yao, Y. Fu, L. Gao, Q. Dong, C. Zhao, M. Leng, F. Ma, W. Liang, L. Wang, S. Jin, J. Han, L. Zhang, J. Etheridge, J. Wang, Y. Yan, E. H. Sargent and J. Tang, Nature, 2018, 563, 541–545 CrossRef CAS PubMed
.
- X. Y. Zhu and V. Podzorov, J. Phys. Chem. Lett., 2015, 6, 4758–4761 CrossRef CAS
.
- G. N. Papatheodorou, J. Chem. Phys., 1976, 66, 2893–2900 CrossRef
.
- J. A. Steele, P. Puech, M. Keshavarz, R. Yang, S. Banerjee, E. Debroye, C. W. Kim, H. Yuan, N. H. Heo, J. Vanacken, A. Walsh, J. Hofkens and M. B. J. Roeffaers, ACS Nano, 2018, 12, 8081–8090 CrossRef CAS PubMed
.
- D. S. Jiang, H. Jung and K. Ploog, J. Appl. Phys., 1988, 64, 1371–1377 CrossRef CAS
.
- R. Lin, Q. Guo, Q. Zhu, Y. Zhu, W. Zheng and F. Huang, Adv. Mater., 2019, 31, 1905079 CrossRef CAS
.
- G. Wu, C. Zhou, W. Ming, D. Han, S. Chen, D. Yang, T. Besara, J. Neu, T. Siegrist, M. H. Du, B. Ma and A. Dong, ACS Energy Lett., 2018, 3, 1443–1449 CrossRef CAS
.
- R. Roccanova, A. Yangui, H. Nhalil, H. Shi, M.-H. Du and B. Saparov, ACS Appl. Electron. Mater., 2019, 1, 269–274 CrossRef CAS
.
- P. Cheng, L. Sun, L. Feng, S. Yang, Y. Yang, D. Zheng, Y. Zhao, Y. Sang, R. Zhang, D. Wei, W. Deng and K. Han, Angew. Chem., 2019, 131, 16233–16237 CrossRef
.
- B. Yang, X. Mao, F. Hong, W. Meng, Y. Tang, X. Xia, S. Yang, W. Deng and K. Han, J. Am. Chem. Soc., 2018, 140, 17001–17006 CrossRef CAS PubMed
.
- S. J. Yu, M. W. Kang, H. C. Chang, K. M. Chen and Y. C. Yu, J. Am. Chem. Soc., 2005, 127, 17604–17605 CrossRef CAS PubMed
.
- J. Vogelsang, R. Kasper, C. Steinhauer, B. Person, M. Heilemann, M. Sauer and P. Tinnefeld, Angew. Chem., Int. Ed., 2008, 47, 5465–5469 CrossRef CAS PubMed
.
- D. J. Norris, A. Sacra, C. B. Murray and M. G. Bawendi, Phys. Rev. Lett., 1994, 72, 2612 CrossRef CAS PubMed
.
- M. T. Trinh, M. Y. Sfeir, J. J. Choi, J. S. Owen and X. Zhu, Nano Lett., 2013, 13, 6091–6097 CrossRef CAS PubMed
.
- D. B. Straus, S. Hurtado Parra, N. Iotov, J. Gebhardt, A. M. Rappe, J. E. Subotnik, J. M. Kikkawa and C. R. Kagan, J. Am. Chem. Soc., 2016, 138, 13798–13801 CrossRef CAS PubMed
.
- Z. Yuan, C. Zhou, Y. Tian, Y. Shu, J. Messier, J. C. Wang, L. J. Van De Burgt, K. Kountouriotis, Y. Xin, E. Holt, K. Schanze, R. Clark, T. Siegrist and B. Ma, Nat. Commun., 2017, 8, 14051 CrossRef CAS
.
- C. Dzorkpata, S. Thapa, H. Zhu, A. Grigoriev, D. Babaian, S. Guha and P. Zhu, J. Alloys Compd., 2024, 1005, 176064 CrossRef CAS
.
- E. F. Schubert and J. K. Kim, Science, 2005, 308, 1274–1278 CrossRef CAS PubMed
.
- C. Hou, X. Liu, C. Wan, B. Li, T. Lu, C. Ge, Y. Song, A. Wang, Y. Kang and Q. Dong, Chem. Mater., 2023, 35(24), 10635–10644 CrossRef CAS
.
- J. Li, Y. Sheng, G. Tong, H. Zhu, X. Tao, C. Wu, Y. Chang, Z. Tang, J. Yang and S. Zhang, Adv. Opt. Mater., 2023, 11(16), 2300100 CrossRef CAS
.
- Y. Peng, J. Guo, J. Guo, J. Wu, N. Zhang, G. Zhao, J. Hou, G. Zhang, Y. Liu and Y. Fang, J. Mater. Chem. C, 2024, 12(33), 12874–12881, 10.1039/D4TC01168F
.
- Y. Sheng, P. Chen, Y. Gao, Y. He, J. Li, Muhammad, X. Xie, C. Cheng, J. Yang and Y. Chang, ACS Appl. Mater. Interfaces, 2024, 16(15), 19175–19183 CrossRef CAS PubMed
.
- Z. Li, Z. Deng, A. Johnston, J. Luo, H. Chen, Y. Dong, R. Sabatini and E. H. Sargent, Adv. Funct. Mater., 2022, 32(18), 2111346 CrossRef CAS
.
- X. Li, D. Wang, Y. Zhong, F. Jiang, D. Zhao, S. Sun, P. Lu, M. Lu, Z. Wang and Z. Wu, Adv. Sci., 2023, 10(20), 2207571 CrossRef CAS PubMed
.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4tc03776f |
| ‡ Y. D. and Z. L. contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2025 |