Multimodal anti-counterfeiting inks: modern use of an ancient pigment in synergy with a persistent phosphor†
Francesco
Armetta
 ab,
Alessandro Lo
Bianco
ab,
Alessandro Lo
Bianco
 c,
Vitalii
Boiko
c,
Vitalii
Boiko
 d,
Dariusz
Hreniak
d,
Dariusz
Hreniak
 *d and
Maria Luisa
Saladino
*d and
Maria Luisa
Saladino
 *a
*a
aDepartment of Biological, Chemical and Pharmaceutical Sciences and Technologies (STEBICEF)-University of Palermo, Viale delle Scienze, Bld.17, Palermo I-90128, Italy. E-mail: marialuisa.saladino@unipa.it
bInstitute for Chemical and Physical Processes IPCF-Messina, CNR, Viale Ferdinando Stagno d’Alcontres 37, I-98158 Messina, Italy
cDepartment of Physic and Chemistry “E. Segrè”, University of Palermo, Viale delle Scienze, Bld.17, Palermo I-90128, Italy
dDivision of Optical Spectroscopy, Institute of Low Temperature and Structure Research, Polish Academy of Sciences, ul. Okólna 2, Wrocław PL-50-422, Poland. E-mail: d.hreniak@intibs.pl
First published on 13th November 2024
Abstract
Multi-level luminescent, transparent and non-permanent inks for anti-counterfeiting systems and security were developed. The inks emit radiation at different wavelengths based on the type of radiation used to illuminate them, providing multiple layers of safety. The red persistent phosphor Y2O2S:Eu3+,Mg2+,Ti4+ (YOS) was mixed with Egyptian Blue (EB) and dispersed in an aqueous solution of arabic gum. Imaging techniques, excitation and emission spectra, the study of luminescence over time and the duration time were used to verify that the obtained systems showed the desired optical characteristic both in the infrared and in the visible spectral ranges. The two luminescent materials act synergistically: when illuminated with UV light, YOS emits red photons over time and EB, absorbing this energy, emits infrared photons. The resulting emission characteristics are non-obvious and can be designed at the ink developing stage. The tests performed on several substrates showed that the developed luminescent inks are well suited for use in the field of security and anti-counterfeiting.
1. Introduction
In an era where counterfeiting poses a growing threat to businesses and security, multi-level luminescent anti-counterfeiting systems have emerged as a powerful weapon in the battle against fraudulent replication.1–4 These advanced technologies employ formulations with luminescence properties to create intricate and nearly impossible-to-replicate security markings. Multi-level luminescent systems involve the use of various luminescent materials which can encompass a spectrum of colors, compositions, and activation methods, resulting in highly intricate and unique security patterns.5–7 Their complexity and versatility make them an ideal choice for protecting valuable assets and ensuring the integrity of products and brand identities. These systems can generate peculiar luminescent effects when exposed to specific light sources, such as ultraviolet (UV) or infrared (IR) light. Examples include emission visible only under UV light or hidden motifs revealed under particular conditions, adding an additional security level. The complexity of multi-level luminescent markings makes them exceptionally challenging to reproduce without perfect knowledge of the specific materials and components used. Multi-level luminescent anti-counterfeiting systems find application in various industries, including the production of banknotes, passports, pharmaceutical product security labels, luxury brand identifiers, and more.5,8 This level of security can be crucial for safeguarding valuable products, official documents, and high-end goods.In the innovation of security measures, some of the key challenges are: to integrate both chemical and physical units to produce large-area and high-quality inks; to obtain “real” multilevel inks, to produce formulations on-demand (ad hoc), to produce green and eco-sustainable formulations, to produce multilevel systems capable of conjugating conventional security levels to additional properties (i.e. transparency, permanence/no-permanence, time); to develop new solutions for difficult surfaces in difficult environments; to integrate integration with digital technologies. There is in fact a growing emphasis on developing eco-friendly alternatives that maintain security while minimizing environmental impact.9–18
Among the various strategies employed to combat counterfeiting, the use of lanthanide (Ln)-based light conversions, including downshifting and up-conversion, as well as persistent phosphors, has gained significant traction.19–24 These materials play a crucial role in enhancing security features, and their adoption in commercial anti-counterfeiting measures has proven to be effective.4,25–27 Lanthanide-based light conversion materials have unique properties that make them valuable in anti-counterfeiting applications. Persistent phosphors (PersL) are materials that continue to emit light after being exposed to an external light source, adding an additional level of security by enabling the verification of a product's authenticity through its afterglow.28–31
Therefore, the aim of this work was to develop multilevel luminescent, easily removable and non-toxic inks capable of generating a specific emission spectrum using different excitation wavelengths. It is thus important that one phosphor, when mixed with another one, can act as a sensitizer for the luminescence of the second one. Since an important criterion for such materials is their chemical stability implying the invariability of their optical properties, among other things, when the labelled objects are exposed to fire, it is therefore advantageous to use favourably thermally stable inorganic materials as both phosphors. At the same time, it is important that in the case of the sensitizing phosphor, the excitation is efficient and advantageously broadband. On the other hand, already the emission signature of the first phosphor should be specific, preferably with narrow emission lines that allow appropriate coding of the signal under analysis.
To reach this goal, Egyptian Blue32 (EB), an ancient blue pigment, was chosen as the NIR-emitting phosphor when excited with red light.33–35 EB, a striking and enduring pigment, holds a unique place in the annals of art and history. With origins dating back over four millennia, it is regarded as one of the world's oldest synthetic pigments.36 While we now possess a fundamental understanding of its composition, the exact methodology employed by the Egyptians remains a subject of ongoing study and debate among historians and archaeologists.37–40 No recipes for EB production have been uncovered in any known Egyptian texts to date.32,41 EB is a crystalline compound essentially composed of cuprorivaite (CaCuSi4O10). EB is thermodynamically stable, degrades at very high temperatures (∼1000 °C), is resistant to acid attacks, stable in an alkaline environment, non-toxic and emits in the IR region, when illuminated with a red lamp with excellent quantum yield.42,43 However, the most important drawback of using this pigment in the presented concept is its market cost. In order to overcome this undoubtedly significant disadvantage, a simple but effective method of obtaining this pigment through a solid-state reaction process has been developed. On the other hand, the key to achieving adequate efficiency of the reabsorption process by EB is the use of the longest possible red emission time allowing intensification of its emission in the NIR. The role of such a sensitizing phosphor, which upon UV excitation emits red light falling in the EB absorption range, was assigned to yttrium oxysulfide doped with europium (Eu3+), titanium (Ti4+), and magnesium (Mg2+), Y2O2S:Eu3+,Ti4+,Mg2+ (YOS).44–49 Doped Y2O2S is a phosphor that plays a crucial role in modern lighting and display technologies. This phosphor, known for its remarkable luminescence properties, with high-energy efficiency, has found applications in anticounterfeiting and various fields, including fluorescent lamps, cathode-ray tube (CRT) displays, LED-based displays, and solid-state lighting. It helps in reducing energy consumption while providing excellent illumination, making it environmentally friendly. Y2O2S is renowned for its outstanding luminescence properties. One of the notable features of this phosphor is its ability, depending on the doping used, to emit light in a range of colors. In addition, Y2O2S is known for its long-lasting luminescence, ensuring that devices and lighting systems remain operational for extended periods without a significant decline in performance.
As the medium, arabic gum (GA) was chosen for the preparation of the ink. GA is a natural rubber (complex blend of polysaccharides and glycoproteins), fully edible, very cheap and well known as a binder in paint (watercolors) and inks.50 Its optical transparency allows it to maintain the high effectiveness of the luminescent properties of the phosphors.51 The weight percentage of 0.1% for EB compared to the total weight of the ink was chosen to prevent the ink from becoming blue upon application or acquiring a color that would not make it transparent.
This study was carried out in order to optimize the effect of sensibilization between the two compounds (YOS and EB) and to obtain an ink with the highest efficiency. Multispectral imaging techniques such as UV fluorescence and visible induced luminescence (VIL) were used to preliminary investigate the optical properties. The luminescence properties were determined by luminescence spectroscopy (through emission (PL) and excitation (PLE) spectra, and duration time measurements).
2. Materials and inks preparation
The reagents, information on commercial Y2O2S:Eu3+,Ti4+,Mg2+ (YOS), the method of the preparation of EB and the list of apparatus used for the material characterization are reported in the ESI.† EB was obtained by solid-state synthesis by using CaCO3, Cu2CO3(OH)2, natron and silica gel as reagents and calcining at 950 °C in a furnace for 24 hours (see the XRD pattern reported in Fig. S1 of the ESI†). The solid mixtures YOS/EB were prepared by mixing in a mortar the YOS and EB powders in a weight ratio of 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 3
1, 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 2
1, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. For the ink preparation, the GA aqueous solution at 33 w/w% was obtained at 70 °C on a heated magnetic stirrer to accelerate the GA dissolution, preventing the formation of lumps. The resulting solution, having a honey-like color, was thus filtered to remove impurities present in the powdered gum. Then, the four YOS/EB mixtures were blended into the GA solution.
1. For the ink preparation, the GA aqueous solution at 33 w/w% was obtained at 70 °C on a heated magnetic stirrer to accelerate the GA dissolution, preventing the formation of lumps. The resulting solution, having a honey-like color, was thus filtered to remove impurities present in the powdered gum. Then, the four YOS/EB mixtures were blended into the GA solution.
3. Results and discussion
3.1 Solid mixtures of YOS/EB
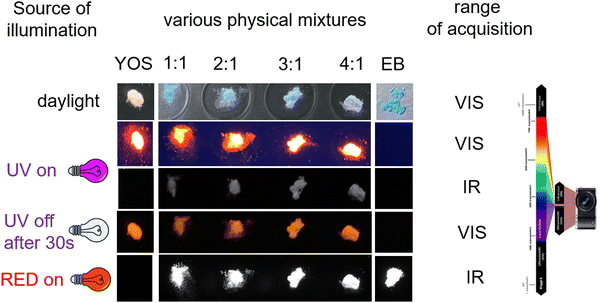
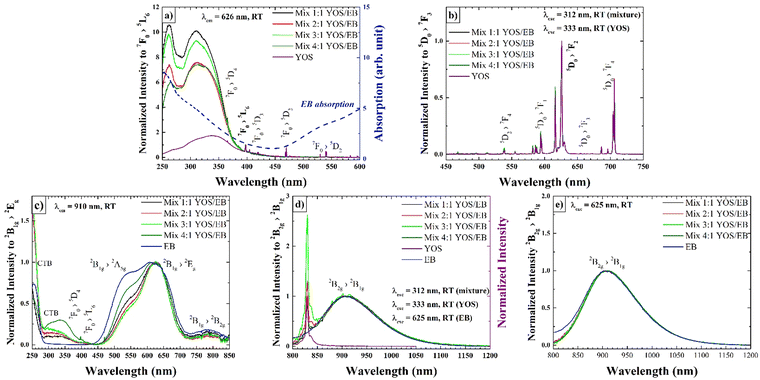
Under daylight, the color of the resulting mixture of pigment and phosphor was blue and slightly decreased in intensity with the relative increase in YOS content (Fig. 1). The physical mixtures were illuminated with different sources and photos were acquired in order to evaluate the emission in the visible and IR ranges. When irradiated with a 365 nm UV LED as the source, the mixtures emitted both in the red region of the visible spectrum and in the IR ones. This is evident observing the red emission in the VIS range and the emission in the IR range, revealed as signals by the detector of the photo camera. When irradiated with a 640 nm RED source, only the emission in the IR region is observed (Fig. 1). In addition, the emission both in VIS and in the IR range is evident after 30 seconds.In order to evaluate the optical properties of the mixtures, the excitation (PLE) and emission (PL) spectra as well as the lifetime were acquired. The spectra of EB and YOS were used as references (Fig. S2 and S3 of ESI† (ref. 45)). The excitation spectra at λem = 626 and 910 nm and the emission spectra at λexc = 312 and 625 nm are reported in Fig. 2a–e.
The excitation spectrum of the YOS/EB mixtures acquired at λem = 626 nm (Fig. 2a) shows a slight apparent shift towards higher energies (∼6–7 nm) of the O2−(2p) → Eu3+ charge transfer band at around 268 nm and a significant shift towards higher energies (∼26 nm) of the S2−(3p) → Eu3+ charge transfer band at about 340 nm compared to the YOS powder used as a reference. In the literature, variations of charge transfer bands are usually attributed to nanoscale dimensions52 or the presence of dopants in Y2O2S.53 However, in this case, it is the result of a superposition of the EB absorption band (its characteristic is plotted in Fig. 2a for better understanding, without intensity correction) and CT bands, which apparently shifts the maxima of both bands of the YOS charge transition toward the UV.
Interestingly, the relative intensity of the CT bands in the excitation spectra, recorded for the mixtures (relative to the intensity of the 7F0 → 5L6 transition), increases with increasing EB content. The intensity increases of this band, as its maximum shifts toward increasing EB absorption, can be explained by energy transfer between the two components of the mixture. On the other hand, the emission spectra acquired at λexc = 312 nm (Fig. 2b) do not show any significant changes in visible emission between the various mixtures and compared to pristine YOS phosphor. The emission spectrum in Fig. 2d allows for the evaluation of EB's near-infrared emission following energy transfer from YOS (spectra acquired at λexc = 312 nm). In all four cases, it is possible to observe the band centered at 910 nm, typical of the 2B2g → 2B1g transition of EB, confirming the energy transfer from the YOS phosphor to EB; the spectrum also contains a relatively intense and narrow (compared to the broadband 2B2g → 2B1g emission) emission at 830 nm which corresponds to the Eu3+ emission attributed to the 5D0 → 7F6 transition.48
Excitation spectra acquired at λem = 910 nm (Fig. 2c) confirm this; they include charge transfer bands and peaks related to europium transitions, which are not present in the EB excitation spectrum. Furthermore, the ratio between the area of bands centered at 320 nm, responsible for the synergistic effect between YOS and EB, and the ones centered at 625 nm proportionally increases with the increase in YOS/EB ratio, indicating that with increasing YOS, the energy transfer phenomenon is favored (Fig. 3). The linear trend allows us to predict the behaviour of mixtures with higher YOS/EB ratios. Fig. 2e shows the EB emission obtained by exciting at a wavelength corresponding to the maximum of the excitation spectrum (λexc = 625 nm) where no difference between the emission spectrum of pristine EB and the mixture is observed.
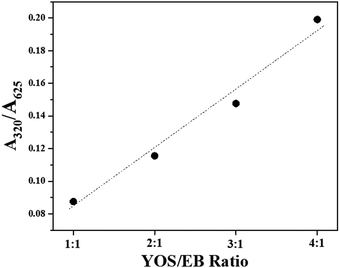 | ||
| Fig. 3 Ratio between the area of bands (integral intensity) at 320 nm and 625 nm (A320/A625). Dashed line is only a guide for eyes. | ||
Relaxation profiles of YOS in mixtures, of EB in mixtures and of EB following YOS-stimulated excitation, are reported in Fig. S4 (ESI†). The lifetimes of EB in the mixtures, referenced to the 2B2g → 2B1g transition of EB at 910 nm (λexc = 625 nm), are found to be slightly shorter than those of the reference EB. Notably, the lifetimes of the 2B2g → 2B1g of the EB transition, following excitation by YOS (λexc = 312–333 nm range for mix 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 YOS/EB), are approximately 3 times longer than those of EB. On the other hand, the rise time of the mixtures, referred to the signal's rise phase for the 2B2g → 2B1g transition at 910 nm (λexc = 312–333 nm), is approximately 270 μs for all four mixtures, about 220 μs longer than the rise time of EB when irradiated in the UV, indicating that the excitation times of EB depend on both the rise time and the lifetime of YOS (Table 1 and Fig. S6 of the ESI†).
1 YOS/EB), are approximately 3 times longer than those of EB. On the other hand, the rise time of the mixtures, referred to the signal's rise phase for the 2B2g → 2B1g transition at 910 nm (λexc = 312–333 nm), is approximately 270 μs for all four mixtures, about 220 μs longer than the rise time of EB when irradiated in the UV, indicating that the excitation times of EB depend on both the rise time and the lifetime of YOS (Table 1 and Fig. S6 of the ESI†).
| Sample | λ exc; λem (nm) | Rise time (μs) |
|---|---|---|
| YOS/EB | 333; 910 | ∼270 |
| YOS | 333; 626 | ∼100 |
| EB | 625; 910 | ∼46 |
| YOS/EB | 625; 910 | ∼46 |
The persistent luminescence spectra (PersL) were acquired to assess the presence of differences in persistence behaviour between YOS and its mixtures and to evaluate the possibility of exciting EB over time through the PersL of YOS. The graphs in Fig. S5a and c of the ESI† were obtained by plotting the integral intensity values of the peaks at 626 nm and 900 nm at various acquisition times. Fig. S5b and d of the ESI† show the normalized curves for (a) and (c), respectively. The absolute intensity of the afterglow decay at 626 nm is higher for pure YOS and decreases with the YOS/EB ratio. The normalized graph (Fig. S5b of ESI†) indicates that the curve's shape and, thus the afterglow, is not dependent on the YOS/EB ratio. YOS is capable of transferring energy and exciting EB over time, stimulating its emission for several seconds (Fig. S5c and d of ESI†). No variations in this behaviour are observed with the YOS/EB ratio, but a higher quantity of YOS compared to EB enhances the intensity over time (Fig. S6c of the ESI†).
3.2 YOS/EB inks
A photo of the developed inks is shown in Fig. 4, where the emissions observed for the solid mixtures under the UV and RED lights are maintained.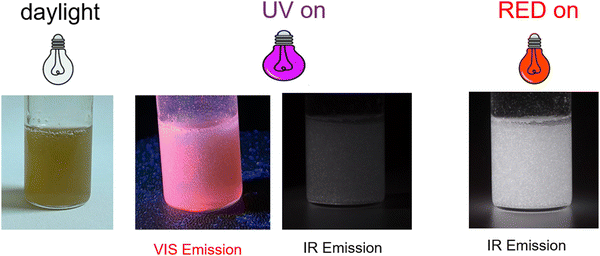 | ||
| Fig. 4 Photos of the GA based inks, when illuminated with different lamps VIS light; LED UV lamp (365 nm); red LED lamp (640 nm). | ||
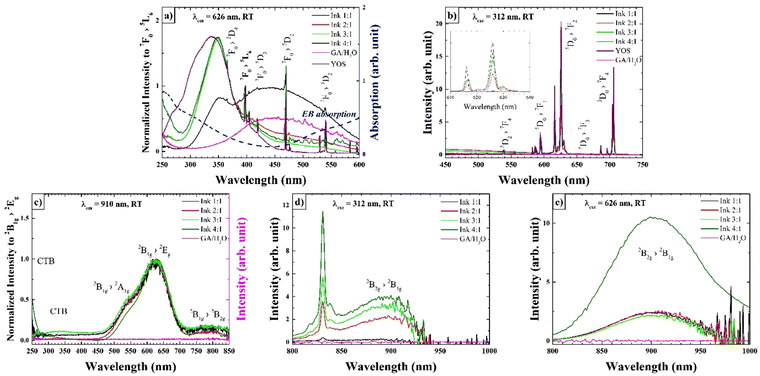
The excitation spectra of the inks acquired at λem = 626 nm show the characteristic absorption bands of YOS (Fig. 5a). The spectral position of the narrow bands corresponding to electronic transitions within the Eu3+ 4f configuration remains unchanged as for EB/YOS mixtures but, in contrast to the mixture, a red shift in the position of CT bands relative to pristine YOS is noticeable for GA ink solutions. As a result, the recorded band maximum associated with the S2−(3p) → Eu3+ is shifted by about 15 nm, appearing around ∼350 nm. The shift is due, on the one hand, to the already limited energy transfer between EB and YOS in solution compared to their solid mixtures, and on the other hand, to the increase in the absorption of GA, which has a maximum in the blue range as plotted additionally in Fig. 5a and in Fig. S4 of the ESI.† An increase in the intensity of the excitation spectrum with the YOS amount can be observed, as well as in the emission spectra (Fig. 5c). The broad low-intensity band present in the excitation spectrum (Fig. 5a) due to the UV absorption of the GA solution affects the UV/blue excitation efficiency, but does not significantly affect the YOS emission itself, except in the case of INK 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, where the phosphor emission intensity is very low. The normalized excitation spectra exhibit the characteristic bands typical of EB, and a low-intensity band at around 320 nm due to YOS (Fig. 5c). However, the presence of the IR emission band 2B2g → 2B1g of EB is a demonstration that energy transfer between YOS and EB is possible even in solution (Fig. 5d). As observed for the excitation spectra, increasing the YOS amount, the IR emission increases. The YOS emission peak at 830 nm is present under excitation with λexc = 312 nm (Fig. 5d). The excitation spectrum at λem 910 nm (Fig. 5b) and the IR emission spectrum at λexc 312 nm (Fig. 5d) for the GA solution do not exhibit any luminescence, indicating that the medium does not contribute to the emission processes. The spectrum in Fig. 5e shows the IR emission spectrum of the inks at λexc = 625 nm, typical of EB. In this case, the spectra of the INK 1
1, where the phosphor emission intensity is very low. The normalized excitation spectra exhibit the characteristic bands typical of EB, and a low-intensity band at around 320 nm due to YOS (Fig. 5c). However, the presence of the IR emission band 2B2g → 2B1g of EB is a demonstration that energy transfer between YOS and EB is possible even in solution (Fig. 5d). As observed for the excitation spectra, increasing the YOS amount, the IR emission increases. The YOS emission peak at 830 nm is present under excitation with λexc = 312 nm (Fig. 5d). The excitation spectrum at λem 910 nm (Fig. 5b) and the IR emission spectrum at λexc 312 nm (Fig. 5d) for the GA solution do not exhibit any luminescence, indicating that the medium does not contribute to the emission processes. The spectrum in Fig. 5e shows the IR emission spectrum of the inks at λexc = 625 nm, typical of EB. In this case, the spectra of the INK 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, INK 2
1, INK 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, and INK 3
1, and INK 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 inks were acquired under the same conditions, and the emission from the 2B2g → 2B1g transition has the same intensity in all three cases, as one would expect considering the equal quantity of EB present in these inks.
1 inks were acquired under the same conditions, and the emission from the 2B2g → 2B1g transition has the same intensity in all three cases, as one would expect considering the equal quantity of EB present in these inks.
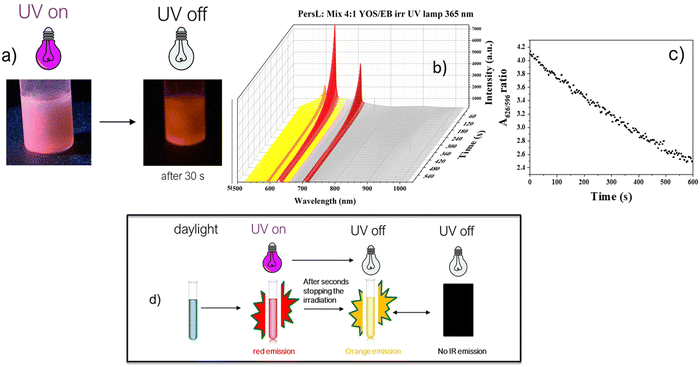
To underline this, the emission of the inks continues after the UV lamp is turned off, even if, as observed for the powders, the color of the emission becomes orange (Fig. 6a). The PersL spectrum also shows that the intensity of the peaks gradually decreases over seconds, although the rate of intensity change is not the same for all peaks. In detail, the value of the ratio A626/A596 decreases with time45 (Fig. 6c). The change in this ratio is correlated with the shift in the color of the persistent luminescence from red to orange in the investigated time. The observed change in the colour of the emission, evident in photographs taken with a photo camera (Fig. 1, 6a and 8), is confirmed also by measurements of PersL spectra (Fig. 6b). It can be seen that, with time, the emission colour changes from red (which is dominant for samples under UV showing PL and PersL) to increasingly orange (PersL), which dominates the recorded optical images as the UV exposure is turned off. Since this change is evident for both the YOS itself and its systems tested in this work with other components, this effect is related not to the effect of changing the environment of the phosphor particle. Since the emission is observed from the same 5D0 excited level and the phosphor itself does not undergo any structural changes during the PersL process, the colour change is due both to the mixing with previously reported emission associated with the presence of titanium ions in Y2O2S:Ti,53 which, when detrapping the carrier, can change its intensity (Fig. 6c) as a result of changes in Ti3+ → Ti(IV) valence and additionally, in the system studied here, to the stronger reabsorption by the phosphor of the Eu3+ part of the spectrum, which is related to longer wavelength transitions (primarily 5D0 → 7F4, but also 5D0 → 7F2) relative to the transition corresponding to the orange colour (5D0 → 7F1) by EB absorption (2B1g → 2Eg), which may increase the orange effect of the visible PersL signal.
These results can be considered as an additional level of security for the ink when the UV lamp is turned off. On the other hand, it is not possible to observe the IR afterglow as seen in solid mixtures, as its intensity is evidently too low to be visualized in the ink. A comprehensive scheme of the emission when the UV lamp is turned-off is reported in Fig. 6d.
In order to evaluate the stability of the inks, the turbidimetric measurements on GA solutions with YOS, with EB, and without powders were performed. The absorbance of the inks was unchanged for 60 minutes, indicating that the dispersions are stable at least in this time range (Fig. 7). In addition, the inks appear homogeneous after agitation, in which the Tyndall effect can be easily observed, using a red laser (Fig. 7).
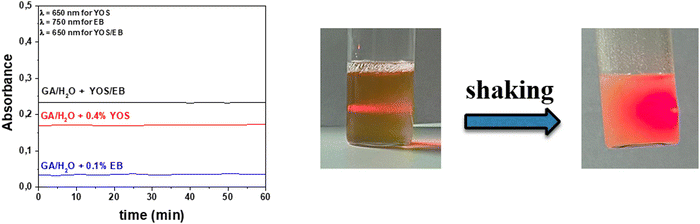 | ||
| Fig. 7 (Left) Absorbance of inks monitored for 60 min. (Right) Photos of the inks before and after shaking. | ||
4. Application of the YOS/EB inks
As a proof of concept, an ink containing a 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 YOS/EB mixture in order to bring the testing conditions as close as possible to real applications was tested on a variety of substrates commonly present in the heritage field, such as black cardboard, canvas, wood and ceramics. The ink was applied with a brush, as is done when applying any substance to the surface of works of art, e.g. in the process of conservation. The ink looks homogeneous on all substrate surfaces and is a few microns in thickness. Once applied, the ink is not visible to the naked eye because it is transparent and colorless (Fig. S8 of the ESI†). In addition, the solid mixture was also mixed into the lime for application on stone substrates. Illuminating the samples with a UV lamp (λexc = 365 nm) and then photographing in the visible and with a red lamp (λexc = 640 nm) and photographing in the IR spectral ranges, the typical red colour of YOS and at the same time the IR fluorescence induced by EB are observed (Fig. 8). Turning off the UV light the emission in the visible range switched to orange.
1 YOS/EB mixture in order to bring the testing conditions as close as possible to real applications was tested on a variety of substrates commonly present in the heritage field, such as black cardboard, canvas, wood and ceramics. The ink was applied with a brush, as is done when applying any substance to the surface of works of art, e.g. in the process of conservation. The ink looks homogeneous on all substrate surfaces and is a few microns in thickness. Once applied, the ink is not visible to the naked eye because it is transparent and colorless (Fig. S8 of the ESI†). In addition, the solid mixture was also mixed into the lime for application on stone substrates. Illuminating the samples with a UV lamp (λexc = 365 nm) and then photographing in the visible and with a red lamp (λexc = 640 nm) and photographing in the IR spectral ranges, the typical red colour of YOS and at the same time the IR fluorescence induced by EB are observed (Fig. 8). Turning off the UV light the emission in the visible range switched to orange.
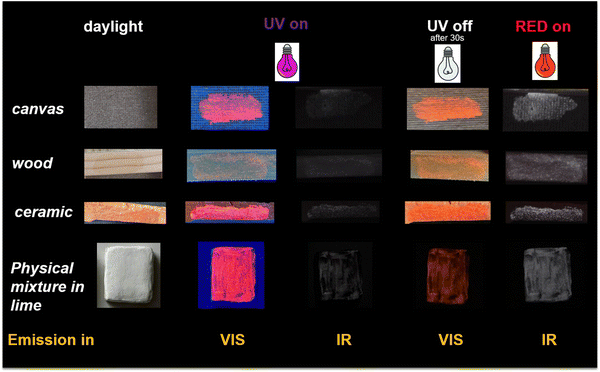 | ||
| Fig. 8 Photos of the inks applied on various supports, when illuminated with different lamps (uncoloured: white light; violet: LED UV lamp at 365 nm; red: red LED lamp at 640 nm). | ||
5. Conclusions
This work concerned the preparation of inks based on phosphors that can find application in various fields and in particular in the anti-counterfeiting sector. The aim of this study was to obtain a transparent ink which, depending on the radiation used to excite it, could emit at different wavelengths, specifically red, orange and infrared emission. It was possible to obtain multi-level (3 levels) anti-counterfeiting inks based on Egyptian blue and yttrium oxide sulphur.Arabic gum was chosen because of the ease of application, non-hazardousness to health, and optical transparency to maintain high effectiveness of the luminescence properties of the phosphors. In addition, the optical properties are affected by the phosphor ratio, indicating that the inks can be used ad hoc for the specific needs of a customer. The ink was successfully developed with the following features: transparency, effectiveness on various types of substrates, stability lasting at least one hour with the ability to regenerate after shaking, easy removability, and low toxicity. The chosen substrates are frequently encountered in the field of cultural heritage, where all the above characteristics are essential together with the possibility of easy removal.
Author contributions
FA and MLS: conceptualization, methodology, and writing – review and editing. ALB: synthesis, preparations, and obtained XRD patterns. VB and DH: optical property analysis (PL, PLE and PersL spectra). All authors contributed to the article and approved the submitted version.Data availability
The datasets generated during the current study are available from the corresponding author on reasonable request.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
F. A. thanks MIUR for the Project PON Ricerca e Innovazione 2014–2020 – Avviso DD 407/2018 “AIM Attrazione e Mobilità Internazionale” (AIM1808223). A. L. B. thanks the University of Palermo for supporting his research work through the progetto “Viaggi e soggiorni di studio degli studenti” A. A. 2022–2023.References
- J. Gooch, B. Daniel, V. Abbate and N. Frascione, Taggant Materials in Forensic Science: A Review, TrAC, Trends Anal. Chem., 2016, 83, 49–54, DOI:10.1016/j.trac.2016.08.003.
- Q. Kuang, X. Hou, C. Du, X. Wang and D. Gao, Recent Advances in the Anti-Counterfeiting Applications of Long Persistent Phosphors, Phys. Chem. Chem. Phys., 2023, 25(27), 17759–17768, 10.1039/D3CP01818K.
- H. Zhao, X. Qin, L. Zhao, S. Dong, L. Gu, W. Sun, D. Wang and Y. Zheng, Invisible Inks for Secrecy and Anticounterfeiting: From Single to Double-Encryption by Hydrochromic Molecules, ACS Appl. Mater. Interfaces, 2020, 12(7), 8952–8960, DOI:10.1021/acsami.0c00462.
- A. Abdollahi, H. Roghani-Mamaqani, B. Razavi and M. Salami-Kalajahi, Photoluminescent and Chromic Nanomaterials for Anticounterfeiting Technologies: Recent Advances and Future Challenges, ACS Nano, 2020, 14(11), 14417–14492, DOI:10.1021/acsnano.0c07289.
- H. Huang, H. Li, J. Yin, K. Gu, J. Guo and C. Wang, Butterfly-Inspired Tri-State Photonic Crystal Composite Film for Multilevel Information Encryption and Anti-Counterfeiting, Adv. Mater., 2023, 35(17), 2211117, DOI:10.1002/adma.202211117.
- W. Wu, H. Liu, J. Yuan, Z. Zhang, L. Wang, S. Dong and J. Hao, Nanoemulsion Fluorescent Inks for Anti-Counterfeiting Encryption with Dual-Mode, Full-Color, and Long-Term Stability, Chem. Commun., 2021, 57(40), 4894–4897, 10.1039/D1CC00555C.
- F. Armetta, V. Boiko, D. Hreniak, R. C. Ponterio and M. L. Saladino, Luminescent YPO4:Eu@PVA Dispersions for Anti-Counterfeiting Ink Applications, Mater. Lett., 2023, 333, 133653, DOI:10.1016/j.matlet.2022.133653.
- A. Abdollahi, B. Ghasemi, S. Nikzaban, N. Sardari, S. Jorjeisi and A. Dashti, Dual-Color Photoluminescent Functionalized Nanoparticles for Static-Dynamic Anticounterfeiting and Encryption: First Collaboration of Spiropyran and Coumarin, ACS Appl. Mater. Interfaces, 2023, 15(5), 7466–7484, DOI:10.1021/acsami.2c22532.
- L. E. MacKenzie and R. Pal, Circularly Polarized Lanthanide Luminescence for Advanced Security Inks, Nat. Rev. Chem., 2021, 5(2), 109–124, DOI:10.1038/s41570-020-00235-4.
- J. H. R. Monica, J. Sharma and P. Y. Dave, Anticounterfeiting Technology – A Luminescent Path: Short Review, J. Adv. Chem. Sci., 2021, 706–710, DOI:10.30799/jacs.233.21070103.
- K. Muthamma, D. Sunil and P. Shetty, Carbon Dots as Emerging Luminophores in Security Inks for Anti-Counterfeit Applications – An up-to-Date Review, Appl. Mater. Today, 2021, 23, 101050, DOI:10.1016/j.apmt.2021.101050.
- T. M. D. Cao, T. T. G. Le, S. Turrell, M. Ferrari, Q. V. Lam and T. T. V. Tran, Luminescent Ink Based on Upconversion of NaYF4:Er,Yb@MA Nanoparticles: Environmental Friendly Synthesis and Structural and Spectroscopic Assessment, Molecules, 2021, 26(4), 1041, DOI:10.3390/molecules26041041.
- X. Jaramillo-Fierro, G. Cuenca and J. Ramón, Comparative Study of the Effect of Doping ZnTiO3 with Rare Earths (La and Ce) on the Adsorption and Photodegradation of Cyanide in Aqueous Systems, Int. J. Mol. Sci., 2023, 24(4), 3780, DOI:10.3390/ijms24043780.
- S. Kalytchuk, Y. Wang, K. Poláková and R. Zbořil, Carbon Dot Fluorescence-Lifetime-Encoded Anti-Counterfeiting, ACS Appl. Mater. Interfaces, 2018, 10(35), 29902–29908, DOI:10.1021/acsami.8b11663.
- H. Chen, H. Hu, B. Sun, H. Zhao, Y. Qie, Z. Luo, Y. Pan, W. Chen, L. Lin, K. Yang, T. Guo and F. Li, Dynamic Anti-Counterfeiting Labels with Enhanced Multi-Level Information Encryption, ACS Appl. Mater. Interfaces, 2023, 15(1), 2104–2111, DOI:10.1021/acsami.2c17870.
- W. Yao, R. Lan, K. Li and L. Zhang, Multiple Anti-Counterfeiting Composite Film Based on Cholesteric Liquid Crystal and QD Materials, ACS Appl. Mater. Interfaces, 2021, 13(1), 1424–1430, DOI:10.1021/acsami.0c18132.
- Z. Yang, X. Zhao, J. Liu, J. Wen, F. Zhang, X. Guo, K. Zhang, J. Zhang, A. Wang, R. Gao, Y. Wang and Y. Zhang, Designed Growth of AgNP Arrays for Anti-Counterfeiting Based on Surface-Enhanced Raman Spectroscopy Signals, ACS Appl. Mater. Interfaces, 2022 DOI:10.1021/acsami.2c12124.
- L. Xu, J. Chen, J. Song, J. Li, J. Xue, Y. Dong, B. Cai, Q. Shan, B. Han and H. Zeng, Double-Protected All-Inorganic Perovskite Nanocrystals by Crystalline Matrix and Silica for Triple-Modal Anti-Counterfeiting Codes, ACS Appl. Mater. Interfaces, 2017, 9(31), 26556–26564, DOI:10.1021/acsami.7b06436.
- Z. Chen, H. Zhu, J. Qian, Z. Li, X. Hu, Y. Guo, Y. Fu, H. Zhu, W. Nai, Z. Yang, D. Li and L. Zhou, Rare Earth Ion Doped Luminescent Materials: A Review of Up/Down Conversion Luminescent Mechanism, Synthesis, and Anti-Counterfeiting Application, Photonics, 2023, 10(9), 1014, DOI:10.3390/photonics10091014.
- J. Dong, X. Ni, R. Meng, W. Kong, S. Liang and J. Zhou, A Rare-Earth Spectral Traceability Anti-Counterfeiting Detection Technology, Optoelectron. Lett., 2022, 18(9), 535–540, DOI:10.1007/s11801-022-2048-z.
- J. Chen, L. Pei, W. Shi, M. A. Saleem, C. Jiao, H. Zhang and J. Wang, Application of Rare Earth Marking on Anti-Counterfeiting Waterless/Less-Water Dyeing Technology, AATCC J. Res., 2024, 11(1), 3–11, DOI:10.1177/24723444231206855.
- X. Zhou, L. Ning, J. Qiao, Y. Zhao, P. Xiong and Z. Xia, Interplay of Defect Levels and Rare Earth Emission Centers in Multimode Luminescent Phosphors, Nat. Commun., 2022, 13(1), 7589, DOI:10.1038/s41467-022-35366-3.
- J. Li, D. Xia, M. Gao, L. Jiang, S. Zhao and G. Li, Invisible Luminescent Inks and Luminescent Films Based on Lanthanides for Anti-Counterfeiting, Inorg. Chim. Acta, 2021, 526, 120541, DOI:10.1016/j.ica.2021.120541.
- D. V. Pereira; Y. G. Mello; H. D. S. Barud; M. Cavicchioli and S. J. L. Ribeiro, Anti-Counterfeiting Strategy Based on Rare Earths and Biopolymers, In Latin America Optics and Photonics (LAOP) Conference 2022, paper W4A.46, Optica Publishing Group, 2022, p. W4A.46 DOI:10.1364/LAOP.2022.W4A.46.
- M. Li, W. Liu, T. Yang, Q. Xu, H. Mu, J. Han, K. Cao, M. Jiao, M. Liu, S. Zhang, X. Tan and C. Yang, Multi-Color UCNPs/CsPb(Br1−xIx)3 for Upconversion Luminescence and Dual-Modal Anticounterfeiting, Opt. Express, 2023, 31(2), 2956–2966, DOI:10.1364/OE.476991.
- J. Andres, R. D. Hersch, J.-E. Moser and A.-S. Chauvin, A New Anti-Counterfeiting Feature Relying on Invisible Luminescent Full Color Images Printed with Lanthanide-Based Inks, Adv. Funct. Mater., 2014, 24(32), 5029–5036, DOI:10.1002/adfm.201400298.
- W. Ren, G. Lin, C. Clarke, J. Zhou and D. Jin, Optical Nanomaterials and Enabling Technologies for High-Security-Level Anticounterfeiting, Adv. Mater., 2020, 32(18), 1901430, DOI:10.1002/adma.201901430.
- C. Du, D. Gao, X. Hou, X. Zhang, Q. Pang and S. Yun, J. Mater. Chem. C, 2024, 12, 9284–9292, 10.1039/D4TC01792G.
- X. Zhang, X. Hou, J. Gao, Z. Wang, X. Zhao, C. Xu and D. Gao, J. Mater. Chem. C, 2023, 11, 16631–16637, 10.1039/D3TC03298A.
- D. Gao, Q. Kuang, F. Gao, H. Xin, S. Yun and Y. Wang, Mater. Today Phys., 2022, 27, 100765, DOI:10.1016/j.mtphys.2022.100765.
- D. Gao, J. Gao, F. Gao, Q. Kuang, Y. Pan, Y. Chen and Z. Pan, J. Mater. Chem. C, 2021, 9(46), 16634–16644, 10.1039/D1TC04568G.
- H. Berke, The Invention of Blue and Purple Pigments in Ancient Times, Chem. Soc. Rev., 2006, 36(1), 15–30, 10.1039/B606268G.
- M. Nicola, C. Garino, S. Mittman, E. Priola, L. Palin, M. Ghirardello, V. Damagatla, A. Nevin, A. Masic, D. Comelli and R. Gobetto, Increased NIR Photoluminescence of Egyptian Blue via Matrix Effect Optimization, Mater. Chem. Phys., 2024, 313, 128710, DOI:10.1016/j.matchemphys.2023.128710.
- P. García-Fernández, M. Moreno and J. A. Aramburu, Origin of the Exotic Blue Color of Copper-Containing Historical Pigments, Inorg. Chem., 2015, 54(1), 192–199, DOI:10.1021/ic502420j.
- L. M. Seymour, M. Nicola, M. I. Kessler, C. L. Yost, A. Bazzacco, A. Marello, E. Ferraris, R. Gobetto and A. Masic, On the Production of Ancient Egyptian Blue: Multi-Modal Characterization and Micron-Scale Luminescence Mapping, PLoS One, 2020, 15(11), e0242549, DOI:10.1371/journal.pone.0242549.
- C. Malacrino; A. Arcudi; F. Armetta; D. Giuffrida and B. Fazzari, Gli affreschi provenienti dalle terme romane di Reggio Calabria. Gli Affreschi Provenienti Dalle Terme Romane Reggio Calabr. Dalla Conoscenza Al Restauro Giorn. Studi IGIIC Stacchi E Strappi Dipinti Murali Kermes ( 2023), 127–128, pp. 154–158.
- P. Dariz and T. Schmid, Trace Compounds in Early Medieval Egyptian Blue Carry Information on Provenance, Manufacture, Application, and Ageing, Sci. Rep., 2021, 11(1), 11296, DOI:10.1038/s41598-021-90759-6.
- M. Nicola, L. M. Seymour, M. Aceto, E. Priola, R. Gobetto and A. Masic, Late Production of Egyptian Blue: Synthesis from Brass and Its Characteristics, Archaeol. Anthropol. Sci., 2019, 11(10), 5377–5392, DOI:10.1007/s12520-019-00873-w.
- A. Kostomitsopoulou Marketou, The Materialisation of Colour: Reconstructing Egyptian Blue Manufacture on Late Hellenistic Kos, Norw. Archaeol. Rev., 2022, 55(1), 21–37, DOI:10.1080/00293652.2022.2052746.
- Proceedings of the Tenth International Congress of Egyptologists, University of the Aegean, Rhodes, 22–29 May 2008, ed. Kousoulis, P. and Lazaridis, N., Orientalia Lovaniensia analecta; Peeters: Leuven, 2015.
- I. Kakoulli, Egyptian Blue in Greek Painting between 2500 and 50 BC. In Mine Microsc. Adv. Study Anc. Technol., 2009, pp. 101–112 Search PubMed.
- T. Katsaros, I. Liritzis and N. Laskaris, Identification of Theophrastus’ Pigments Egyptios Yanos and Psimythion from Archaeological Excavations: A Case Study, ArcheoSciences, 2010, 34, 69–80, DOI:10.4000/archeosciences.2632.
- C. Grifa, L. Cavassa, A. D. Bonis, C. Germinario, V. Guarino, F. Izzo, I. Kakoulli, A. Langella, M. Mercurio and V. Morra, Beyond Vitruvius: New Insight in the Technology of Egyptian Blue and Green Frits, J. Am. Ceram. Soc., 2016, 99(10), 3467 CrossRef CAS.
- X. Wang, Z. Zhang, Z. Tang and Y. Lin, Characterization and Properties of a Red and Orange Y2O2S-Based Long Afterglow Phosphor, Mater. Chem. Phys., 2003, 80(1), 1–5, DOI:10.1016/S0254-0584(02)00097-4.
- M. Itoh and Y. Inabe, Optical Properties and Electronic Structure of Yttrium Oxysulfide, Phys. Rev. B: Condens. Matter Mater. Phys., 2003, 68(3), 035107, DOI:10.1103/PhysRevB.68.035107.
- W. Li, Y. Liu and P. Ai, Synthesis and Luminescence Properties of Red Long-Lasting Phosphor Y2O2S:Eu3+,Mg2+,Ti4+ Nanoparticles, Mater. Chem. Phys., 2010, 119(1), 52–56, DOI:10.1016/j.matchemphys.2009.07.037.
- T. Hoshina, S. Imanaga and S. Yokono, Charge Transfer Effects on the Luminescent Properties of Eu3+ in Oxysulfides, J. Lumin., 1977, 15(4), 455–471, DOI:10.1016/0022-2313(77)90044-8.
- M. Tanaka, G. Nishimura and T. Kushida, Contribution of J mixing to the 5D0-7F0 transition of Eu3+ ions in several host matrices, Phys. Rev. B: Condens. Matter Mater. Phys., 1994, 49(24), 16917–16925, DOI:10.1103/PhysRevB.49.16917.
- J. M. de Carvalho, C. C. Santos Pedroso, I. Pompermayer Machado, J. Hölsä, L. C. Veloso Rodrigues, P. Głuchowski, M. Lastusaari and H. Felinto Brito, Persistent luminescence warm-light LEDs based on Ti-doped RE2O2S materials prepared by rapid and energy-saving microwave-assisted synthesis, J. Mater. Chem. C, 2018, 6, 8897–8905, 10.1039/C8TC01826J.
- N. Prasad, N. Thombare, S. C. Sharma and S. Kumar, Gum Arabic – A Versatile Natural Gum: A Review on Production, Processing, Properties and Applications, Ind. Crops Prod., 2022, 187, 115304, DOI:10.1016/j.indcrop.2022.115304.
- M. H. El-Newehy, A. Aldalbahi, B. M. Thamer and M. Moydeen Abdul Hameed, Preparation of Smart Glue Using Strontium Aluminate and Polylactic Acid Oligomer Grafted Arabic Gum, J. Photochem. Photobiol. Chem., 2024, 447, 115193, DOI:10.1016/j.jphotochem.2023.115193.
- Z. Fu, Y. Geng, H. Chen, S. Zhou, H. K. Yang and J. H. Jeong, Combustion Synthesis and Luminescent Properties of the Eu3+-Doped Yttrium Oxysulfide Nanocrystalline, Opt. Mater., 2008, 31(1), 58–62, DOI:10.1016/j.optmat.2008.01.008.
- Z. Hong, P. Zhang, X. Fan and M. Wang, Eu3+ Red Long Afterglow in Y2O2S:Ti,Eu Phosphor through Afterglow Energy Transfer, J. Lumin., 2007, 124(1), 127–132, DOI:10.1016/j.jlumin.2006.02.008.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4tc04228j |
| This journal is © The Royal Society of Chemistry 2025 |