Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Atmospheric mercury concentration variations at Syowa Station, Lützow-Holm Bay, East Antarctica and contributing factors†
Koyomi
Nakazawa
 *a,
Osamu
Nagafuchi
*a,
Osamu
Nagafuchi
 *b,
Akihiro
Mitsui
c,
Tomoaki
Watanabe
c,
Naoko
Hishida
c,
Megumu
Tsujimoto
*b,
Akihiro
Mitsui
c,
Tomoaki
Watanabe
c,
Naoko
Hishida
c,
Megumu
Tsujimoto
 de and
Satoshi
Imura
de and
Satoshi
Imura
 ef
ef
aToyama Prefectural University, 5180 Kurokawa, Imizu City, 939-0398, Toyama, Japan. E-mail: nakazawa@pu-toyama.ac.jp; Fax: +81-766-56-0396; Tel: +81-766-56-7500
bFukuoka Institute of Technology, 3-30-1 Wajiro-Higashi, Higashi-ku, Fukuoka City, Fukuoka, 811-0295, Japan. E-mail: nagafuchi@bene.fit.ac.jp; Fax: +81-92-606-0636; Tel: +81-92-606-1077
cNippon Instruments Corporation, 14-8, Akaoji-Cho, Takatsuki-shi, 569-1146, Osaka, Japan
dKeio University, 5322 Endo, Fujisawa-shi, Kanagawa 252-0882, Japan
ePolar Science Program, The Graduate Institute for Advanced Studies, SOKENDAI, 10-3, Midori-cho, Tachikawa-shi, Tokyo 190-8518, Japan
fNational Institute of Polar Research, 10-3, Midori-cho, Tachikawa-shi, Tokyo 190-8518, Japan
First published on 12th November 2024
Abstract
In January 2022, gaseous elemental Hg (GEM) concentrations were continuously monitored at Syowa Station on East Ongul Island, located ∼4 km from the continent on the eastern coast of Lützow-Holm Bay, to examine atmospheric Hg concentrations during the summer in the southeastern Antarctic region. Atmospheric GEM ranged from 0.36 to 1.83 ng m−3 average value: 1.01 ± 0.21 ng m−3 and increased during the day and decreased at night. While maintaining these diurnal variations, GEM concentrations increased to 1.99 and 1.55 ng m−3 on January 2–3 and 17–20, 2022, respectively. During both events, the low-pressure system approached the Syowa Station, and the 72 hours backward trajectory analysis revealed that the air mass originated from open water surfaces, implying that Hg evasion from the sea surface increased the atmospheric GEM concentration. To investigate the causes of diurnal variation causes—excluding these two events mentioned—Hg concentrations in the soil [n = 102, 2.61 ± 3.16 (0.14–19.0) ng g−1], snow, glacier, and ice sheet around Syowa Station (n = 19, 0.45–5.60 ng L−1), as well as in the atmosphere on the fast ice around the station (0.54–1.10 ng m−3), were measured. The results revealed that sources such as ornithogenic soil from the penguin rookery around the station, open water surfaces, and the gaseous oxidized Hg transported inland by katabatic winds did not contribute to the daytime GEM concentration increases. The cause of the summer diurnal variation at Syowa Station was unidentified and warrants further investigation.
Environmental significanceIn 2017, the Minamata Convention on Mercury came into force, marking a significant global effort to address mercury pollution. Understanding the global dynamics of mercury has since become a critical environmental science issue. Antarctica, as one of the last pristine environments on Earth, remains free from most human activities, making it a unique region for studying natural mercury cycles. In particular, the ice-free coastal areas of Antarctica have shown signs of mercury bioaccumulation. Therefore, investigating the atmospheric mercury dynamics and identifying the key factors influencing its variations in these coastal regions is crucial for advancing our understanding of global mercury pollution and its potential impact on such fragile ecosystems. |
1 Introduction
Mercury (Hg) is a globally recognized environmental pollutant that has adverse effects on wildlife and humans, even in low concentrations. In response to growing global concerns about Hg management, the Minamata Convention was enacted in 2017, emphasizing the urgency for global management of Hg.1 Hg is released from both natural and anthropogenic sources. In the atmosphere, Hg exists primarily in three forms: gaseous elemental Hg (GEM), gaseous oxidized Hg (GOM), and particulate bound Hg, with GEM accounting for >95% of atmospheric Hg.2 The lifespan of GEM released into the atmosphere from both natural and anthropogenic sources is ∼1 year.3,4 Due to weather conditions, it can be efficiently transported globally, making it a pollutant of global concern.4 Although GEM is the most abundant form of Hg in the atmosphere, it can be oxidized to form highly reactive GOM, which is rapidly deposited onto land.5Antarctica is the only continent that retains some of the most pristine environments and is largely unaffected by human activities.6 Generally, the ice-covered continent surface is considered chemically inactive; however, it is highly photochemically active under sunlight.7 Observations from 2011 to 2015 and model validations indicate that extensive GEM oxidation by high quantities of oxidants such as O3 and NOx in the air boundary layer over the Antarctic ice sheet produces GOM, which then accumulates on the snow surface. This is followed by GOM photoreduction in the presence of sunlight, which releases GEM back into the atmosphere, causing diurnal variations in atmospheric GEM concentration.6 Furthermore, regions along the Antarctic coast, heavily impacted by katabatic winds, are influenced by the GOM-rich air masses generated over the inland of the Antarctic ice sheet.6
According to reports from the Dumont d'Urville station (DDU), located ∼1 km from the Antarctic mainland, GOM-rich air masses moved from the inland Antarctica deposit onto the snow surface and were re-emitted as GEM. Moreover, ocean eversion and Hg released from penguin guano-derived soils also regulate Hg concentrations, resulting in similar diurnal variations in GEM levels observed during the summer at this station.8 In contrast, no GEM diurnal variations have been observed at the Troll station, ∼200 km inland and at a height of 1275 m, likely due to the absence of contributing sources or sinks.8,9
Studies on atmospheric Hg in Antarctica are limited,6,10–13 and it is unclear whether the diurnal variations observed at DDU are unique or occur in other coastal Antarctic regions. However, considering the increase in snow Hg concentrations from the inland of Antarctica to the coast7 and the increased bioaccumulation in soil, moss, and lichen collected from ice-free coastal areas,14 the coastal Antarctic ecosystem may become an Hg sink. Understanding the Hg dynamics in the coastal regions of Antarctica is crucial for ecosystem conservation.
Here, continuous atmospheric Hg observations were conducted in January 2022 at the Syowa Station to identify any variations in atmospheric Hg concentrations. Additionally, snow and soil Hg concentrations were measured at several locations within and around the Syowa Station, East Ongul Island, 4 km from the coast of Lützow-Holm Bay in East Antarctica. The island was surrounded by fast ice during the observations since it is unaffected by katabatic winds and lacks notable penguin nesting sites. The aim of this study was to determine whether the summer diurnal variations observed at DDU were also present at Syowa Station. Additionally, the study sought to evaluate whether these variations were attributable to open water surface evasion, local GEM sources at Syowa Station, or the cyclic deposition of GOM and re-emission of GEM from air masses influenced by katabatic winds.
2 Materials and methods
2.1 Site description and sample collection
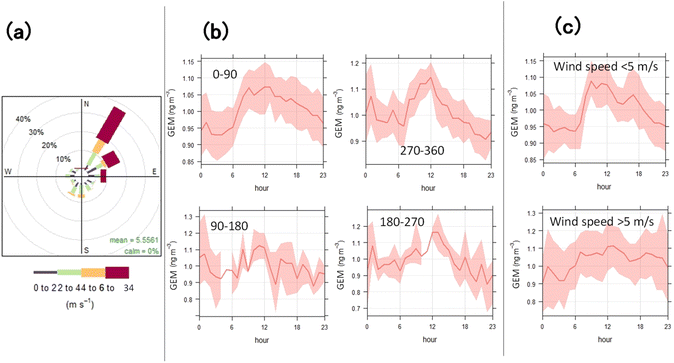
GEM was monitored at the fundamental observation building within Syowa Station (69°00′14.4′′ S, 39°34′46.7′′ E) and on the observation deck of the icebreaker Shirase, anchored in the fast ice (69°05′22.6′′ S, 39°27′06.6′′ E) [Fig. S1(b) and (c)†]. Syowa Station has various facilities, including administrative, power generation, residential, facilities.15 At Syowa Station—the only known anthropogenic Hg source in Lützow-Holm Bay—there are two power generation facilities and one waste incineration facility which are Hg point sources.15Here, Hg monitors (AM-5, Nippon Instruments Corporation) were installed in the fundamental observation building [Fig. S1(c)†],15 at distances of 140 and 180 m from the two power generation buildings and 150 m from the incinerator. The various facilities around Syowa Station are located east and south of the GEM sampling location (Fundamental observation building). The wind direction frequency observed here was <10% from east to SSW [Fig. 1(a)], and the maximum wind speed was 4–6 m s−1, indicating limited impact on the building. Here, Hg was monitored using atomic fluorescence spectroscopy (AFS) with gold amalgamation and argon as the carrier gas, and the limit of detection was 0.1 pg. In studies using the Tekran 2537A (CVAFS method), researchers have measured total gaseous Hg as the sum of GEM and GOM. However, a soda lime filter attached to the inlet removed most of the GOM, allowing for GEM measurement.16,17 Here, a poly tetrafluoroethylene filter with a pore size of 0.45 μm and soda lime were attached to the inlet to measure GEM. Air was sampled using a Teflon tube through an inlet on the 2nd floor of the Fundamental observation building. For quality control, an Hg vapor calibrator (MB-1, Nippon Instruments Corporation) and a Hamilton syringe were used to manually inject saturated Hg vapor from temperature-controlled vessels at the time of installation. An automated calibrator (MGA-1, Nippon Instruments Corporation) was attached to the Hg monitor for automatic calibration every 24 h.
The observation period was from December 30, 2021, to January 30, 2022. Observations on the Shirase were conducted when it was anchored on the fast ice ∼1.5 km from the main unit of Syowa Station (69°00′23.9′′ S, 39°36′59.0′′ E). A portable Hg monitor (EMP-3 Gold+, Nippon Instruments Corporation)—which uses a battery as a power source—was installed on the observation deck at the stern of the Shirase, located ∼15 m above the sea ice surface. This monitor uses gold amalgamation concentrations for atomic absorption spectrophotometry, and by attaching the Gold + unit to the monitor, the detection limit was reduced to 0.002 ng. After creating the calibration curve using a mercury vapor monitor (MB-1, Nippon Instruments Corporation), a 30 μL sample was isolated, and the recovery rate was determined (n = 5, with 99.2% recovery). This process was conducted to verify the precision and accuracy of the measurements as part of the quality assurance/quality control (QA/QC) procedures.
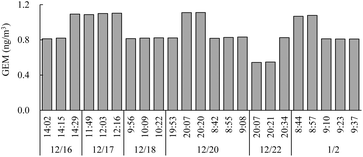
The Shirase was anchored in an area covered with ice during the survey, with open water only at the stern, where the ice was broken by icebreaking navigation. Observations were conducted on December 16–18, 20, and 22, 2021, and January 2, 2022. Since members of the Japanese Antarctic Research Expedition (JARE) dwell at Syowa Station and seldom return to the Shirase during the summer, observations were feasible only when the author stayed there.
2.2 Collection and analysis of snow, glacier, and ice sheet samples
Snow, glacier, and ice sheet samples (n = 16) were collected from both East and West Ongul Island within Syowa Station and several coastal areas of Antarctica surveyed by JARE (Fig. S1 and S3†). The collection period lasted from December 28, 2021, to February 8, 2022, and samples were collected at locations and times that were surveyed by the author. Fresh snow was collected at Syowa Station within 0–1 day of snowfall (Table 1). The samples were collected in Ziploc plastic bags and transported and frozen until further analysis. Snow crusts were broken using a stainless-steel shovel before collection.| Site name | Sample | The days after snowing (d) | n | Location | Elevationb (m) | T-Hg | D-Hg | P-Hg | Sampling pointc | |
|---|---|---|---|---|---|---|---|---|---|---|
| Longitude | Latitude | Average ± standard deviation (min–max) ng L−1 | ||||||||
| a Unknown. b Dataset obtained from Google earth. c This sampling points are linked to Fig S1. | ||||||||||
| East Ongul Is. | ||||||||||
| Syowa station | Snow | 0–1 | 4 | −69.006794 | 39.572806 | 29 | 0.55 ± 0.24 (0.37–0.91) | 0.19 ± 0.11 (0.05–0.30) | 0.35 ± 0.18 (0.20–0.61) | 7,9 |
| Snow | 13–20 | 3 | −69.006794 | 39.572806 | 29 | 2.31 ± 0.38 (2.07–2.75) | 0.42 ± 0.23 (0.16–0.48) | 1.90 ± 0.61 (1.51–2.60) | 8,10,11 | |
| West Ongul Is. | Snow | 13 | 1 | −69.022742 | 39.557156 | 0 | 0.45 | 0.32 | 0.13 | 6 |
| Skallen | Snow | —a | 1 | −69.67587 | 39.41193 | 121 | 5.60 | 0.29 | 5.31 | 5 |
| Tama misaki cape | Snow | —a | 1 | −68.72504 | 40.47261 | 70 | 3.69 | 0.76 | 2.92 | 1 |
| Tottsuki misaki | Glacier/ice sheet | —a | 1 | −68.911203 | 39.819489 | 9 | 0.64 | 0.54 | 0.11 | 2 |
| Langovde glacier | Glacier/ice sheet | —a | 3 | −69.20281 | 39.82299 | 662 | 1.96 ± 0.39 (1.51–2.20) | 0.51 ± 0.12 (0.41–0.65) | 1.46 ± 0.51 (0.87–1.79) | 3 |
| S16 | Glacier/ice sheet | —a | 2 | −69.028903 | 40.060569 | 584 | 0.78 ± 0.02 (0.76–0.79) | 0.45 ± 0.04 (0.42–0.48) | 0.33 ± 0.06 (0.29–0.37) | 4 |
The samples were then transferred to beakers on a clean bench, melted at 20 °C, and immediately analyzed. The beakers used for melting were pre-cleaned with acid, rinsed with ultrapure water (18.2 MΩ), and dried before use. The samples were pretreated with BrCl (0.5 mL/100 mL of the sample) for at least 3 h before measurement to oxidize organic Hg and particulate Hg (P-Hg) to Hg(II), which reacts with tin chloride. For a 5 mL sample, 1.1 mL of a mixed reagent of 5 N NaOH and 1000 ppm Cu2+ (CuSO4·5H2O) was added, followed by 0.3 mL of 10% SnCl2 for reduction vaporization.
Atomized Hg was concentrated in a gold trap tube and quantified using reduction vaporization cold atomic fluorescence spectrophotometry (RA3000FG+, Nippon Instruments Corporation). Total Hg (T-Hg) and dissolved Hg (D-Hg) were measured, and P-Hg was calculated from the difference between T-Hg and D-Hg. For quantification of D-Hg, the filtrate obtained by filtering the sample through a 0.45 μm poly tetrafluoroethylene filter (ADVANTEC, 25HP045AN) was used.18 The detection limits were 0.1 ng L−1. Three measurements were performed for each sample, and the coefficient of variation of the measurement results was <10%. QA/QC was performed using NIST standard reference material 1641e Hg in water, with a recovery rate of 95.8% (n = 3).
2.3 Observation and quantification of Hg in soil
Soil samples were collected from East Ongul Island from December 21–27, 2007 (JARE 49, summer period) at intervals of 100 or 200 m in a mechanically meshed pattern (n = 102) (Fig. S2†). The samples were collected in Spitz tubes using sterilized plastic spoons, transported and frozen until further analysis. Before analysis, the samples were sieved through a 500 μm mesh sieve, and fractions <500 μm were freeze-dried (FDU-1200, Tokyo Rika Kikai Co. Ltd) and then ground using an agate mortar (n = 3). The amount of soil Hg was determined using a heat-vaporization cold-vapor atomic absorption spectrometer (MA-2000 Nippon Instruments). Standard reference materials (CRM 482, trace elements in lichen) were analyzed for QA/QC, and Hg recovery was 97 ± 4.6%.2.4 Air mass back trajectories
To understand air mass pathways, a backward trajectory analysis was conducted using the hybrid simple particle Lagrangian integrated trajectory (HYSPLIT-4) model provided by the National Oceanic and Atmospheric Administration (NOAA) on the web (Air Resources Laboratory–HYSPLIT).19 The latitude and longitude of the Fundamental observation building at Syowa Station were set as the initial coordinates of the starting point, and the starting altitude was set to 500 m. A backward analysis spanning 72 h was performed at a rate of one trajectory per hour.2.5 Meteorological and sea ice information
Meteorological observations were conducted at a meteorological station inside the Fundamental observation building, and the data are publicly available.20 Sea ice data were obtained from the University of Bremen.21,223 Results and discussion
3.1 GEM concentrations in Antarctica
During the survey period, the average GEM concentration recorded using the Hg monitor at the Fundamental observation building was 1.01 ± 0.21 (range: 0.36–1.83) ng m−3 [Fig. 2(a)]. This is comparable to the concentrations reported for other regions of Antarctica, such as 0.69 ± 0.35 ng m−3 at the Concordia Station on the Dome C site (French/Italian Station: DC) station during the summer,8 0.9 ± 0.3 ng m−3 at the Italian Antarctic Station in Terra Nova Bay,12 1.06 ± 0.24 ng m−3 at the German Antarctic Research Station Neumayer,9 and 0.93 ± 0.19 ng m−3 at the Norwegian Antarctic Research Station Troll.9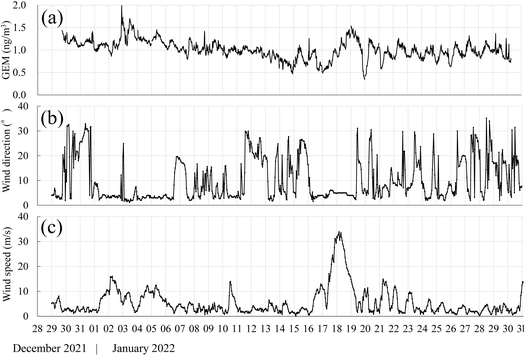 | ||
| Fig. 2 GEM concentration, wind direction, and wind speed observed at Syowa Station, Antarctica during Dec 2021 to Jan 2022. (a) GEM concentration, (b) wind direction, (c) wind speed. | ||
Summer diurnal variations at the inland DC station and the considerable influence of katabatic winds at DDU Station resulted in average summer GEM concentrations of 0.78 ± 0.46 ng m−3 and 0.88 ± 0.32 ng m−3, respectively, lower than the GEM concentrations at the Fundamental observation building.
The lower average GEM concentration at the DC station during summer is assumed to be due to intense GEM oxidation by reactive bromine species present in the atmospheric boundary layer.6 The oxidized GEM converts to GOM and undergoes dry deposition on the snow surface; however, GEM is released back into the atmosphere because of GOM photoreduction in the top layer of the snow.8 Hg concentrations in the snow increased from 4.2 (inland) to 194.4 ng L−1 (coastal), suggesting that GOM is carried from the Antarctic interior to the coast.8
At Syowa Station (East Ongul Island), T-Hg snow concentrations collected immediately (0–1 day) and after (13–20 days) snowfall were 0.55 ± 0.24 (n = 4) and 2.31 ± 0.38 ng L−1 (n = 3), respectively (Table 1). The T-Hg snow concentration (2.31 ng L−1) collected 13–20 days after snowfall at Syowa Station was 4.2 times higher than that of snow collected immediately after snowfall. The percentage of P–Hg relative to T-Hg in snow 0–1 and 13–20 days after snowfall was 64% (0.35 ± 0.18 ng L−1) and 82% (1.90 ± 0.61 ng L−1), respectively. The P-Hg percentage was greater at 13–20 days than that at 0–1 day. In contrast, the proportion of P-Hg (0.13 ng L−1) to T-Hg (0.45 ng L−1) in snow collected 13 days after snowfall on West Ongul Island was 29%, where the ground was mostly covered with snow at the time of the survey.
The percentage increase of P-Hg in snow samples at Syowa Station when snow melts and the land revises may have been influenced by the contribution of sand dust. Additionally, snow, glacier, and ice sheet samples in Lützow-Holm Bay collected with unknown days following snowfall had T-Hg concentrations ranging from 0.64 to 5.60 ng L−1, in contrast higher T-Hg concentrations were reported by Angot et al.8 in snow, glacier, and ice sheet samples collected along the Antarctic coast.
Syowa Station lies 4 km from the Antarctic continent, and katabatic winds have limited effects as they do not reach the station.23 A 72 hours trajectory analysis with the 500 m above sea level. Upper level of the Fundamental observation building as the starting point further confirmed that the majority of the air masses during the survey period originated from the coast, and that katabatic wind influences were minimal. The average wind speed during the survey period was 5.51 m s−1,20 comparable to previous observations at Syowa Station.24 Given the higher average summer GEM concentration (1.01 ± 0.21 ng m−3) observed at the Fundamental observation building compared with that at the DC (0.78 ± 0.46 ng m−3) and DDU (0.88 ± 0.32 ng m−3) stations, it can be concluded that the inflow of GOM-rich air masses from inland Antarctica during the summer has minimal impact around Syowa Station.
3.2 Variability in GEM concentration and factors contributing to its increase
GEM concentration fluctuations during the observation period showed a distinct diurnal variation with lower concentrations until 6 a.m., followed by an increase and then decrease after 3 p.m. [Fig. 2(a)]. Two distinct GEM concentration spike events were observed on January 2–3 and January 17–20, 2022. Peak GEM concentrations were the highest recorded during the entire survey period—1.99 and 1.71 ng m−3 on January 2 and 3, respectively. These values were 1.7 to 2.0 times higher than the average GEM concentration (1.01 ng m−3) of the survey period.During these GEM spike events, the predominant wind direction was north-northeast, and low-pressure systems impacted the area surrounding Syowa Station which recorded precipitation from January 1–6, including uncommon rainfall on January 2 and 3, whereas the rest of the period experienced snowfall. The period from January 17–20 was designated as a “Class B blizzard” by JARE criteria, with visibility <1 km and wind speeds >15 m s−1 for more than 12 hours, resulting in a restriction on outdoor activities. In January 2022, the area surrounding Syowa Station generally had fast ice up to ∼68 °S, with open water north of this.
A 72 hours back trajectory analysis revealed that the air masses during these events originated from ∼45 °S, suggesting that they were transported from open water surfaces. The concentration of T-Hg in seawater near Casey Station (Australia) was an order of magnitude greater than that in the open ocean.25 Legrand et al.26 observed that at the DDU French station, where the open sea is seen throughout the summer, atmospheric dimethylsulfide (DMS) and chloride ions increase during sea breeze events. Furthermore, ice melt was found to increase total gaseous Hg concentrations in the Arctic.27 A previous study documented an increase in DMS concentration at Syowa Station during meteorological disturbances,28,29 implying that the GEM concentration increase on January 2–3 and January 17–20, 2022, could be attributed to emissions from seawater surfaces caused by a low pressure. Throughout the survey period, Syowa Station was surrounded by sea ice; however, an increase in atmospheric GEM concentrations was observed when air masses arrived from open water surfaces. According to Angot et al.,6 the ocean is one of the sources of atmospheric GEM along the Antarctic coast.
3.3 Diurnal variations and local GEM sources
Excluding the GEM spike episodes of January 2–3 and January 17–20, 2022, a clear diurnal variation in GEM concentration was observed throughout the survey period [Fig. 2(a)]. This diurnal cycle, observed regardless of wind direction, exhibited a trend similar to that observed at DDU Station by Angot et al.,8 implying that the observed diurnal variation is related to local sources of GEM occurring around noon. This is the second report of diurnal variation in Antarctic coastal areas, indicating that this phenomenon may be unique to the coastal regions of Antarctica.Because Syowa Station is situated on East Ongul Island, which is surrounded by the sea on all sides, Hg emissions from the ocean into the atmosphere could have influenced the atmospheric GEM concentrations observed here. However, during the one-month survey period, Syowa Station surroundings were covered with sea ice, with open water only at the rear, where the Shirase breached the ice. When the Shirase was anchored in the fast ice near the base, atmospheric Hg concentrations measured at the stern ranged from 0.54 to 1.10 ng m−3, comparable with those at Syowa Station and approximately at the background concentration level of 0.9 ng m−3 for the Southern Hemisphere30 (Fig. 3). Koga et al.29 reported that DMS concentrations were below detectable levels when the Shirase was near Syowa Station and surrounded by fast ice. This indicates that DMS emissions from fast ice are likely negligible. Diurnal variation in atmospheric GEM concentrations was observed regardless of wind direction [Fig. 1(b)], indicating that Hg emissions from the ocean were limited. The area around Syowa Station was surrounded by fast ice, which is unlikely to contribute notably to the diurnal variation of GEM observed at the Fundamental observation building.
In Antarctica, where there are no Hg emission sources except volcanos, seal mummies and skeletons have been identified as a source of Hg emissions to the environment.31 There are also high Hg concentrations in catchment area sediments near penguin colonies in the Ross Sea region32 and in ornithogenic soils—formed by guano accumulation—on King George Island.33 More than 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Adélie penguins nest near DDU Station, and the decrease in GOM on the snow surface in early and late summer—with the release of GEM from ornithogenic soils intensified by the increase in noon temperatures—may contribute to the diurnal variation from November to February.7 However, there are no large penguin nesting sites around Syowa Station, and only 1–2 instances of breeding—timing and scale unknown—have been confirmed in the past near the survey site located ∼3 km southwest and 1 km northwest of the Fundamental observation building.34 Throughout the study duration, wind prevailed from northwest to north northwest [Fig. 1(a)],20 and the impact of ornithogenic soils, if any trace existed at the sites where breeding was observed in the past, was considered insignificant.
000 Adélie penguins nest near DDU Station, and the decrease in GOM on the snow surface in early and late summer—with the release of GEM from ornithogenic soils intensified by the increase in noon temperatures—may contribute to the diurnal variation from November to February.7 However, there are no large penguin nesting sites around Syowa Station, and only 1–2 instances of breeding—timing and scale unknown—have been confirmed in the past near the survey site located ∼3 km southwest and 1 km northwest of the Fundamental observation building.34 Throughout the study duration, wind prevailed from northwest to north northwest [Fig. 1(a)],20 and the impact of ornithogenic soils, if any trace existed at the sites where breeding was observed in the past, was considered insignificant.
At the end of summer, snow melts, and the ground is exposed at Syowa Station. If penguin colonies had previously nested at here, the soil Hg concentration could have been higher because of ornithogenic soil residues resulting from penguin nesting. The average soil Hg concentration measured from the soil collected in December 2007 on East Ongul Island (Table 2) was 2.61 ± 3.16 (0.14–19.0) μg kg−1 (n = 102). The building of Syowa Station and activities of the expedition members are mostly restricted to the north side of East Ongul Island (A-area), with little human access to the south side (B-area) (Fig S2†). The average soil Hg concentrations in A- and B-areas were 3.01 ± 3.47 (0.39–19.0) μg kg−1 and 1.22 ± 0.75 (0.14–3.1) μg kg−1, respectively. Although soil Hg concentrations were higher in the A-area, the difference was not statistically significant (t-test, P > 0.01).
| Site | n | Mercury concentration (ng g−1) | Remarks and references |
|---|---|---|---|
| East Ongul Is. (Syowa station) | |||
| All data | 102 | 2.61 ± 3.16 (0.14–19.0) | Surface soil. This study |
| A-area | 79 | 3.01 ± 3.47 (0.39–19.0) | |
| B-area | 23 | 1.22 ± 0.75 (0.14–3.15) | |
| King George island | 30 | 13.1 | Surface soil. Lu et al., 2012 (ref. 35) |
| North Victoria land | — | 7–96 | Surface soil. Bargagli et al., 1993 (ref. 36) |
| North Victoria land | 43 | 31 ± 19 | Surface soil. Bargagli et al., 2005 (ref. 14) |
| Ross island, Beaufort island | 3 | 150 ± 8.73 | Guano and algae samples. Nie et al., 2012(ref. 32) |
Generally, soil Hg concentrations in Antarctica are very low. The background soil Hg concentration on King George Island is 13 μg kg−1,34 and in North Victoria Land, it ranges from 7 to 96 μg kg−1.14,35 Conversely, Hg concentrations in guano observed in Antarctica have been reported as 150 ± 8.73 ng g−1.32 At DDU, where penguin colonies are located near the base, uric acid from ornithogenic soils is broken down by bacteria, producing oxalic acid.36 This process enhances the photoreduction of GOM species in water and ice. As a result, midday temperature increases lead to an intensified release of GEM from the ornithogenic soils at DDU, contributing to the diurnal Hg emission cycle.8 In contrast, the soil Hg concentration at Syowa Station was observed to be lower than these values. Therefore, even if snow melts and the land around Syowa Station becomes ice-free in the summer, it is unlikely that soil Hg concentrations can contribute to diurnal variation. Syowa Station is an ice-free area, and as mentioned above, the land was exposed in the summer because of snowmelt. However, 98% of the Antarctic continent is covered with snow.
Brooks et al.13 reported an Hg concentration of 67 ± 21 ng L−1 in coastal snow; however, the values here were lower. Given the low Hg concentrations in snow and glacier samples collected at Syowa Station and Lützow-Holm Bay—immediately after snowfall: 0.55 ± 0.24 ng L−1; 13–20 days after snowfall: 2.31 ± 0.38 ng L−1—and the results of the 72 h back trajectory analysis, which showed little evidence of the influence of katabatic winds [Fig. S4(b)†], the mechanism proposed by Angot et al.8 regarding inland GOM-rich air masses being brought to the Antarctic coast along with humidity, as the cause of diurnal variation in the GEM, could not be explained.
The GEM diurnal variation trend was particularly noticeable after January 20, 2022. At Syowa Station, the midnight sun ends in mid-January. At DDU Station, the difference between the day and night GEM concentrations vanishes during polar nights.7 The distinct diurnal variation observed after January 20 is assumed to be caused by a reduction in daylight during the night. During the observation period, katabatic winds were rarely observed, and Hg concentrations in snow, glacier, and ice sheet samples were low. The hypothesis that GOM is transported from penguin excrement, evasion from seawater, and katabatic winds and then deposited in large amounts in snow, leading to diurnal variation due to Hg release, lacks decisive evidence. However, the area surrounding the survey site was covered with a considerable amount of snow. Even if snow Hg concentrations are low, the release of GEM from the snow surface during the day may play a role in the diurnal variation of atmospheric GEM but further investigation is required.
4 Conclusions
Atmospheric GEM concentrations were continuously monitored at the Fundamental observation building of Syowa Station in January 2022. Diurnal variation was observed throughout the study period, with GEM concentrations increasing during the day and decreasing at night. Two episodes of high GEM concentrations were observed on January 2–3 and January 17–20, 2022, possibly caused by Hg emissions from the sea surface owing to the low-pressure system. To investigate the cause of the diurnal variation, excluding these periods, the following were determined: soil Hg concentrations within Syowa Station; Hg concentrations in snow, glaciers, and ice sheets in and around the ice-free areas of Syowa Station; and the influence of atmospheric Hg on the fast ice covering the area around the base. However, none of these sources contributed to an increase in GEM concentration during the day. Nevertheless, since the majority of the Antarctic continent is covered with snow, further investigation on emissions from snow may be needed despite the low Hg concentration in the snow. Additionally, diurnal variation during the summer has only been reported at the DDU Station. Similar diurnal variations at Syowa Station in summer suggest that this may be a characteristic of Antarctic coastal regions, and studies in coastal areas may be important.Data availability
Data for this article are provided as a ESI.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
For their assistance with the field surveys, we thank Takuro Kayashima (Ministry of the Environment, Japan), Masanori Murakami (Nippon Marine Enterprises, Ltd), and Takashi Ishikawa (Japan Meteorological Agency). Also, we express our gratitude to the members of the 63rd and 49th Japanese Antarctic Research Expedition (JARE 63 and JARE 49) for their cooperation with the survey. We are grateful to Editage (https://www.editage.com) for English language editing. We greatly appreciate the constructive and valuable comments on the manuscript from two anonymous reviewers. This study was partly supported by the Comprehensive Research Funding for Environmental Studies (JRMEERF20205R03) funded by Koyomi Nakazawa, Grant-in-Aid for Challenging Research (Exploratory) funded to Osamu Nagafuchi (19K22937), Grant-in-Aid for Scientific Research (C) funded to Koyomi Nakazawa (18K04413), and Grant-in-Aid for Scientific Research (B) funded to Satoshi Imura (19H04321).References
- UN Environment, Environment Program Chemicals and Health Branch, Global Mercury Assessment 2018, Geneva, 2019 Search PubMed.
- S. E. Lindberg and W. J. Stratton, Atmospheric mercury speciation: Concentrations and behavior of reactive gaseous mercury in ambient air, Environ. Sci. Technol., 1998, 32, 49–57 CrossRef CAS.
- E. G. Slemer, R. Brunke and J. Ebinghaus, Kuss, Worldwide trend of atmospheric mercury since 1995, Atmos. Chem. Phys., 2011, 11, 4779–4787 CrossRef.
- S. E. Lindberg and W. J. Stratton, A synthesis of progress and uncertainties in attributing the sources of mercury in deposition, Ambio, 2007, 36, 19–32 CrossRef CAS PubMed.
- O. Lindqvst and H. Rodhe, Atmospheric mercury–a review, Tellus, 1985, 37B, 136–159 CrossRef.
- H. Angot, O. Magand, D. Helmig, P. Ricaud, B. Quennehen, H. Gallée, M. Guasta, F. Sprovieri, N. Pirrone, J. Savarino and A. Dommergue, New insights into the atmospheric mercury cycling in central Antarctica and implications on a continental scale, Atmos. Chem. Phys., 2016, 16, 8249–8264 CrossRef CAS.
- D. Davis, J. B. Nowak, G. Chen, M. Buhr, R. Arimoto, A. Hogan, F. Eisele, L. Mauldin, D. Tanner, R. Shetter, B. Lefer and P. McMurry, Unexpected high levels of NO observed at South Pole, Geophys. Res. Lett., 2001, 28, 3625–3628 CrossRef CAS.
- H. Angot, I. Dion, N. Vogel, M. Legrand, O. Magand and A. Dommmergue, Multi-year record of atmospheric mercury at Dumont d'Urville, east Antarctic coast: Continental outflow and oceanic influences, Atmos. Chem. Phys., 2016, 16, 8265–8279 CrossRef CAS.
- K. A. Pfaffhuber, T. Berg, D. Hirdman and A. Stohl, Atmospheric mercury observations from Antarctica: Seasonal variation and source and sink region calculations, Atmos. Chem. Phys., 2012, 12, 3241–3251 CrossRef CAS.
- R. Ebinghaus, H. H. Kock, C. Temme, J. W. Einax, A. G. Lowe, A. Richter, J. P. Burrows and W. H. Schroeder, Antarctic springtime depletion of atmospheric mercury, Environ. Sci. Technol., 2002, 36, 1238–1244 CrossRef CAS PubMed.
- C. Temme, J. W. Einax, R. Ebinghaus and W. H. Schroeder, Measurements of atmospheric mercury species at a coastal site in the Antarctic and over the South Atlantic ocean during polar summer, Environ. Sci. Technol., 2003, 37, 22–31 CrossRef CAS PubMed.
- F. Sprovieri, N. Pirrone and M. Edgecock, Intensive atmospheric mercury measurements at Terra Nova Bay in Antarctica during November and December 2000, J. Geophys. Res., 2002, 107(D23), 4222 CrossRef.
- S. B. Brooks, R. Arimoto and S. E. Lindberg, Springtime atmospheric mercury speciation in the McMurdo, Antarctica coastal region, Atmos. Environ., 2008, 42, 2885–2893 CrossRef CAS.
- R. Bargagli, C. Agnorelli, F. Borghini and F. Monaci, Enhanced deposition and bioaccumulation of mercury in Antarctic terrestrial ecosystems facing a coastal polynya, Environ. Sci. Technol., 2005, 39, 8150–8155 CrossRef CAS PubMed.
- NiPR Editing, Directory of Syowa Station, 2021, 2021, (in Japanese) Search PubMed.
- A. Steffen, W. Schroeder, J. Bottenheim, J. Narayan and J. D. Fuentes, Atmospheric mercury concentrations: measurements and profiles near snow and ice surfaces in the Canadian Arctic during Alert 2000, Atmos. Environ., 2002, 36, 2653–2661 CrossRef CAS.
- A. Steffen, T. Douglas, M. Amyot, P. Ariya, K. Aspmo, T. Berg, J. Bottenheim, S. Brooks, F. Cobbett, A. Dastoor, A. Dommergue, R. Ebinghaus, C. Ferrari, K. Gardfeldt, M. E. Goodsite, D. Lean, A. J. Poulain, C. Scherz, H. Skov, J. Sommar and C. Temme, A synthesis of atmospheric mercury depletion event chemistry in the atmosphere and snow, Atmos. Chem. Phys., 2008, 8, 1445–1482, DOI:10.5194/acp-8-1445-2008.
- USEPA, Method 1631, Revision E: Mercury in Water by Oxidation, Purge and Trap, and Cold Vapor Atomic Fluorescence Spectrometry, 2002 Search PubMed.
- NOAA HYSPLIT model HP, https://www.ready.noaa.gov/hypub-bin/trajtype.pl?runtype=archive(2024/4/6 accessed) Search PubMed.
- Japan Meteorological Agency, Database of Previous Meteorological Datasets, https://www.data.jma.go.jp/obd/stats/etrn/index.php?prec_no=99%26block_no=89532%26year=%26month=%26day=%26view= > (2023/10/8 accessed) Search PubMed.
- University of Bremen, Sea Ice Remote Sensing, <Sea Ice Concentration (AMSR-E/AMSR2) (uni-bremen.de)> accessed 2024/2/6 Search PubMed.
- G. Spreen, L. Kaleschke and G. Heygster, Sea ice remote sensing using AMSR-E 89 GHz channels, J. Geophys. Res., 2008, 113, C02S03–2008 CrossRef.
- Y. Morita, Winds of katabatic origin observed at Syowa station, Nankyoku shiryo, 1968, 31, 21–32 Search PubMed.
- K. Sato and N. Hirasawa, Statistics of Antarctic surface meteorology based on hourly data in 1957-2007 at Syowa station, Polar Sci., 2007, 1, 1–15 CrossRef.
- D. Cossa, L.-E. Heimbürger, D. Lannuzel, S. R. Rintoul, E. C. V. Butler, A. R. Bowie, B. Averty, R. J. Watson and T. Remenyi, Mercury in the Southern Ocean, Geochim. Cosmochim., 2011, 75, 4037–4052 CrossRef CAS.
- M. Legrand, X. Yang, S. Preunkert and N. Theys, Year-round records of sea salt, gaseous, and particulate inorganic bromine in the atmospheric boundary layer at coastal (Dumont d'Urville) and central (Concordia) East Antarctic sites, J. Geophys. Res.: Atmos., 2016, 121, 997–1023 CrossRef CAS.
- J. Yu, Z. Xie, H. Kang, Z. Li, C. Sun, L. Bian and P. Zhang, High variability of atmospheric mercury in the summertime boundary layer through the central Arctic Ocean, Sci. Rep., 2014, 4, 6091 CrossRef CAS PubMed.
- S. Koga, I. Nagao, H. Tanaka and H. Mouri, Methanesulfonate and non-sea-salt sulfate concentration in aerosols at Syowa Antarctica, J. Meteorol. Soc. Jpn., 1999, 77, 155–164 CrossRef.
- S. Koga, D. Nomura and M. Wada, Variation of dimethylsulfide mixing ratio over the Southern Ocean from 36°S to 70°S, Polar Sci., 2014, 8, 306–313 CrossRef.
- F. Slemr, L. Martin, C. Labuschangnem, T. Mkololo, H. Angot, O. Magand, A. Dommergue, P. Garat, M. Ramonet and J. Bieser, Atmospheric mercury in the Southern Hemisphere- Part 1: Trend and inter-annual variations in atmospheric mercury at Cape Point, south Africa, in 2007-2017, and on Amsterdam island in 2012-2017, Atmos. Chem. Phys., 2020, 20, 7683–7692 CrossRef CAS.
- O. Zvěřina, P. Coufalík, K. Brat, R. Červenka, J. Kuta, O. Mikeš and J. Komárek, Leaching of mercury from seal carcasses into Antarctic soils, Environ. Sci. Pollut. Res., 2017, 24, 1424–1431 CrossRef PubMed.
- Y. Nie, X. Liu, L. Sun and S. D. Emslie, Effect of penguin and seal excrement on mercury distribution in sediments from the Ross Sea region, East Antarctica, Sci. Total Environ., 2012, 433, 132–140 CrossRef CAS PubMed.
- P. D. A. Renato, F. M. M. Roverto, E. G. R. S. Carlos, N. B. S. Felipe and C. W. Claudia, Hg distribution and speciation in Antarctic soils of the Fildes and Ardley peninsulas, King George Island, Antarct. Sci., 2012, 24, 395–407 CrossRef.
- A. Kato and Y. Ropert-Coudert, Rapid increase in Adélie penguin populations in the Lützow-Holm Bay area since the mid 1990s, Polar Biosci., 2006, 20, 55–62 Search PubMed.
- Z. Lu, M. Cai, J. Wang, H. Yang and J. He, Baseline values for metals in soils of Fildes Peninsula, King George Island, Antarctica: The extent of anthropogenic pollution, Environ. Monit. Assess., 2012, 184, 7013–7021 CrossRef CAS PubMed.
- R. Bargagli, E. Battisti, S. Focardi and P. Formichi, Preliminary data on environmental distribution of mercury in northern Victoria Land, Antarctica, Antarct. Sci., 1993, 5, 3–8 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4va00166d |
| This journal is © The Royal Society of Chemistry 2025 |