Open Access Article
Open Access ArticleEffect of the foliar application of biogenic-ZnO nanoparticles on physio-chemical analysis of chilli (Capsicum annum L.) in a salt stress environment
Muhammad
Adnan
a,
Faisal
Mahmood
*b,
Zhenhua
Zhao
*a,
Hamza
Khaliq
a,
Muhammad
Usman
a,
Tahir
Muhammad
c and
Ghulam Abbas
Ashraf
 *ad
*ad
aKey Laboratory of Integrated Regulation and Resource Development on Shallow Lake of Ministry of Education, College of Environment, Hohai University, Nanjing 210098, P. R. China. E-mail: Adnanbuzdar2014@hhu.edu.cn; zzh4000@126.com; hamzakhosa01@hhu.edu.cn; itxusman85@gmail.com; ga_phy@yahoo.com
bDepartment of Environmental Sciences, Government College University Faisalabad, 38040, Pakistan. E-mail: faisalmahmood@gcuf.edu.pk
cCollege of Hydrology and Water Resources, Hohai University, Nanjing 210098, China. E-mail: engineerkakar95@cau.edu.cn
dNew Uzbekistan University, Mustaqillik Ave. 54, Tashkent, 100007, Uzbekistan
First published on 17th December 2024
Abstract
Chilli (Capsicum annuum L.) plants are cultivated globally and are valued for their culinary use. One of the major challenges in agriculture is soil salinity, which drastically cuts down crop productivity. However, no information has been reported concerning the effects of biogenic zinc oxide nanoparticles (ZnO NPs), applied as a foliar spray, on the physio-chemical properties of chilli plants under salt stress conditions. The nanoparticles were synthesized using an extract from Acacia nilotica leaves, which acted as a stabilizing and reducing agent. The characteristics of the synthesized nanoparticles were analyzed using various techniques including UV-visible spectroscopy, X-ray diffraction, Fourier transform infrared spectroscopy, scanning electron microscopy and X-ray photoelectron spectroscopy. The pot experiment utilized a salinity level of 50 mM NaCl and tested five concentrations of ZnO NPs (0, 25, 50, 75 and 100 ppm). The results demonstrated that the highest concentration (100 ppm) significantly enhanced growth parameters, including the shoot length (38.6%) and root length (25.5%) compared to the control. Additionally, biochemical parameters such as chlorophyll content (23.3%) and phenolic content (12.5%) enhanced zinc accumulation by 38.7% and decreased oxidative stress malondialdehyde (MDA) by 54.4% and hydrogen peroxide (H2O2) by 33.1% as compared to the control. We can conclude that foliar application of 100 ppm of the synthesized biogenic-ZnO NPs may increase chilli growth in a salt-stress environment.
Environmental significanceIncreasing environmental issues, such as soil salinity, pose substantial danger to worldwide agricultural sustainability and the availability of food. Traditional agricultural practices frequently fail to appropriately tackle these difficulties and might worsen environmental harm due to excessive dependence on artificial fertilizers and pesticides. This study investigates the foliar application of biogenic-zinc oxide (ZnO) NPs on plant leaves to enhance plant resistance and productivity in environments with high salt levels. The results of our study indicate that biogenic-ZnO NPs have a substantial positive impact on important physiological and biochemical characteristics of chilli plants, including their growth rates, chlorophyll synthesis, and antioxidant enzyme activities. Our work highlights an effective approach to reduce the use of detrimental agricultural chemicals, which in turn healthily promotes the development of crops and encourages the adoption of farming techniques that are more environmentally sustainable. Utilizing biogenic-ZnO NPs enhances crop resilience to environmental shocks and supports global efforts to implement environmentally friendly agricultural technologies that protect natural resources and preserve ecological balance. |
Introduction
Soil salinity poses a profound obstacle in agriculture, especially in dry and semi-dry areas, where it greatly diminishes crop yield.1 An estimated 10 percent of the world's cultivable land area is affected by salt stress and the amount is increasing day by day.2 Salinized soil hampers plant growth by restricting water absorption, obstructing nutrient assimilation, and compromising metabolic functions.3 This poses a significant risk to global food security, particularly for crops like chilli (Capsicum annuum L.), which are widely cultivated for their culinary and economic value. Chilli belongs to the Solanaceae family, which also includes tomatoes, eggplants, and potatoes.4,5 This crop is perennial and grows well in both tropical and temperate climates, and thus it can be grown all year around.6 Globally chilli production is 31 million tons and it spans over 1.93 million hectares; about 0.2 million tons of chilies are produced annually in Pakistan.7 During 2022–23 the production of chilli crops was 82 thousand tons with a cultivated area of 31 thousand hectares, and there was a decrease in the production of chilli by 43.1%.8Root causes behind the low yield, include biotic (pests and diseases) and abiotic (water scarcity, low/high temperature and salinity) pressure.9 Maximum salt concentration causes osmotic pressure and water deficiency all around the plasma membrane, which minimizes the water content and leads to significant physiological changes that restrict plant growth.10 Furthermore, the photosynthesis rate has been reduced due to salinity which consequently decreases the stomatal conductance, inhibits photochemical capacity, and slows down the metabolic process in carbon uptake.11 Minimizing salinity via breeding salt-tolerant plants and providing a low-salt water source and drainage to remove salt from the plant's root zone are two potential approaches; however, both possibilities are expensive and time-consuming.12 Many techniques are used to make the smallest possible size of nanoparticles (NPs). Mainly, four methods, chemical, physical, photochemical, and organic approaches are used to prepare NPs.13 Chemical, physical and photochemical processes are costly and not eco-friendly. Worldwide, scientists are looking to devise methods to synthesize NPs that are eco-friendly, economical and easy to apply; among such techniques is the biogenic method.14 In the biogenic approach, NPs are synthesized by using plant extract, and bacteria, algae, and fungi are used as the capping and reducing agents.15
The process of synthesizing nanoparticles using environmentally friendly methods has a direct impact on plants and their natural chemicals, like enzymes, amino acids, and vitamins, which act as reducing agents during the generation of NPs. ZnO NPs have gained popularity in agriculture due to their ability to promote the production of secondary metabolites and provide other advantages.16 Zinc plays a vital role in facilitating various enzymatic activities and serves as an essential cation in some biochemical processes, including those of carbohydrates and photosynthetic pigments.17 The ZnO foliar application approach is effective and safe.18 Nutrients are absorbed through the leaf cuticles and stomatal openings of leaves, as well as the cell wall of the epidermal cells and the plasma membrane, via active transport during foliar application.19 According to recent data, ZnO NPs are highly effective bioactive substances that can affect the transcriptome, tissue differentiation, proteome, and metabolism in plants.20 In addition, zinc has an important function in mitigating sodium buildup and improving the potassium-to-sodium ratio in plants subjected to salt-induced stress.21 However, lipid peroxidation, H2O2 content, and membrane impairment in plants subjected to salt stress were all decreased by ZnO application.22 The utilization of biogenic-ZnO NPs has the potential to significantly impact plant development, photosynthesis, protein synthesis, respiration and reproduction of plants.23 Further investigation is needed to understand the interplay between salt and zinc deficiency and how it affects plant development and yield.24
Despite the demonstrated potential of ZnO NPs in improving plant stress tolerance, their precise impact on chilli plants under salt stress has not been thoroughly investigated, especially when used as a foliar spray. The limited research on the specific interactions between biogenically produced ZnO nanoparticles (NPs) and the physiological and biochemical responses of chilli plants, as well as their potential long-term effects on plant health and productivity, underscores the need for further study. Therefore, ZnO NPs were biogenically synthesized and characterized and applied to chilli plants in the hope to reduce the adverse effects of salinity. The aim of this study is to evaluate the effects on growth metrics, biochemical indicators, and oxidative stress markers, and to investigate the feasibility of using foliar application of biogenic-ZnO NPs as a sustainable approach to improve the resilience of chilli plants in a saline environment.
Materials and methods
Growth conditions and plant materials
The Capsicum annum L. f1 hybrid seeds were collected from a local seed market. A pot experiment was carried out. To start a nursery, seeds were planted in little clay containers. The soil samples were collected from agricultural fields. Surface soil, 0–15 cm depth, has been taken in triplicate with auger. Soil samples were taken randomly in polythene bags. The soil samples have been properly combined to provide composite samples that are uniform and homogeneous. The soil was sieved to remove stones and other impurities. Physiochemical properties of soil (electrical conductivity (2.4 dS m−1), potential hydrogen (7.6), and contents of nitrogen (0.6%), phosphorus (11.2 ppm), potassium (154 ppm) and other organic matter (0.68%)) were found out by standard procedures. Nursery was transferred after one month of seed germination to pots for further growth. Every container was filled with 5 kg of soil. 5 seeds were planted in each container. A 50 mM NaCl solution was applied in the soil as salt stress on chilli plants after transplantation of the nursery. The fertilizer was applied in the experimental pots 50 kg ha−1 but for 5 kg soil, 0.5 g of triple super phosphate and muriate of potash was applied. After the transplantation of chilli plants, a biogenic ZnO-NP foliar spray was applied every 15 days. In total 5 applications of ZnO-NP treatments (0, 25, 50, 75 and 100 ppm) were foliar applied in each pot. The number of replicates was three. In the control group, plants were subjected to the application of distilled water and provided with tap water for nourishment. Some characteristics, such as the fresh weight of the root and shoot, were measured right after harvesting the plants, which occurred 60 days after transplantation. Using an electrical balance, three plants from each pot were used to measure their average fresh weight. After 24 hours in an oven, the electrical balance was used to quantify the dry weight (g) of the root and shoot. The shoot and root length of the plant were measured with measuring tape. Leaves and roots of three plants were taken from each pot and washed carefully with distilled water and then stored in falcon tubes at 40 °C for further examination.Synthesis of biogenic-ZnO NPs
Acacia nilotica leaves were collected from nearby gardens, and carefully washed and dried in sunlight for dust particle removal. Afterwards, a pestle and mortar was used to crush the leaves into a fine powder. Subsequently, a quantity of 20 g of Acacia nilotica leaf powder was combined with 100 mL of distilled water and agitated at a temperature of 100 °C for 15 minutes. The solution underwent filtration using Whatman filter paper No. 1. Subsequently, zinc acetate was utilized as a precursor in the synthesis of ZnO nanoparticles, with the prepared plant extract serving as the reducing and stabilizing agent. A solution of zinc acetate with a concentration of 0.21 M was prepared in deionized water. 40 mL of Acacia nilotica leaf extract was added drop by drop while stirring the solution for 4 hours at 70 °C. After that, the solution was placed on a hot plate for 1 hour and finally, the solution underwent calcination for 4 hours at 400 °C.25Biochemical parameter analysis
The chlorophyll concentrations were measured. 0.1 g of ground leaves and 2–3 mL of 80% methanol were taken. At 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm this solution was centrifuged, and the extract was taken, allowed to settle down and stored. A spectrophotometer was utilized at three distinct wavelengths such as 663, 645, and 480 nm, respectively to determine the chlorophyll-a, chlorophyll-b and carotenoid contents.26 The sample of fresh leaves (0.25) g was taken and 5 mL of acetone was added to ground fresh plant leaves. The solution was left to settle for 24 hours. The spectrophotometer was set at 463 nm to determine absorbance. The phenolic contents were determined by comparison with a standard curve at distinctive phenolic concentrations.
000 rpm this solution was centrifuged, and the extract was taken, allowed to settle down and stored. A spectrophotometer was utilized at three distinct wavelengths such as 663, 645, and 480 nm, respectively to determine the chlorophyll-a, chlorophyll-b and carotenoid contents.26 The sample of fresh leaves (0.25) g was taken and 5 mL of acetone was added to ground fresh plant leaves. The solution was left to settle for 24 hours. The spectrophotometer was set at 463 nm to determine absorbance. The phenolic contents were determined by comparison with a standard curve at distinctive phenolic concentrations.
Antioxidant enzyme analysis
According to ref. 27 methods, “superoxide dismutase (SOD)” contents were calculated. The reaction mixture was prepared using 100 mL of methionine, 650 mL of phosphate buffer, 50 mL of riboflavin, and 100 mL of enzyme extract and subsequently, NBT (nitroblue tetrazolium) was added to the mixture. The reduction was conducted for approximately 10 min under the illumination of a 15 (W) fluorescent lamp from a distance of approximately 13 cm. To achieve uniform illumination, a digital lux meter (Lutron Lx-101, Taichung, Taiwan) was utilized to regulate the illumination within 6500–7500 lx, and the absorbance was measured using a spectrophotometer at a wavelength of 560 nm. The method given in ref. 28 was used to calculate “ascorbate peroxide (APX)” activity. The test tubes were prepared with 2.2 mL of phosphate buffer (pH 7.0), 0.3 mL of methionine, and 0.2 mL of EDTA, and were thoroughly shaken to ensure proper mixing. After this 50 μL enzyme extract and 0.1 mL (NBT) were added to it and shaken again. All the tubes were placed under light for about 7 min. Subsequently, the spectrophotometer was used to measure the absorbance at a wavelength of 560 nm. The “catalase (CAT)” and “peroxidase (POD)” activity were analysed by the procedure described.29ZnO accumulation analysis
A mixture containing HNO3 and HClO4, 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, was employed for digestion of dry fine ground chilli plant leaves (0.5 g) and left overnight. Plant samples were dried in an oven at 80 °C. The digestion process will be carried out by following the protocol introduced in ref. 30 for 3–4 hours until fumes appear. The absorbance of ZnO was quantified using an atomic absorption spectrophotometer.
1, was employed for digestion of dry fine ground chilli plant leaves (0.5 g) and left overnight. Plant samples were dried in an oven at 80 °C. The digestion process will be carried out by following the protocol introduced in ref. 30 for 3–4 hours until fumes appear. The absorbance of ZnO was quantified using an atomic absorption spectrophotometer.
Reactive oxygen species (ROS) analysis
The method given in ref. 31 was used to calculate the H2O2 concentration. In this method, 0.1% (w/v) trichloroacetic acid (TCA) and 0.1 g of fresh plant sample were homogenized in an ice bath. 3 mL of Kl was mixed with 0.1 mL potassium phosphate buffer (pH 7.0) and then 0.1 mL of supernatant was added to it, shaken well and left for 30 min at room temperature. The absorbance was evaluated with the help of a spectrophotometer at 390 nm. By comparison with the standard curve, distinctive H2O2 concentration was measured. The MDA contents were determined using the prescribed procedure given in ref. 32. In this technique, 0.25 g fresh plant samples were mixed with 5 mL TCA and centrifuged at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 15 min. After this, in a test tube, 1.5 mL of 0.6% tributaric acid (TBA) and 1.5 mL supernatant were taken and shaken properly. After shaking for 30 min, it was placed in a water bath at 100 °C and absorbance was measured at 532, 600 and 450 nm.
000 rpm for 15 min. After this, in a test tube, 1.5 mL of 0.6% tributaric acid (TBA) and 1.5 mL supernatant were taken and shaken properly. After shaking for 30 min, it was placed in a water bath at 100 °C and absorbance was measured at 532, 600 and 450 nm.
Statistical data analysis
Statistically significant differences between treatments were determined from the data obtained throughout the experiment using one-way analysis of variance (ANOVA) and the appropriate statistical tools. The mean values of each treatment were compared by the CRD test (p ≤ 0.05). Graphs were plotted using Microsoft Excel, GraphPad Prism 9.0, and Origin Program.Results
Characterization of biogenic-ZnO NPs
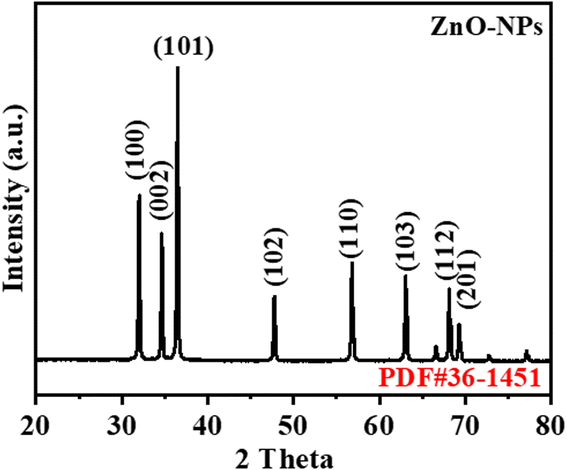
The XRD pattern of the product is illustrated in Fig. 1. The peaks in the figure are 2θ = 31.79, 34.42, 36.25, 47.53, 56.60, 62.86, 67.96 and 69.09. The identified peaks correspond to the (100), (002), (101), (102), (110), (103), (112), and (201) cross-lattice planes, respectively, and these planes were determined to be characteristic of the hexagonal wurtzite structure in the produced nanoparticles. The observed peaks appear to be following the reference data.33The FT-IR spectrum of the biogenic-ZnO NPs is recorded. FT-IR spectrum analysis was performed by referring to the reported study34 as shown in Fig. 2(A). A significant absorption band was discovered at a wavenumber of 414.82 cm−1, which can be attributed to the stretching vibration of Zn–O bonds. In prior investigations on ZnO nanoparticles, researchers were able to successfully examine the FT-IR spectra, which exhibited a prominent band at around 400 cm−1. The identification of all detected peaks was based on references from prior scholarly studies, hence validating the obtained results. Prior studies on the synthesis and characterization of ZnO-NPs yielded comparable findings. The peaks seen at 1426 cm−1 and 1613 cm−1 correspond to the symmetric and asymmetric stretching vibration, respectively, of the adsorbed carbonate anion. Meanwhile, the peaks observed at 1029 cm−1 indicate the lattice vibration of carbonate-generated absorption peaks. In addition, the 3402.82 cm−1 absorption peak corresponds to the stretching of the hydroxyl group. Some extra peaks show that biogenic particles were added in ZnO-NPs.
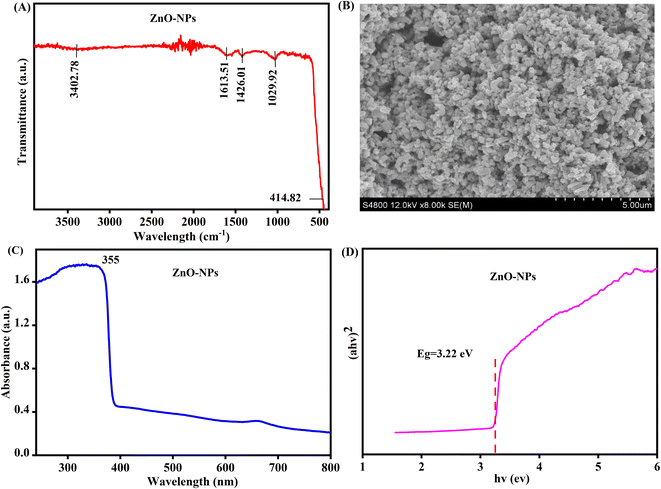 | ||
| Fig. 2 (A) FT-IR spectra, (B) SEM image, (C) UV-visible spectrum, and (D) energy bandgap (Eg) of biogenic-ZnO NPs. | ||
The surface morphology of ZnO NPs was examined using scanning electron microscopy. The SEM image in Fig. 2(B) shows the irregular shape nanoparticles formed with a diameter range of 11–25 nm.35Fig. 2(C) shows the UV-visible spectrum of ZnO nanoparticles which presented a strong peak at 355 nm which features the congenital band gap of ZnO absorption.36Fig. 2(D) illustrates the conversion of the optical absorption, denoted as α, into (αhv)2 and its representation as a function of energy, E, and a linear section with a tangential slope, indicating stronger absorption, is extrapolated. The point at which this line crosses the X-axis is often referred to as the optical bandgap for the given system. The observed bandgap value is about 3.22 eV.37
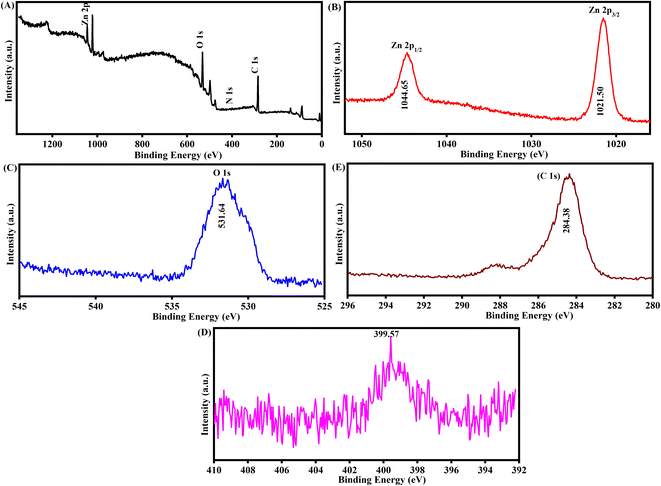
Fig. 3(A) presents further evidence for the purity and composition of biogenic-ZnO NPs, as determined using XPS spectrum analysis. The XPS spectrum samples indicated the existence of Zn, O, C, as well as a N reference. Fig. 3(B) illustrates the high-resolution spectrum of Zn 2p. The binding energy values of Zn 2p1/2 and 2p3/2 are 1044.65 and 1021.50 eV, respectively. Fig. 3(C) shows that the peak position at 531.64 eV can be attributed to oxygen O 1s-in the lattice of ZnO.38Fig. 3(E) shows the XPS spectra of C 1s. The 284.3 eV binding energy of C 1s is usually used as an internal reference in the spectrum.39 As shown in Fig. 3(D), the N 1s peak, which is centred at 399.57 eV, has a significantly reduced intensity in the sample that has been exposed to oxygen, indicating the presence of molecularly chemisorbed nitrogen. Conversely, the intensity of the N 1s peak in the sample irradiated in a nitrogen environment is higher.40
Physical parameter analysis
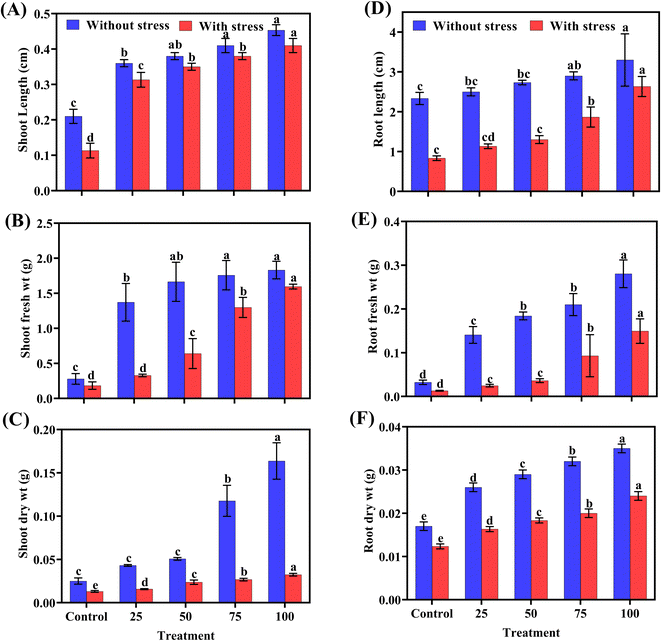
The application of ZnO NPs significantly improved the physical growth parameters of chilli plants under salt stress. The data presented in Fig. 4 indicate that at a concentration of 100 ppm, the plants showed a notable increase in both shoot and root length. Specifically, the maximum shoot length increased from 5.6 cm under salt-stress conditions to 7.76 cm under normal conditions. Similarly, the root length increased from 2.63 cm under stress to 3.3 cm normally. This improvement was also reflected in the fresh and dry weights of both shoots and roots, with the maximum values observed at the highest nanoparticle concentration compared to the control (Table 1).| Concentration (ppm) | Shoot length (cm) | Shoot fresh weight (g) | Shoot dry weight (g) | Root length (cm) | Root fresh weight (g) | Root dry weight (g) |
|---|---|---|---|---|---|---|
| a The ± symbol represents standard deviation. Different letters indicate significant differences among treatments at p ≤ 0.05. | ||||||
| 0 (control) | 2.3 ± 4.5 e | 0.18 ± 0.27 e | 0.01 ± 0.02 e | 0.83 ± 2.33 e | 0.01 ± 0.03 e | 0.012 ± 0.017 e |
| 25 | 2.8 ± 6.4 d | 0.32 ± 1.37 d | 0.01 ± 0.04 d | 1.13 ± 2.50 d | 0.02 ± 0.14 d | 0.016 ± 0.026 d |
| 50 | 3.1 ± 7.0 c | 0.63 ± 1.66 c | 0.02 ± 0.05 c | 1.30 ± 2.73 c | 0.03 ± 0.18 c | 0.018 ± 0.029 c |
| 75 | 4.4 ± 7.5 b | 1.29 ± 1.75 b | 0.02 ± 0.10 b | 1.86 ± 2.90 b | 0.09 ± 0.21 b | 0.020 ± 0.032 b |
| 100 | 5.6 ± 7.7 a | 1.59 ± 1.83 a | 0.03 ± 0.16 a | 2.63 ± 3.30 a | 0.14 ± 0.28 a | 0.024 ± 0.035 a |
Biochemical parameter analysis
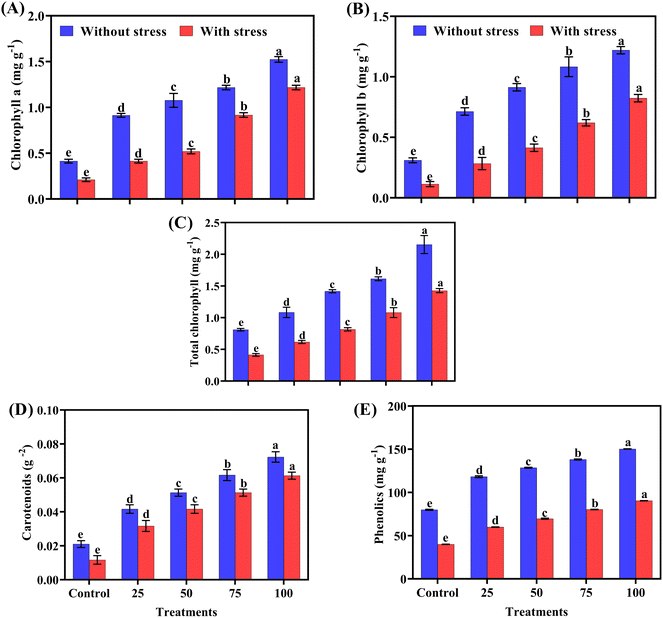
As shown in Fig. 5 foliar application of biogenic ZnO NPs enhanced the biochemical parameters of the chilli plants, particularly under salt stress conditions. Chlorophyll content, a key indicator of plant health, significantly increased at 100 ppm of ZnO NP application. Chlorophyll a increased from 1.21 mg g−1 under salt stress to 1.52 mg g−1 normally, and chlorophyll b from 0.82 mg g−1 to 1.22 mg g−1. The total chlorophyll content and carotenoids also improved, supporting better photosynthetic efficiency and stress response. The maximum phenolic content was observed at the highest NP concentration compared to the control.Antioxidants enzyme analysis
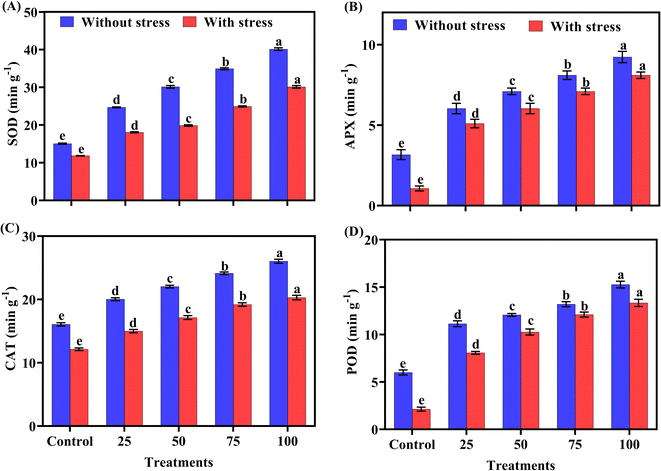
The applied amendments significantly impacted the antioxidant enzyme activity. Statistical analysis in Fig. 6(A)–(D) showed that maximum SOD, APX, CAT and POD activity were observed at 100 ppm (ZnO NPs) in normal and salt-stress treatments, respectively, while minimum antioxidant enzymes were observed in the control treatment. Antioxidant enzymes are gradually enhanced with increasing concentration of biogenic ZnO-NPs as compared to the control (Table 3).| Concentration (ppm) | Chlorophyll a (mg g−1) | Chlorophyll b (mg g−1) | Total chlorophyll (mg g−1) | Carotenoid (gm−2) | Phenolic (mg g−1) |
|---|---|---|---|---|---|
| a The ± symbol represents standard deviation. Different letters indicate significant differences among treatments at p ≤ 0.05. | |||||
| 0 (control) | 0.21 ± 0.41 e | 0.11 ± 0.31 e | 0.41 ± 0.81 e | 0.01 ± 0.02 e | 39.9 ± 79.9 e |
| 25 | 0.4 1 ± 0.91 d | 0.28 ± 0.71 d | 0.61 ± 108 d | 0.03 ± 0.04 d | 59.9 ± 118.1 d |
| 50 | 0.52 ± 1.07 c | 0.41 ± 0.91 c | 0.81 ± 1.41 c | 0.04 ± 0.05 c | 69.6 ± 128.5 c |
| 75 | 0.91 ± 1.21 b | 0.62 ± 1.08 b | 1.08 ± 1.61 b | 0.05 ± 0.06 b | 80.2 ± 138.1 b |
| 100 | 1.21 ± 1.52 a | 0.82 ± 1.22 a | 1.42 ± 2.15 a | 0.06 ± 0.07 a | 90.3 ± 150.2 a |
| Concentration (ppm) | SOD (min g−1) | APX (min g−1) | CAT (min g−1) | POD (min g−1) |
|---|---|---|---|---|
| a The ± symbol represents standard deviation. Different letters indicate significant differences among treatments at p ≤ 0.05. | ||||
| 0 (control) | 11.8 ± 15.0 e | 1.06 ± 3.16 e | 12.1 ± 16.0 a | 2.13 ± 6 e |
| 25 | 18.0 ± 24.7 d | 5.1 ± 6.03 d | 15 ± 20.0 d | 8.06 ± 11.1 d |
| 50 | 19.8 ± 30.1 c | 6.03 ± 7.1 c | 17.1 ± 22.0 c | 10.2 ± 12.0 c |
| 75 | 24.9 ± 34.9 b | 7.1 ± 8.1 b | 19.2 ± 24.1 b | 12.1 ± 13.2 b |
| 100 | 30.1 ± 40.1 a | 8.1 ± 9.23 a | 20.3 ± 26.0 a | 13.3 ± 15.2 a |
ZnO accumulation in chilli plants
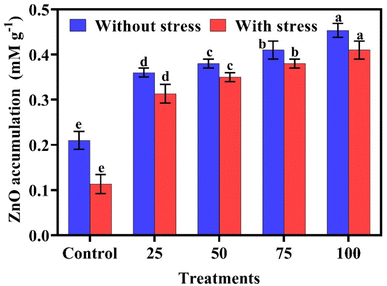
Statistical analysis in Fig. 7 showed that maximum ZnO accumulation (0.45 mM g−1 and 0.41 mM g−1) was observed at 100 ppm in normal and stress treatments, and a minimum uptake of ZnO in plants (0.21 mM kg−1 and 0.11 mM g−1) was observed in control treatments. ZnO accumulation in plants was increased gradually with the increasing application of biogenic ZnO NPs.Reactive oxygen species (ROS)
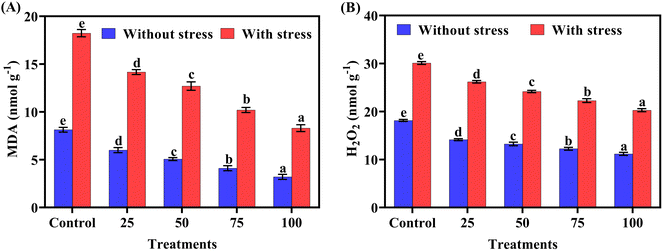
The results regarding the impact of applied treatment on reactive oxygen species are reported in Fig. 8(A) and (B). Foliar application of biogenic-ZnO NPs has decreased MDA and H2O2 content statistically under salt stress conditions. Statistical analysis showed that minimum MDA contents of 3.2 nmol g−1 and 8.3 nmol g−1 and those of H2O2 of 11.2 nmol g−1 and 20.2 nmol g−1 were observed at 100 ppm (ZnO NPs) in normal and salt-stress treatments, respectively. Maximum malondialdehyde and H2O2 contents (18.2, 30.1 nmol g−1) were observed in the control treatment. ROS activity gradually decreased with increasing concentration of biogenic-ZnO NPs as compared to the control.Principal component analysis (PCA)
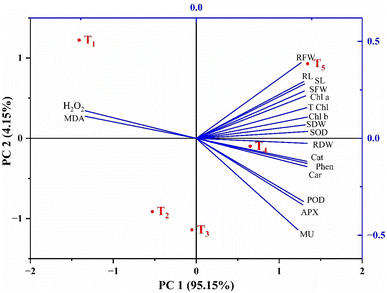
The study demonstrated the impact of treatment of certain amounts of biogenic-ZnO NPs on chilli plants in both normal and stressful situations, as shown in (Fig. 9). The cumulative variance was calculated, with one component accounting for 95.15%, and the second 4.15%. Additionally, noticeable variation in the chosen levels of biogenic ZnO between the control and salt stress conditions in chilli plants was observed. T5 shows the highest coordinate on the PCA-biplot. The analysis also revealed that a notable variation was present among attributes; almost all attributes are correlated with each other. There was a negative correlation between MDA and H2O2, while other characters are positively correlated.Discussion
Salinity is a major obstacle to plant development and productivity, especially in arid and semi-arid regions.41 ZnO NPs are more soluble and reactive compared to ordinary ZnO fertilizers due to their nanoscale size and larger surface area.42 Thus, substituting Zn fertilizer with ZnO NPs could enhance the availability of Zn for meeting the nutritional needs of crops.43 Our research aimed to produce biogenic-ZnO NPs with a more uniform shape and high dispersion using a synthesis method involving Acacia nilotica leaf extract and zinc acetate. Techniques such as XRD, SEM, FT-IR, UV-vis spectroscopy and XPS were employed to analyze the properties and effectiveness of the synthesis procedure by demonstrating the formation of single crystalline ZnO NPs with a hexagonal wurtzite structure. The FT-IR experiments provided convincing evidence of ZnO synthesis and confirmed that the plant extract acts as a capping and stabilizing agent through various phytochemicals. The analysis of the results demonstrates that the precursors have significantly influenced the surface morphology and structure of ZnO NPs.33The results show that the growth parameters of chilli plant as compared to the control significantly decreased with salinity stress. Growth parameters such as the shoot and root length, and fresh and dry weight of both shoots and roots in normal and salt-stressed plants has increased with the application of ZnO NPs at 100 ppm (Table 1). Plants take up nutrients through the xylem and phloem. In the absence of stress, plants can easily take up nutrients. However, under salinity stress plants are unable to take up nutrients due to the shortage of water. Metabolic activities are also affected due to the shortage of nutrients and water within the plant cell. After some period in stressed plants, a defense system of oxidative species is present in plants whose threshold varies from crop to crop, which damages the crop under stress conditions.44 Foliar application might enhance the overall development by stomatal opening and take up more zinc;45 biogenic-ZnO NPs also improved physiological and biochemical parameters under salt-stress conditions.46
Due to salt stress biochemical parameters were negatively affected in stressed plants. While in plants without stress, this content showed a positive increase. Whereas, biogenic-ZnO NPs were applied in the form of foliar application with a maximum increase in biochemical parameters such as chlorophyll a, chlorophyll b, total chlorophyll, carotenoids and phenolic content observed in normal and stress treatments (Table 2). The photosynthesis rate depends upon many factors like water availability, chlorophyll and light. Water is broken down into ions in the light reaction phase. For the reduction of NADP+ to NADPH hydrogen ions are carried to the dark phase. Shortage of hydrogen ions occurred when the shortage of water occurred. Photosynthesis is reduced due to a shortage of water. Chlorophyll enhances its light spectra due to carotenoid assistance. Phenolics maintain the structural integrity of plants and also protect the plants against stress.47
Antioxidant activity (POD, APX, SOD and CAT) also decreased in pots where salt stress was applied. Our findings showed that values of antioxidant parameters were reduced under salt stress conditions as compared to without stressed plants. However, foliar application of biogenic-ZnO NPs upgrades the antioxidant level in without stressed plants and stress treatments. Antioxidant levels increased in stressed plants with increasing concentrations of biogenic ZnO NPs (Table 3). Against oxidative stress, antioxidants have a natural defense mechanism to defend the plants. Antioxidants protect the plant to some extent and cope with reactive oxygen species and scavenging species under stress.48
Our findings showed that the plants grown under salt stress had a lower accumulation of ZnO. Salt stress leads to ion imbalance, osmotic stress, and ionic toxicity and also disturbs the metal uptake rates and transport mechanisms within the plants. The application of biogenic ZnO NPs through foliar application increased the digestion of ZnO in plants with increasing concentration. The maximum ZnO uptake was observed at 100 ppm in normal and stress treatments, while minimum digestion of ZnO was observed in control treatments. Nutrients applied via foliar spray are absorbed directly through the leaves because of stomatal openings, and nutrients are rapidly transported to various parts of the plants. Higher concentration of ZnO NPs has the potential to cause toxicity, affecting both the plant and the consumers. Nevertheless, when present in low amounts, ZnO NPs can function as a micronutrient, thereby potentially providing advantages to the plant without inflicting any damage. A study indicates that ZnO can be a hazardous nanomaterial when used in quantities over 50 mg L−1.49,50 However, the foliar application of biogenic ZnO NPs might have a positive impact on the uptake of ZnO in the shoots and roots of the plant particularly at 100 ppm.51
Oxidative stress (MDA and H2O2) was increased under salt stress conditions. The use of biogenic ZnO NPs by foliar spray decreased the reactive oxygen species growth. Maximum growth was observed in 100 ppm treatment in both without stress and stress plants as compared to the control treatment (Table 4). Under stress, scavenging species (MDA and H2O2) may generate and damage plant cells with the help of rupturing cell membranes. This scavenging also induces oxidative stress in plants, while by the foliar application of biogenic-ZnO NPs scavenging species such as MDA and H2O2 significantly reduced in both normal and stress treatments. These scavenging species work against antioxidants. The reason behind this scavenging species germination is that salt stress also causes water stress, water stress produces free radicles due to oxidative damage which activates lipid peroxidation, and lipid peroxidation produces MDA in the plant.52 Salt stress boosted reactive oxygen species and decreased the antioxidant activity in the chilli crop. By absorbing reactive oxygen species and improving the activity of antioxidants such as SOD, POD, CAT, and APX. ZnO nanoparticles have many effective activities. Application of biogenic-ZnO NPs decreases MDA and H2O2 and increases the antioxidants under both stressed and without stress conditions. Scavenging species activity decreases with increasing concentration of biogenic-ZnO NPs.53
| Concentration (ppm) | ZnO uptake (mM g−1) | MDA (nmol g−1) | H2O2 (nmol g−1) |
|---|---|---|---|
| a Values are means of three replicates. The ± symbol represents standard deviation. Different letters indicate significant differences among treatments at p ≤ 0.05. | |||
| 0 (control) | 0.11 ± 0.21 e | 18.2 ± 8.1 e | 30.1 ± 18.1 e |
| 25 | 0.31 ± 0.36 d | 14.1 ± 6 d | 26.2 ± 14.1 d |
| 50 | 0.35 ± 0.38 c | 12.2 ± 5.06 c | 24.1 ± 13.2 c |
| 75 | 0.38 ± 0.41 b | 10.2 ± 4.1 b | 22.2 ± 12.2 b |
| 100 | 0.41 ± 0.45 a | 8.3 ± 3.2 a | 20.2 ± 11.1 a |
Conclusion
Chilli plant exposure to salt stress resulted in a considerable decrease in many plant development metrics, as well as biochemical and physiological indicators. The foliar application of biogenic-ZnO NPs significantly ameliorated the detrimental effects of salinity on chilli plants. Particularly at 100 ppm, these NPs notably increased growth parameters such as plant height and biomass, and biochemical indices such as chlorophyll a, chlorophyll b, total chlorophyll, carotenoids, and phenolic contents, including levels of antioxidants such as SOD, APX, CAT and POD and zinc content, while effectively reducing oxidative stress indicators such as MDA and H2O2. These findings highlight the capacity of foliar application of ZnO NPs synthesized via eco-friendly techniques, as a promising strategy that may improve the resilience and productivity of chilli plants under saline conditions. This approach not only conforms to a sustainable agricultural methodology by employing green synthesis techniques but it may also offer a feasible resolution to the worldwide issue of soil salinity, which presents a substantial risk to crop productivity and food security.Data availability
Data will be available to readers on request.Author contributions
Muhammad Adnan: writing – original draft, investigation, methodology, formal analysis, data curation, funding acquisition; Faisal Mahmood: investigation, validation, methodology and formal analysis; Zhenhua Zhao: writing – review & editing, conceptualization, methodology, resources, supervision, project administration, funding acquisition; Hamza Khaliq, Muhammad Usman, and Tahir Muhammad: investigation, validation, methodology and formal analysis; Ghulam Abbas Ashraf: review & editing, investigation, validation.Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.Acknowledgements
We acknowledge the support and contributions from our colleagues and mentors, who provided invaluable insights and guidance throughout this research.References
- K. Hussain, A. Majeed, K. Nawaz and M. F. Nisar, Curr. Res. J. Biol. Sci., 2009, 1, 135–138 CAS.
- K. Zhani, B. F. Mariem, M. Fardaous and H. Cherif, J. Stress Physiol. Biochem., 2012, 8, 236–252 Search PubMed.
- S. J. Roy, S. Negrão and M. Tester, Curr. Opin. Biotechnol., 2014, 26, 115–124 CrossRef CAS PubMed.
- S. M. Arain and M. A. Sial, Pak. J. Bot., 2022, 54, 817–822 CAS.
- R. Arimboor, R. B. Natarajan, K. R. Menon, L. P. Chandrasekhar and V. Moorkoth, J. Food Sci. Technol., 2015, 52, 1258–1271 CrossRef CAS PubMed.
- A. Saxena, R. Raghuwanshi, V. K. Gupta and H. B. Singh, Front. Microbiol., 2016, 7, 1527 Search PubMed.
- F. Hussain and M. Abid, Int. J. Biol. Biotechnol., 2011, 8, 325–332 Search PubMed.
- Anonymous 2022–2023, Economic survey of Pakistan, Economic advisory wing, Minestry of finance, 2023 Search PubMed.
- S. Naz and S. Perveen, Pak. J. Bot., 2021, 53, 1209–1217 CAS.
- H. Hniličková, F. Hnilička, M. Orsák and V. Hejnák, Plant, Soil Environ., 2019, 65, 90–96 CrossRef.
- B. Gupta and B. Huang, Int. J. Genomics, 2014, 701596 Search PubMed.
- A. Doğru and S. Canavar, Academic Platform Journal of Engineering and Science, 2020, 8, 155–174 Search PubMed.
- M. Bhat, B. Chakraborty, R. S. Kumar, A. I. Almansour, N. Arumugam, D. Kotresha, S. S. Pallavi, S. B. Dhanyakumara, K. N. Shashiraj and S. Nayaka, J. King Saud Univ., Sci., 2021, 33, 101296 CrossRef.
- M. Zahoor, N. Nazir, M. Iftikhar, S. Naz, I. Zekker, J. Burlakovs, F. Uddin, A. W. Kamran, A. Kallistova, N. Pimenov and F. A. Khan, Water, 2021, 13, 1–28 CrossRef.
- S. Nayak, S. P. Sajankila and C. V. Rao, J. Microbiol., Biotechnol. Food Sci., 2018, 7, 641–645 CrossRef CAS.
- Z. Noohpisheh, H. Amiri, A. Mohammadi and S. Farhadi, Plant Biosyst., 2021, 155, 267–280 CrossRef.
- L. Tang, Y. Hamid, D. Liu, M. J. I. Shohag, A. Zehra, Z. He, Y. Feng and X. Yang, Environ. Int., 2020, 145, 106122 CrossRef CAS.
- R. Ahmed, M. K. Uddin, M. A. Quddus, M. Y. A. Samad, M. A. M. Hossain and A. N. A. Haque, Horticulturae, 2023, 9(2), 162 CrossRef.
- M. R. Ali, H. Mehraj and A. F. M. Jamal Uddin, Journal of Bioscience and Agriculture Research, 2015, 6, 512–517 CrossRef.
- A. Iranbakhsh, Z. Oraghi Ardebili and N. Oraghi Ardebili, Plant Responses to Nanomater: Recent Interventions, and Physiological and Biochemical Responses, 2021, pp. 33–93 Search PubMed.
- F. Nadeem, M. Azhar, M. Anwar-ul-Haq, M. Sabir, T. Samreen, A. Tufail, H. U. M. Awan and W. Juan, J. Soil Sci. Plant Nutr., 2020, 20, 2059–2072 CrossRef CAS.
- M. N. Iqbal, R. Rasheed, M. Y. Ashraf, M. A. Ashraf and I. Hussain, Environ. Sci. Pollut. Res., 2018, 25, 23883–23896 CrossRef CAS.
- H. Marschner, Marschner's mineral nutrition of higher plants, Academic Press, 2011 Search PubMed.
- I. Tolay, PLoS One, 2021, 16, 1–12 CrossRef PubMed.
- J. Singh, S. Kaur, G. Kaur, S. Basu and M. Rawat, Green Process. Synth., 2019, 8(1), 272–280 CAS.
- B. Aron, A manual for analysis of the Thematic Apperception Test: a method and technique for personality research, 1949 Search PubMed.
- C. Beauchamp and I. Fridovich, Anal. Biochem., 1971, 44, 276–287 CrossRef CAS.
- A. Krivosheeva, D.-L. Tao, C. Ottander, G. Wingsle, S. L. Dube and G. Öquist, Planta, 1996, 200, 296–305 CrossRef CAS.
- B. Chance and A. C. Maehly, Methods Enzymol., 1955, 2, 764–775 Search PubMed.
- M. Z. ur Rehman, M. Rizwan, A. Ghafoor, A. Naeem, S. Ali, M. Sabir and M. F. Qayyum, Environ. Sci. Pollut. Res., 2015, 22, 16897–16906 CrossRef.
- V. Velikova, I. Yordanov and A. Edreva, Plant Sci., 2000, 151, 59–66 CrossRef CAS.
- R. S. Dhindsa, P. Plumb-Dhindsa and T. A. Thorpe, J. Exp. Bot., 1981, 32, 93–101 CrossRef CAS.
- S. Fakhari, M. Jamzad and H. Kabiri Fard, Green Chem. Lett. Rev., 2019, 12, 19–24 CrossRef CAS.
- K. Yang, D. Lin and B. Xing, Langmuir, 2009, 25, 3571–3576 CrossRef CAS PubMed.
- C. Vidya, S. Hiremath, M. N. Chandraprabha, M. L. Antonyraj, I. V. Gopal, A. Jain and K. Bansal, International Journal of Current Engineering and Technology, 2013, 1, 118–120 Search PubMed.
- S. Talam, S. R. Karumuri and N. Gunnam, Int. Scholarly Res. Not., 2012, 372505 Search PubMed.
- R. M. Sahani, C. Kumari, A. Pandya and A. Dixit, Sci. Rep., 2019, 9, 11354 CrossRef CAS.
- F. J. Manjón, B. Mari, J. Serrano and A. H. Romero, J. Appl. Phys., 2005, 97(5), 053516 CrossRef.
- C. D. Wagner, W. M. Riggs, L. E. Davis, J. F. Moulder and G. E. Muilenberg, Handbook of X-ray Photoelectron Spectroscopy: A Reference Book of Standard Data for Use in X-ray Photoelectron Spectroscopy, 1979 Search PubMed.
- F. Esaka, K. Furuya, H. Shimada, M. Imamura, N. Matsubayashi, H. Sato, A. Nishijima, A. Kawana, H. Ichimura and T. Kikuchi, J. Vac. Sci. Technol., A, 1997, 15, 2521–2528 CrossRef CAS.
- J. R. Acosta-Motos, M. F. Ortuño, A. Bernal-Vicente, P. Diaz-Vivancos, M. J. Sanchez-Blanco and J. A. Hernandez, Agronomy, 2017, 7, 1–38 CrossRef.
- P. Borm, F. C. Klaessig, T. D. Landry, B. Moudgil, J. Pauluhn, K. Thomas, R. Trottier and S. Wood, Toxicol. Sci., 2006, 90, 23–32 CrossRef CAS.
- M. Adil, S. Bashir, S. Bashir, Z. Aslam, N. Ahmad, T. Younas, R. M. A. Asghar, J. Alkahtani, Y. Dwiningsih and M. S. Elshikh, Front. Plant Sci., 2022, 13, 1–9 Search PubMed.
- M. A. Jenks, P. M. Hasegawa, S. M. Jain and M. Foolad, Advances in molecular breeding toward drought and salt tolerant crops, Springer, 2007 Search PubMed.
- M. Movahhedy-Dehnavy, S. A. M. Modarres-Sanavy and A. Mokhtassi-Bidgoli, Ind. Crops Prod., 2009, 30, 82–92 CrossRef CAS.
- L. V. Subbaiah, T. N. V. K. V. Prasad, T. G. Krishna, P. Sudhakar, B. R. Reddy and T. Pradeep, J. Agric. Food Chem., 2016, 64, 3778–3788 CrossRef CAS.
- N. Taran, V. Storozhenko, N. Svietlova, L. Batsmanova, V. Shvartau and M. Kovalenko, Nanoscale Res. Lett., 2017, 12, 1–6 CrossRef CAS PubMed.
- R. Mittler, Trends Plant Sci., 2002, 7, 405–410 CrossRef CAS PubMed.
- A. Kahru and H.-C. Dubourguier, Toxicology, 2010, 269, 105–119 CrossRef CAS.
- M. Faizan, J. A. Bhat, C. Chen, M. N. Alyemeni, L. Wijaya, P. Ahmad and F. Yu, Plant Physiol. Biochem., 2021, 161, 122–130 CrossRef CAS.
- W. M. Semida, A. Abdelkhalik, G. F. Mohamed, T. A. Abd El-Mageed, S. A. Abd El-Mageed, M. M. Rady and E. F. Ali, Plants, 2021, 10, 1–18 Search PubMed.
- H. Zhang, J. Feng, T. Fei, S. Liu and T. Zhang, Sens. Actuators, B, 2014, 190, 472–478 CrossRef CAS.
- L. Foroutan, M. Solouki, V. Abdossi and B. A. Fakheri, International Journal of Basic Sciences in Medicine, 2018, 3, 178–187 CrossRef.
| This journal is © The Royal Society of Chemistry 2025 |