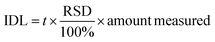Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Treated wastewater reuse for crop irrigation: a comprehensive health risk assessment†
Solomon
Ofori
 *a,
Ylenia
Di Leto
b,
Štěpánka
Smrčková
a,
Marco Antonio
Lopez Marin
*a,
Ylenia
Di Leto
b,
Štěpánka
Smrčková
a,
Marco Antonio
Lopez Marin
 a,
Giuseppe
Gallo
bc,
Iveta
Růžičková
a and
Jiří
Wanner
a
a,
Giuseppe
Gallo
bc,
Iveta
Růžičková
a and
Jiří
Wanner
a
aDepartment of Water Technology and Environmental Engineering, Faculty of Environmental Technology, University of Chemistry and Technology, Technická 5, 166 28 Prague 6 – Dejvice, Prague, Czech Republic. E-mail: solofori@yahoo.com
bDepartment of Biological, Chemical and Pharmaceutical Sciences and Technologies, Palermo University, 90128, Palermo, Italy
cNBFC, National Biodiversity Future Center, Piazza Marina 61, 90133, Palermo, Italy
First published on 25th November 2024
Abstract
The use of treated effluent/wastewater (TWW) for crop irrigation is gaining prominence globally due to growing freshwater scarcity. However, there are still questions about the safety of such a practice. This study sought to investigate and evaluate the health risks associated with the use of TWW for crop irrigation by assessing the potential risks arising from pathogens, heavy metals/potentially toxic elements (PTEs), micropollutants or pharmaceuticals and antibiotic resistance genes (ARGs), using tomato, carrot and cabbage as test crops. The levels of copper bioaccumulated in TWW irrigated crops were 25 mg kg−1 for tomato, 30 mg kg−1 for carrot and 20 mg kg−1 for cabbage, while those of the control (tap water) were 30 mg kg−1 for tomato, 40 mg kg−1 for carrot and 65 mg kg−1 for cabbage, respectively. Arsenic, cadmium and lead levels were below the detection limit for all treatments. The hazard quotient (HQ) and hazard index (HI) of copper and zinc were below 1 (adults) for TWW irrigated crops. Escherichia coli, Clostridium perfringens, coliform and thermotolerant bacteria were not detected on the fruits of tomato plants irrigated with TWW. All analysed pharmaceuticals were below the limit of detection except gabapentin, which was 3 μg kg−1 in TWW irrigated tomatoes. tetA, ermB, blaTEM, sul2, sul3 and qnrS genes were found in the metagenomic DNA extracted from TWW- and tap-irrigated cabbage. The results indicate no potential non-carcinogenic health risk for adult consumers and no microbial contamination of the tomato fruits under TWW irrigation. No difference was observed in the presence and distribution of the ARGs between TWW- and tap-irrigated crops, suggesting no contribution to the diffusion of different ARGs due to irrigation. Altogether, these findings highlight that health risk assessment of TWW for crop irrigation should focus on the quality of the TWW and on soil characteristics, which may contribute to risk exposure of different types of contaminants.
Environmental significanceThe growing scarcity of freshwater for agricultural use calls for the use of alternative water sources aside from freshwater resources to help achieve environmental sustainability. Treated wastewater is considered a viable substitute for freshwater for crop irrigation. However, due to the perceived potential risks associated with treated wastewater, its use for crop irrigation should be done with care to protect public health and maintain the environmental integrity of the different environmental compartments. The study improves our understanding of the risks associated with water reuse, an important component in the promotion of environmental sustainability. It also sheds light on how the practice of water reuse could impact human health taking into account the different environmental compartments such as water, soil and biota. |
1 Introduction
The use of treated wastewater (TWW) for irrigation is gaining prominence globally due to growing freshwater scarcity. Rapid population growth, urbanization and industrialization have led to increased demand for and depletion of freshwater resources.1 Various water management strategies have been developed to meet the growing water demands and to ensure water sustainability. These include sea or saline water de-salinization, water conservation measures such as efficient water use technologies (for example, pressured irrigation systems) and treated wastewater irrigation. As almost 70% of the global water resources are abstracted for agricultural use, the reuse of treated wastewater (TWW) for crop irrigation can significantly reduce the amount of water extracted from freshwater sources.2 Globally, the history of using treated wastewater reveals the feasibility of wastewater reuse in agriculture3 and countries such as Israel and Tunisia have demonstrated the sustainable use of TWW in agriculture.4,5 TWW irrigation could be essential for maintaining food security and promoting agriculture, especially in semi-arid and arid regions with limited freshwater sources.6 Reusing TWW for irrigation presents many benefits such as the supply of nutrients, an increase in crop yield and constant irrigation water supply.1 Such practice could help protect environmental quality and alleviate or minimize the pressure on limited freshwater sources for agricultural irrigation.7 However, there is a perceived risk associated with the practice, which has fueled a lack of public acceptance in some regions of the globe.8,9 There is a reluctance to accept agricultural products produced using TWW.10 This perceived risk is associated with the composition or the quality of the water. TWW could be a reservoir of pathogens, organic, non-biodegradable and persistent pollutants such as potentially toxic elements (PTEs) or heavy metals, bacteria, viruses and contaminants of emerging concern (CECs) that could potentially enter the human food chain through irrigation.9,11–13Metallic elements with a density greater than 5 g cm−3 are referred to as heavy metals and these include lead (Pb), copper (Cu), cadmium (Cd), arsenic (As), chromium (Cr), etc.14 Irrigating crops with TWW could lead to the accumulation of these elements in arable soils and their bioaccumulation and biomagnification in the food chain.1,15 Rezapour et al. investigated the bioavailability and accumulation of five heavy metals (zinc (Zn), nickel (Ni), copper, lead and cadmium) in winter wheat crops and calcareous soils irrigated with TWW.16 The authors reported a significant accumulation of heavy metals in the soil and a considerable build-up in the wheat crops. Significant accumulation was noted in the wheat roots when compared to the shoot and grains. In vegetables, heavy metals could be taken up by the roots and accumulate in the edible parts.17 Such bioaccumulation may pose a threat to public health since the human body could absorb the heavy metals/PTEs through food ingestion and skin contact with the soil.14,18 Bioaccumulation of these elements in the bones, liver and kidneys to harmful levels could lead to serious health problems.14 Malfunctions of cell respiration, nerves, kidneys and muscles are all associated with heavy metal/PTE toxicity.19
Another risk associated with the use of TWW for irrigation is microbial contamination of food. Depending on the nature of the treatment processes, TWW could harbour a significant amount of pathogenic and indicator microorganisms such as Enterococci, Escherichia coli, Coliforms, Clostridium perfringens, Salmonella spp., etc., which could pose serious health risks to humans and agricultural animals.20 Several studies involving TWW irrigation have reported higher microbial content above the local or international wastewater reuse guidelines.21–23 A significant number of faecal enterococci, E. coli and coliforms were found in a secondary effluent used for irrigating tomatoes and broccoli plants.23 Pathogen internalization could occur through root uptake and leaf contact as a result of exposure of the crops to pathogens by the irrigation water. Interactions between the irrigated crops and the exposed pathogens vary among different cultivars of the same crop species.24
The exposure of humans to pathogens under treated wastewater irrigation occurs through direct contact with the water (in the case of farm workers) and mouth ingestion of contaminated food crops (consumers). Diarrhea and extraintestinal diseases are some health risks the public could encounter if E. coli contamination of food occurs through irrigation.25 With health risk barrier management strategies such as disinfection, drip irrigation and post-harvest food washing, these health risks could be eliminated or reduced to the barest minimum.
In recent times, the risk of exposure to CECs associated with TWW irrigation has gained attention. These are groups of organic compounds and substances with known or perceived ecological and health risks, comprising antibiotic-resistant genes (ARGs) and antibiotic-resistant bacteria (ARB), antibiotics, personal care products, endocrine disrupting compounds (EDCs), pharmaceuticals and their metabolites.6,9,13,26 The presence of CECs in TWW has been reported in the literature.27–29 Diaz-Sosa et al. detected atenolol, caffeine, carbamazepine, tramadol and sulfamethoxazole together with other pharmaceuticals in the secondary TWW of the Prague central wastewater treatment plant.28 ARGs such as sul1, ermB, uidA, mefC, and tetX have also been detected in TWW from Portugal, Denmark, the Czech Republic, the Netherlands and Israel.29 These findings elucidate the biological safety risk associated with the practice of TWW irrigation. Studies have shown that TWW irrigation could lead to ARG dissemination in soil microbiota, while others have reported the opposite.6,30 A study by Fatta-Kassinos et al. highlighted the bioaccumulation of CECs in soil and crops irrigated with TWW.26 The accumulation of antibiotics in agroecosystems and their potential uptake by food crops is a public health concern.13
Several studies have been conducted on risk assessment of TWW irrigation. Sallach et al., Cerqueira et al., Marano et al., Gudda et al., and Liu et al. focused on antibiotics and ARGs; Razapour et al., Chen et al., and Mosa et al. focused on heavy metals or PTEs; Forslund et al., Farhadkhani et al., and Tripathi et al. focused on pathogens or microbial contamination; Yan et al. focused on heavy metals and CECs; and Sallach et al. focused on antimicrobials and pathogens.6,13,16,24,31–40 However, due to the complexity of the potential risks associated with the practice, no single study has evaluated the comprehensive risk of the practice from the point of view of heavy metal/PTE risk, microbial risk and CEC risk, to the best of our knowledge. Previous studies have focused on either one or two of these risk areas. It is hypothesized that TWW irrigation presents little or no health and environmental risk, and incorporation of the aforementioned risk areas in a single study provides a better option for risk assessment. The study, therefore, provides a comprehensive health risk assessment of TWW irrigation considering the potential risk of exposure to heavy metals/PTEs, microbial contamination and contaminants of emerging concern. The objectives are to evaluate (i) the bioaccumulation and bioaccessibility of heavy metals in soil and edible parts of crops (tomato, cabbage, and carrot), (ii) the potential risk of microbial contamination of crops (tomato), (iii) the bioaccumulation of antibiotics or pharmaceuticals in the edible parts of crops and (iv) the presence of ARGs in the edible parts of crops (cabbage), under TWW irrigation. The outcome of the study will contribute greatly to the assessment of the suitability of TWW for irrigation and ensure public safety concerning the practice.
2 Methods
2.1 Experimental design
The study involved irrigating three different vegetable crops with secondary effluent and tap water cultivated in a greenhouse under a tropically mimicked environment. Secondary effluent was obtained from a municipal wastewater treatment plant (WWTP) in the Czech Republic and tap water from the University of Chemistry and Technology, Prague. Crop treatments were made up of secondary effluent-irrigated crops (SE) and tap water-irrigated crops (Tap). Tap water-irrigated crops (Tap) represented the control group. The test crops were cabbage (Brassica oleracea L.), carrot (Daucus carota subsp. sativus) and cherry tomato (Solanum lycopersicum) and the seeds were purchased from a commercial supermarket. Cherry tomato and cabbage seeds were subject to seeding on a filter paper placed in a Petri dish. The sprouted seeds were transferred to a nursing substrate in the laboratory and later transported to the greenhouse for planting. Carrot seeds were sown directly into the soil in the greenhouse without nursing. Each treatment (tomato and cabbage) consisted of six pots, three for each plant except carrot which consisted of 4 pots. Each pot had a height of 20 cm and a volume of 3.4 L (0.0034 m3) and was filled with soil to about 85% of the volume. The soil is composed of clay (14.0%), silt (37.2%), and sand (48.7%) fractions, falling into the loam soil classification. The pots were set up on a growth bench in a greenhouse and illuminated for 12 hours during the day with a Growth Spectrum Advanced 600 W lamp (GIB Lighting, Berlin, Germany). The time was adjusted to a 9 h daytime setting after 66 days. This was done to reduce the rapid rate at which water evaporated from the soil and plants. The lamp has a photon flux (100 h) of 740 μmol s−1, a color temperature of 8000 K, a light intensity of 48![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 lm, and a nominal power of 600 W. Since the lamp created a high rate of evaporation, irrigation was often done once a day to provide a sufficient supply of water. The average growth temperature and relative humidity were 26 °C and 35%, respectively. No compost, manure, fertilizer, or other soil amendment was employed during the experiment. The sowing of the seeds occurred in September 2021, and the matured crops were harvested in February of the following year. The experimental design is from the studies of Ofori et al.41,42 and a detailed description can be found in these articles. A second study on antibiotic resistance gene dissemination involved irrigating cabbage crops with secondary effluent and tap water. The experiment was conducted in a growth room at 24 °C temperature and 75% relative humidity. The seeds were sown directly in loam soil contained in a pot (the same dimensions as aforementioned) and the growth period lasted for about three months. The young cabbage plants were harvested after this period and analysed for the presence of ARGs. Evaluation of the health risk in both studies focused primarily on oral ingestion of the edible part of the vegetable crops and exposure to contaminated soil.
000 lm, and a nominal power of 600 W. Since the lamp created a high rate of evaporation, irrigation was often done once a day to provide a sufficient supply of water. The average growth temperature and relative humidity were 26 °C and 35%, respectively. No compost, manure, fertilizer, or other soil amendment was employed during the experiment. The sowing of the seeds occurred in September 2021, and the matured crops were harvested in February of the following year. The experimental design is from the studies of Ofori et al.41,42 and a detailed description can be found in these articles. A second study on antibiotic resistance gene dissemination involved irrigating cabbage crops with secondary effluent and tap water. The experiment was conducted in a growth room at 24 °C temperature and 75% relative humidity. The seeds were sown directly in loam soil contained in a pot (the same dimensions as aforementioned) and the growth period lasted for about three months. The young cabbage plants were harvested after this period and analysed for the presence of ARGs. Evaluation of the health risk in both studies focused primarily on oral ingestion of the edible part of the vegetable crops and exposure to contaminated soil.
2.2 Determination of the potential risk of heavy metals or potentially toxic elements
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (v/w) ratio, a 0.01 M CaCl2 extractant was used to extract the elements from the soil according to the procedures of Houba et al. and Motsara and Roy.43,44 CaCl2 solution was added to the soil sample, shaken mechanically for about 2 hours and filtered through a filter paper of 150 mm diameter with a pore size of about 15 μm (Papírna Perštejn s.r.o., Czech Republic). The Pb, Zn and Cd levels in the soil extract were analysed by atomic absorption spectroscopy (AAS-Agilent 280FS AA, Agilent Technologies).
1 (v/w) ratio, a 0.01 M CaCl2 extractant was used to extract the elements from the soil according to the procedures of Houba et al. and Motsara and Roy.43,44 CaCl2 solution was added to the soil sample, shaken mechanically for about 2 hours and filtered through a filter paper of 150 mm diameter with a pore size of about 15 μm (Papírna Perštejn s.r.o., Czech Republic). The Pb, Zn and Cd levels in the soil extract were analysed by atomic absorption spectroscopy (AAS-Agilent 280FS AA, Agilent Technologies).
The procedures for the plant biomass sample preparation and extraction followed those of Hunt, and Motsara and Roy.44,45 Harvested biomass was rinsed several times with tap water, followed by 0.2% detergent solution to remove dirt and waxy or greasy coatings. Biomass samples were then washed with 0.1 M HCl, followed by thorough washing with tap water and final rinsing (twice) with distilled water. The samples were air-dried at room temperature in a dust-free environment for about 72 hours and oven-dried at 70 °C for about 48 hours. The dried samples were then ground with a mill, ashed in a furnace and stored for heavy metal analyses. The ash was then dissolved in a 0.5 M HCl solution, shaken and filtered through a filter paper with a pore size of about 15 μm (Papírna Perštejn s.r.o., Czech Republic) into clean 50 mL tubes. Estimation of the concentration of the heavy metals (Pb, Cu, Zn, As and Cd) in the extract was performed by atomic absorption spectroscopy (AAS-Agilent 280FS AA, Agilent Technologies). The limit of detection was the smallest possible signal that could be differentiated and was determined from the initial phase of the calibration curve. The calibration parameters of the heavy metals are presented as ESI files.†
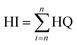 | (1) |
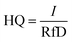 | (2) |
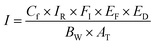 | (3) |
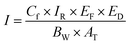 | (4) |
| Input parameters | Cu | Zn |
|---|---|---|
| a Tap-Tom, Tap-Carr, and Tap-Cabb refer to tap water irrigated tomatoes, carrots and cabbage, respectively. SE-Tom, SE-Carr and SE-Cabb refer to secondary effluent irrigated tomatoes, carrots and cabbage, respectively. Tom-Raw, Carr-Raw and Cabb-Raw refer to uncooked tomatoes, carrots and cabbage, respectively. Tom-Cooked, Carr-Cooked and Cabb-Cooked refer to cooked tomatoes, carrot and cabbage, respectively. BW, ED, EF, IR and RfD were obtained from US EPA, Rezapour et al. and Adam et al.14,16,46 | ||
| C f-Tap-Tom (mg kg−1) | 30 | 230 |
| C f-Tap-Carr (mg kg−1) | 40 | 200 |
| C f-Tap-Cabb (mg kg−1) | 65 | 85 |
| C f-SE-Tom (mg kg−1) | 25 | 215 |
| C f-SE-Carr (mg kg−1) | 30 | 165 |
| C f-SE-Cabb (mg kg−1) | 20 | 65 |
| I R-Tom Raw (kg per day) | 0.044 | 0.044 |
| I R-Tom Cooked (kg per day) | 0.046 | 0.046 |
| I R-Carr Raw (kg per day) | 0.018 | 0.018 |
| I R-Carr Cooked (kg per day) | 0.03 | 0.03 |
| I R-Cabb Raw (kg per day) | 0.027 | 0.027 |
| I R-Cabb Cooked (kg per day) | 0.05 | 0.05 |
| E F | 365 | 365 |
| E D-Adult (year) | 70 | 70 |
| E D-Child (year) | 6 | 6 |
| B W-Adult (kg) | 70 | 70 |
| B W-Child (kg) | 15 | 15 |
| A T-Adult (days) | 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 550 550 |
25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 550 550 |
| A T-Child (days) | 2190 | 2190 |
| RfD | 0.04 | 0.3 |
| B W × AT-Adult | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 788 788![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 788 788![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
| B W × AT-Child | 32![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 850 850 |
32![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 850 850 |
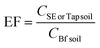
The risks of bioaccessibility and bioaccumulation of heavy metals in irrigated soil were evaluated using a modified enrichment factor (EF) from Rezapour et al.16 EF estimation (eqn (5)) was performed using the initial level of heavy metals in the soil (before irrigation) as the reference.
 | (5) |
2.3 Determination of the potential risk of exposure to pathogens
2.4 Determination of the potential risk of contaminants of emerging concerns
RSD is the relative standard deviation of the peak area obtained from 8 measurements (injection of standards); t (2.999) is the critical value of Student's t-distribution at the 99% confidence level for seven degrees of freedom.
| Parameter | Value (+) | Value (−) |
|---|---|---|
| a The limit of detection (LOD) was estimated using the instrument detection limit (IDL). | ||
| Gas temperature (°C) | 230 | 230 |
| Gas flow (L min−1) | 8 | 8 |
| Nebulizer pressure (psi) | 40 | 40 |
| Sheath gas heater (°C) | 380 | 380 |
| Sheath gas flow (L min−1) | 12 | 12 |
| Capillary voltage (V) | 3000 | 3000 |
Quantification of the targeted pharmaceutical products in the biomass was performed by an external laboratory (Povodi Vltavy) in Pilsen, Czech Republic. Sample preparation involved rinsing the fresh cherry tomatoes with distilled water, storing them in clean tubes and freezing them until transportation to the laboratory for the analyses. The samples of biomass were lyophilized and homogenized by grinding. Subsequently, 0.1 g of each sample was weighed in a 4 mL vial on an analytical balance. Then 1 mL of acetonitrile was added to each sample. An isotope dilution was performed in the next step. Analytes were extracted from biomass by sonification in an ultrasonic bath for 30 minutes. The second extraction of pharmaceuticals was done with 1 mL acid water (water + 0.3% formic acid) for 30 minutes. Both extracts were joined together and centrifuged in vials for 10 min at about 3500 rpm. The last step of preparation was the dilution of the extracts with acid water (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) and transferring the aliquot part of each extract to a 2 mL autosampler vial. Two independent standards with certified concentrations were used for the determination of each analyte, one for calibration and another for standard addition. Standard solutions were prepared both from neat analytes and from commercial solutions with certified concentrations.
3) and transferring the aliquot part of each extract to a 2 mL autosampler vial. Two independent standards with certified concentrations were used for the determination of each analyte, one for calibration and another for standard addition. Standard solutions were prepared both from neat analytes and from commercial solutions with certified concentrations.
Pharmaceuticals were separated and detected by the LC-MS/MS method based on direct injection of the sample extract into the 1290 ultra-high-performance liquid chromatography (UHPLC) coupled with an Agilent 6495B Triple Quad Mass Spectrometer (MS/MS) of Agilent Technologies, Inc. (Santa Clara, CA, USA). The separation was carried out on a Waters Xbridge C18 analytical column (100 mm × 4.6 mm, 3.5 μm particle size). The mobile phase consisted of methanol and water with 0.5 mM ammonium fluoride and 0.02% acetic acid as the mobile phase additives. The flow rate was 0.5 mL min−1. The injection volume was 0.010 mL.
Quantification was done using MassHunter Workstation Version 10.1 software. Each series of samples was verified through calibration control and by maintaining a clean laboratory environment, equipment, and agents. The performance of the analytical system was validated using blank and spiked samples. Every third sample in every batch was processed by the method of standard addition for all analytes, which was used to check the effect of the matrix of the sample and to reset the actual recovery ratio of a specific analyte. The measurements were performed according to the Czech standard ČSN ISO 20179.52 The LOD was estimated from either the calculation of the signal-to-noise ratio or the blank calculation (the mean and standard deviation of a set of 10–15 blanks). Table 3 presents the water quality characteristics of the two irrigation water streams.
| Parameter | Tap water | Secondary effluent |
|---|---|---|
| a Values are concentrations expressed in means plus the standard deviation; LOD is the limit of detection. LOD of arsenic (0.01 mg L−1), cadmium (0.01 mg L−1), copper (0.01 mg L−1), lead (0.05 mg L−1), zinc (0.01 mg L−1), ibuprofen (5.0 ng L−1), naproxen (2.0 ng L−1), diclofenac (0.5 ng L−1), paracetamol (0.08 ng L−1), carbamazepine (0.08 ng L−1), gabapentin (0.2 ng L−1), tramadol (0.2 ng L−1), caffeine (2.0 ng L−1), estriol (5.0 ng L−1), testosterone (0.2 ng L−1), sulfamethoxazole (0.08 ng L−1), trimethoprim (0.2 ng L−1), saccharin (20.0 ng L−1) and warfarin (0.2 ng L−1). Heavy metal and microbial data were obtained from Ofori et al.41,42 | ||
| Heavy metals/PTEs | ||
| Arsenic (mg L−1) | <LOD | <LOD |
| Cadmium (mg L−1) | <LOD | <LOD |
| Copper (mg L−1) | <LOD | <LOD |
| Lead (mg L−1) | <LOD | <LOD |
| Zinc (mg L−1) | 0.04 ± 0.01 | 0.13 ± 0.04 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||
| Contaminants of emerging concern | ||
| Ibuprofen (ng L−1) | <LOD | <LOD |
| Naproxen (ng L−1) | <LOD | 113.75 ± 18.87 |
| Diclofenac (ng L−1) | <LOD | 1950 ± 100.00 |
| Paracetamol (ng L−1) | <LOD | <LOD |
| Carbamazepine (ng L−1) | 11.25 ± 2.47 | 460 ± 14.14 |
| Gabapentin (ng L−1) | 79 ± 1.41 | 1900 ± 81.65 |
| Tramadol (ng L−1) | 0.41 ± 0.01 | 2625 ± 170.78 |
| Caffeine (ng L−1) | 12.50 ± 0.71 | 3.90 ± 2.55 |
| Estriol (ng L−1) | <LOD | <LOD |
| Testosterone (ng L−1) | <LOD | <LOD |
| Sulfamethoxazole (ng L−1) | 0.39 ± 0.10 | 770 ± 112.25 |
| Trimethoprim (ng L−1) | <LOD | 675 ± 64.55 |
| Saccharin (ng L−1) | <LOD | 645 ± 242.83 |
| Warfarin (ng L−1) | <LOD | <LOD |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||
| Microbial characteristics | ||
| Thermotolerant coliform (CFU mL−1) | nd | 5.80 ± 4.15 × 104 |
| Escherichia coli (CFU mL−1) | nd | 2.76 ± 2.33 × 104 |
| Coliform (CFU mL−1) | nd | 9.85 ± 6.52 × 104 |
| Clostridium perfringens (CFU mL−1) | nd | 1.22 ± 0.87 × 103 |
DNA was extracted from the samples using the phenol–chloroform method. The extraction of DNA from the soil and biomass was performed according to the method described by Islam et al. and Di Leto et al. with slight modifications.53,54 The pellet of crude nucleic acids was precipitated by centrifuging at 16000g. On the other hand, the extraction of DNA from water samples was done using a Thermo Scientific Genomic DNA purification kit. The manufacturer's protocol was followed. The concentration of the extracted DNA was measured using a NanoDrop 2000c spectrophotometer (Thermo Fisher Scientific) at 260 nm. The purity of the isolated DNA was assessed using the 260/230 and 260/280 ratios for organic and protein contaminations, respectively. Extracted DNA was used as a template for the PCR analyses of ARGs' genes and of the V3–V4 region of the 16S rRNA gene to verify the amplifiability.55 The obtained amplicons were visualized by agarose gel electrophoresis (1.5–2.5% depending on amplicon size). Gels were stained using GelRed (Invitrogen) and then visualized using UV light.
2.5 Statistical analyses of data
The confidence factor t was determined using Student's t-distribution with a 99% confidence level and n − 1 (8 − 1) degrees of freedom. The critical value of Student's t-distribution at the 99% confidence level for seven degrees of freedom was 2.999. Chart and table representations were created and data analyses were performed using Microsoft Excel 2019. The test of significance of the difference of means between two study variables was performed by Student's t-test (paired) at a confidence interval of 95% (p < 0.05). Analysis of variance (ANOVA) was used to compare the means among multiple variable datasets. To establish the significance (ANOVA) of the difference observed among the means of the different treatments, a confidence level of 95% (p < 0.05) was adopted.3 Results and discussion
3.1 Bioaccessibility and bioaccumulation of heavy metals/PTEs in irrigated crops
The amounts of As, Cd and Pb taken up and bioaccumulated in the edible tissues or biomass of tomatoes, carrots and cabbage were below the detection limit of 0.04 mg L−1, 0.006 mg L−1 and 0.4 mg L−1, respectively. These levels are consistent with the concentrations found in the irrigation water (Table 3) and the soil (refer to Ofori et al.).42 The relatively low levels of As, Cd and Pb in the irrigation water and soil may account for the insignificant amount accumulated in the edible tissues of the test crops. Copper levels in tap water and TWW were also below the limit of detection while those of Zn were 0.04 ± 0.01 mg L−1 and 0.13 ± 0.04 mg L−1, respectively. The quantity of Cu accumulated in crops irrigated with tap water was 0.06 mg L−1 ≈ 30 mg kg−1 for tomato, 0.08 mg L−1 ≈ 40 mg kg−1 for carrot and 0.13 mg L−1 ≈ 65 mg kg−1 for cabbage, while that of treated effluent was 0.05 mg L−1 ≈ 25 mg kg−1 for tomato, 0.06 mg L−1 ≈ 30 mg kg−1 for carrot and 0.04 mg L−1 ≈ 20 mg kg−1 for cabbage, respectively. Tap water irrigated crops accumulated more Cu than treated effluent irrigated crops, with the difference in accumulation being significant for cabbage. The order of accumulation differed between the two irrigation water streams; tap water was cabbage > carrot > tomato; and effluent was carrot > tomato > cabbage. Crop species or physiology, metal translocation and transpiration rate may have influenced the amount of Cu uptake by the different test crops leading to the observed differences in bioaccumulation.15A similar trend of bioaccumulation was also observed for Zn, with tap-water irrigated crops showing higher Zn accumulation. Tap water irrigated crops accumulated 0.46 mg L−1 Zn ≈ 230 mg kg−1 for tomato, 0.4 mg L−1 Zn ≈ 200 mg kg−1 for carrot and 0.17 mg L−1 Zn ≈ 85 mg kg−1 for cabbage, while treated effluent irrigated crops accumulated 0.43 mg L−1 Zn ≈ 215 mg kg−1 for tomato, 0.33 mg L−1 Zn ≈ 165 mg kg−1 for carrot and 0.13 mg L−1 Zn ≈ 65 mg kg−1 for cabbage, respectively. The order of accumulation was the same for both irrigation water, tomato > carrot > cabbage. It was evident that the phenomenon of heavy metal (Cu and Zn) uptake and its accumulation in plant tissues occurred in this study. In a similar study conducted in Iran, the authors also observed an accumulation of Cu and Zn in cabbage after irrigation with wastewater. They reported a higher accumulation of Zn (51.2 mg kg−1) than Cu (10.0 mg kg−1) in wastewater-irrigated cabbage, which is consistent with our results.15 However, the bioaccumulation of these PTEs in the control water (freshwater) irrigated crops was lower than that in the wastewater irrigated cabbage, which is contrary to our findings. Razapour et al. also reported bioaccumulation of Cu and Zn in wheat crops after TWW irrigation, but the mean concentrations were significantly lower compared to the results obtained for the present study.16
The results of the present study suggest a potential risk of exposure of consumers to Cu and Zn. Consuming these irrigated crops could lead to the absorption of Cu and Zn and their biomagnification in the human body. Continuous accumulation in the human body could reach toxic levels and cause serious health problems. Cu and Zn are needed by the human body for vital biological processes, but toxic levels could result in liver cirrhosis, brain damage, anaemia, lethargy and risk of prostate cancer, respectively.56,57
The results of the study showed that the use of the treated effluent posed a relatively lower risk of heavy metal/PTE accumulation in plants than the control (tap water) and that the concentration of Cu and Zn in the irrigation water did not have a direct relationship with the quantity of Cu and Zn accumulated in the edible parts of the crops. We therefore postulate that the main driver that influenced the uptake and the subsequent bioaccumulation of these elements was not the irrigation water but rather other drivers such as plant physiology and soil properties. Factors such as soil properties may have strongly accounted for the uptake and translocation to the edible parts. Such a conclusion is partly consistent with the findings of previous studies. Jalil et al. cited soil characteristics (pH, electrical conductivity, and cation exchange capacity), crop roots and soil interface, metal bioavailability, transpiration rate and metal translocation as factors that may affect the uptake and translocation of metals from soil to crops.15 Other authors cited plant physiology, the quantity of bioavailable heavy metals in the soil, and soil physicochemical attributes as factors that influence the uptake and bioaccumulation of heavy metals or PTEs.58,59 The findings of our study highlight the importance of considering soil, plant and heavy metal characteristics in the evaluation of the risk of bioavailability and bioaccumulation of heavy metals under TWW irrigation.
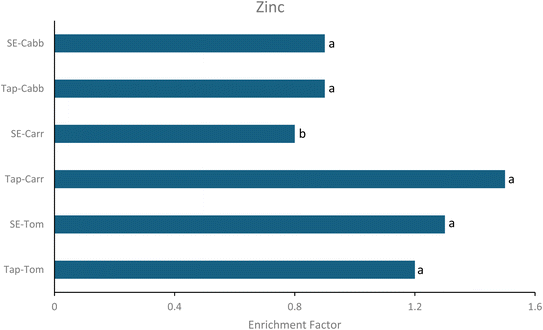
3.2 Zinc accumulation in tap water and TWW irrigated soil
Tap-Tom, Tap-Carr and SE-Tom irrigated soils recorded accumulation of Zn in the order Tap-Carr > SE-Tom > Tap-Tom (Fig. 1). There was a significant (p < 0.05) buildup of Zn in SE-Tom irrigated soil. However, accumulation of Zn did not occur in most of the TWW irrigated soils (SE-Carr and SE-Cabb); instead, a depletion was observed. Comparatively, TWW irrigated soils exhibited no or relatively little accumulation and pose a lower risk of Zn uptake compared to tap water irrigated soils. These findings do not support the general notion held by many that TWW irrigation leads to the accumulation of heavy metals in the soil and will lead to higher uptake of PTEs by crops. Contrary to our results, Rezapour et al. found a significant accumulation of heavy metals in the soil after five years of TWW irrigation.16 Therefore, the results of the current study elucidate an important finding that arable lands subjected to TWW irrigation may not spontaneously make PTEs bioavailable for crops. The order of Zn enrichment or accumulation was Tap-Tom > SE-Tom > Tap-Cabb > Tap-Carr > SE-Carr > SE-Cabb.3.3 Health risk exposure assessment
The result of the non-carcinogenic health risk is presented under two scenarios: (1) risk via ingestion of raw or uncooked vegetables/crops (Table 4, a) and (2) risk via ingestion of cooked vegetables/crops (Table 4, b). Under scenario 1, the hazard quotient (HQ) of Cu in adults ranged from 0.25 to 0.47 for tap-water irrigated crops and 0.19 to 0.39 for TWW-irrigated crops. In children, the HQ for tap-water irrigated crops ranged from 1.20 to 2.92 and 0.90 to 1.83 for TWW irrigated crops (Table 4, a). The latter had a lower hazard quotient than tap-water irrigated crops. A similar trend was observed for Zn. The HQ of Zn in adults ranged from 0.10 to 0.48 and 0.08 to 0.45 for crops irrigated with tap water and TWW, respectively. In children, the HQ ranged from 0.51 to 2.24 for tap water irrigated crops and 0.39 to 2.10 for TWW irrigated crops. Among the TWW irrigated crops, tomatoes recorded the highest quotient in both adults and children for Cu and Zn. In tap water and TWW irrigated crops, the HQ for children was higher than for adults, which is consistent with existing literature.14,16 Rezapour et al. reported HQ values of 0.060 (Zn) and 0.168 (Cu) for adults and 0.132 (Zn) and 0.375 (Cu) for children for wheat grains produced by TWW irrigation.16| Element | Tap-Tom | Tap-Carr | Tap-Cabb | SE-Tom | SE-Carr | SE-Cabb |
|---|---|---|---|---|---|---|
| (a) Raw vegetables | ||||||
| As | HQ | HQ | HQ | HQ | HQ | HQ |
| Adult | nd | nd | nd | nd | nd | nd |
| Child | nd | nd | nd | nd | nd | nd |
| Cd | HQ | HQ | HQ | HQ | HQ | HQ |
| Adult | nd | nd | nd | nd | nd | nd |
| Child | nd | nd | nd | nd | nd | nd |
| Cu | HQ | HQ | HQ | HQ | HQ | HQ |
| Adult | 0.47143 | 0.25714 | 0.62679 | 0.39286 | 0.19286 | 0.19286 |
| Child | 2.20000 | 1.20000 | 2.92500 | 1.83333 | 0.90000 | 0.90000 |
| Pb | HQ | HQ | HQ | HQ | HQ | HQ |
| Adult | nd | nd | nd | nd | nd | nd |
| Child | nd | nd | nd | nd | nd | nd |
| Zn | HQ | HQ | HQ | HQ | HQ | HQ |
| Adult | 0.48190 | 0.17143 | 0.10929 | 0.45048 | 0.14143 | 0.08357 |
| Child | 2.24889 | 0.80000 | 0.51000 | 2.10222 | 0.66000 | 0.39000 |
| Exposure | HI | HI | HI | HI | HI | HI |
| Adult | 0.95333 | 0.42857 | 0.73607 | 0.84333 | 0.33429 | 0.27643 |
| Child | 4.44889 | 2.00000 | 3.43500 | 3.93556 | 1.56000 | 1.29000 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||||
| (b) Cooked vegetables | ||||||
| As | HQ | HQ | HQ | HQ | HQ | HQ |
| Adult | nd | nd | nd | nd | nd | nd |
| Child | nd | nd | nd | nd | nd | nd |
| Cd | HQ | HQ | HQ | HQ | HQ | HQ |
| Adult | nd | nd | nd | nd | nd | nd |
| Child | nd | nd | nd | nd | nd | nd |
| Cu | HQ | HQ | HQ | HQ | HQ | HQ |
| Adult | 0.49286 | 0.42857 | 1.16071 | 0.41071 | 0.32143 | 0.35714 |
| Child | 2.30000 | 2.00000 | 5.41667 | 1.91667 | 1.50000 | 1.66667 |
| Pb | HQ | HQ | HQ | HQ | HQ | HQ |
| Adult | nd | nd | nd | nd | nd | nd |
| Child | nd | nd | nd | nd | nd | nd |
| Zn | HQ | HQ | HQ | HQ | HQ | HQ |
| Adult | 0.50381 | 0.28571 | 0.20238 | 0.47095 | 0.23571 | 0.15476 |
| Child | 2.35111 | 1.33333 | 0.94444 | 2.19778 | 1.10000 | 0.72222 |
| Exposure | HI | HI | HI | HI | HI | HI |
| Adult | 0.99667 | 0.71429 | 1.36310 | 0.88167 | 0.55714 | 0.51190 |
| Child | 4.65111 | 3.33333 | 6.36111 | 4.11444 | 2.60000 | 2.38889 |
The outcome of the assessment of the potential risk posed by a single contaminant suggested that the consumption of the crops irrigated by the TWW does not raise health concerns in adults. The hazard quotient was significantly lower than the threshold limit (1). Adults consuming tomatoes, cabbage and carrots are at no risk of Cu or Zn toxicity.17 However, in children, there is a potential health risk with the consumption of tomatoes. For Cu and Zn, the quotient was greater than 1, implying a health risk concern in children. The study highlights the disparity in the risk of exposure between adults and children to crops irrigated by TWW. Differences in the consumption rate and body weight account for this disparity. A significant outcome of the study is the need to always evaluate the risk of exposure of consumers to agricultural products produced under TWW irrigation by considering the age groups of consumers (adults and children) since their susceptibility to the perceived risk is different.
Assessment of the overall risk of exposure (hazard index) to Cu and Zn through the ingestion pathway revealed no risk of non-carcinogenic health effects (HI < 1) in adults. A contrary outcome was obtained for children. In all the treatments, the health hazard index for children was greater than 1, implying concern for a potential health risk. Therefore, the consumption of the crops irrigated (raw or uncooked) in this study is only safe for adult consumption. It is safer to consume the carrots and cabbage than the tomatoes. The order of risk in adults and children among the different crops was the same: Tap-Tom > SE-Tom > Tap-Cabb > Tap-Carr > SE-Carr > SE-Cabb.
Under scenario 2, the trend of the hazard quotient among the different treatments was very similar to that of scenario 1. The hazard quotient of crops irrigated with tap water ranged from 0.42 to 1.16 for Cu in adults and 2.00 to 5.41 for Cu in children (Table 4, b). In TWW irrigated crops, the HQ of Cu ranged from 0.32 to 0.41 in adults and 1.50 to 1.91 in children (Table 4, b). The HQ of Zn in adults was below 1.0 for all crops under tap and TWW irrigation. Similar results have been published by Kim et al. and Rezapour et al.16,48 Unlike Cu, the HQs of Zn for Tap-Cabb and SE-Cabb in children were below 1.0 (0.94 and 0.72, respectively), while the rest were above the 1.0 safe limit. The results of the assessment of the potential risk posed by a single contaminant indicated that it is safe for adults to consume the edible parts (cooked) of the crops irrigated in this study. However, the consumption of the crops by children is unsafe.
Evaluation of the combined risk of Cu and Zn revealed that the HI of all the treatments for adults was below the threshold limit of 1, except for Tap-Cabb. In contrast, none of the irrigated crops fulfilled the <1 HI safe limit in the case of children. The consumption of the cooked form of all the TWW irrigated crops by adults poses no threat of Cu or Zn toxicity or health risk.17,35
Our results in both scenarios imply that the consumption of raw and cooked tomatoes, carrots and cabbage from the current study poses no non-carcinogenic health risk for adults but is unsafe for children. Comparatively, the HQ and HI of consuming raw tomatoes, carrots and cabbage were lower than those of consuming the cooked counterpart due to the relatively high consumption rate associated with cooked food. These findings are crucial and beneficial as the European Union directives on TWW for irrigation came into effect this year. Member countries can utilize this study's outcome in assessing the suitability of TWW irrigation from a health risk standpoint. Since irrigating crops with TWW is a global phenomenon, the outcome of our study could be replicated in other regions of the globe outside the European Union.
3.4 Risk of exposure to pathogens
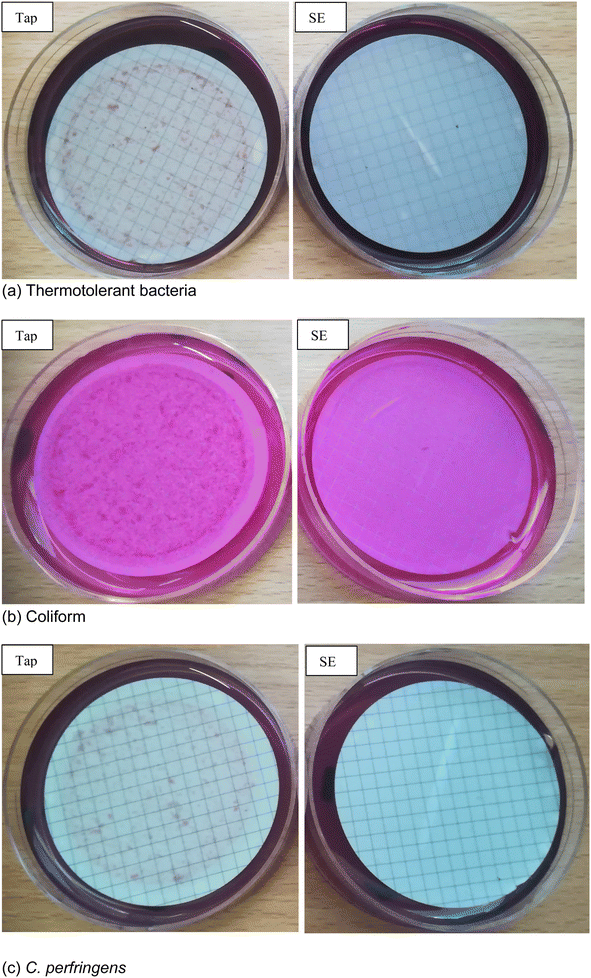
The evaluation of the risk of exposure to pathogens through ingestion of tomato fruits revealed no potential risk to consumers, even though the level of microbial loads of the treated effluent was far above the safety limits stipulated in the EU and WHO directives and guidelines. In Table 5 and Fig. 2, none of the targeted indicator microorganisms were detected on the tomato fruits. This observation is similar to the findings of similar studies reported in the literature.21–23 The type of irrigation system employed in the current study may account for this outcome. The tomato plants were irrigated directly at the base of the plant (analogous to drip irrigation), so there was no direct contact of the aboveground part of the plant with the irrigation water. Since there was no direct contact of the fruits with the water, the transfer of pathogens from the irrigation water to the fruits did not occur. This provides significant knowledge in understanding the pathogenic contamination risk associated with TWW irrigation and elucidates the non-spontaneous microbial contamination risk associated with consuming TWW irrigated crops. The results indicate the possibility of eliminating microbial contamination of TWW irrigated crops by using appropriate irrigation methods. Gatta et al. reported the absence of E. coli and Salmonella spp. on artichoke heads after irrigation with TWW. The authors partly attributed their findings to the drip irrigation system used which prevented fruits or plants from having direct contact with irrigation water.21| Coliform bacteria [CFU 100 mL−1] | Thermotolerant coliform bacteria [CFU 100 mL−1] | Escherichia coli [CFU 100 mL−1] | Clostridium perfringens [CFU 100 mL−1] | |
|---|---|---|---|---|
| Tap | nd | nd | nd | nd |
| SE | nd | nd | nd | nd |
The observed outcome in the present study would have been different if an irrigation method that allows direct contact of fruits with irrigation water had been employed. For instance, in the case of the sprinkler irrigation system, there could have been a possible transfer of pathogens from the treated effluent or wastewater to the tomato fruits since the water could have had direct contact with the tomato fruits. This would have created a risk of pathogen exposure through the ingestion pathway. The assertion is corroborated by existing literature on the microbial risk of TWW irrigation. Makkaew et al. assessed the risk of E. coli contamination in TWW irrigated lettuce under different irrigation configurations and found that the spray type of irrigation system led to contamination of lettuce with E. coli, while the drip type of irrigation did not.60
The high levels of microbial loads in the treated effluent or wastewater (Table 3) pose potential health risks to farmers and farm workers through inhalation and ingestion. There is an occupational exposure pathway since these persons may have direct contact with the irrigation water.61 The microbiological quality of the treated effluent does not fulfil the threshold limits established by EU Regulation 2020/741 and WHO guidelines; therefore, farmers and farm workers working with such irrigation water need to wear appropriate protective gear such as nose masks and gloves to prevent direct contact and transfer of pathogens from the irrigation water.62,63 Considering that the pathogen loads are in the order of 103 and greater, the TWW must be disinfected to reduce the microbial content to an acceptable or safe limit. The application of disinfection processes like ozonation and UV disinfection is efficient in reducing the microbial load to safe limits.9,42 Even though in this study the potential risk of exposure to pathogens via the consumption of tomato fruits did not exist, disinfection of the effluent prior to crop irrigation is strongly recommended. Also, irrigation practices that avoid direct contact of water with edible parts of crops are strongly recommended for wastewater irrigation. This is to ensure maximum consumer safety. These recommendations are not only applicable in the European Union where the study was conducted but also applicable to other geographical locations, especially arid and semi-arid regions where TWW irrigation is relatively predominant.
3.5 Uptake and bioaccumulation of micropollutants/pharmaceuticals
Table 6 presents the bioaccumulation of micropollutants/pharmaceuticals in the edible tissues of the tomato plant. No difference was observed in the amount of micropollutants bioaccumulated in the tap water irrigated tomato fruits and that of the treated effluent irrigated tomato fruits except for gabapentin. The amount of gabapentin accumulated in the latter was 3 μg kg−1 compared to <1.0 μg kg−1 of the former. This might be due to gabapentin's apparent concentration in the soil solution, which was influenced by the quality of the irrigation water, as was also reported by a previous study.64 All the other pharmaceuticals were not quantifiable even though some were present in the TWW at relatively high levels. The concentration of naproxen, diclofenac, carbamazepine, gabapentin, tramadol, sulfamethoxazole, trimethoprim and saccharin in the effluent was 113.75 ± 18.87 ng L−1, 1950 ± 100 ng L−1, 460 ± 14.14 ng L−1, 1900 ± 81.65 ng L−1, 2625 ± 170.78 ng L−1, 770 ± 112.25 ng L−1, 675 ± 64.55 ng L−1, and 645 ± 242.83 ng L−1 and that of tap water was <LOD, <LOD, 11.25 ± 2.47 ng L−1, 79 ± 1.41 ng L−1, 0.41 ± 0.01 ng L−1, 0.39 ± 0.10 ng L−1, <LOD and <LOD, respectively (Table 3). Similar concentrations of naproxen, diclofenac, carbamazepine, gabapentin and trimethoprim in treated effluent have been reported by Diaz-Sosa et al.28| Parameter | Tap water | Secondary effluent |
|---|---|---|
| Ibuprofen (μg kg−1) | <10.0 | <10.0 |
| Naproxen (μg kg−1) | <10.0 | <10.0 |
| Diclofenac (μg kg−1) | <10.0 | <10.0 |
| Paracetamol (μg kg−1) | <2.0 | <2.0 |
| Carbamazepine (μg kg−1) | <1.0 | <1.0 |
| Gabapentin (μg kg−1) | <1.0 | 3.0 |
| Tramadol (μg kg−1) | <1.0 | <1.0 |
| Caffeine (μg kg−1) | <2.0 | <2.0 |
| Erythromycin (μg kg−1) | <10.0 | <10.0 |
| Triclosan (μg kg−1) | <10.0 | <10.0 |
| Sulfamethoxazole (μg kg−1) | <1.0 | <1.0 |
| Trimethoprim (μg kg−1) | <10.0 | <10.0 |
| Saccharin (μg kg−1) | <10.0 | <10.0 |
| Warfarin (μg kg−1) | <1.0 | <1.0 |
Generally, the amount of micropollutants accumulated in the tomato fruit did not correlate with the levels in the treated effluent. The irrigation water quality did not significantly influence the concentration of micropollutants bioaccumulated in the fruits (except for gabapentin), which contrasts with findings reported in some studies. Mordechay et al. concluded that the plant uptake of pharmaceuticals partly depends on the concentration and occurrence of these substances in the irrigation water after the authors found that high-quality irrigation water (low concentration of micropollutants) resulted in crops containing fewer and relatively low concentrations of these pollutants.64 The uptake of pharmaceuticals is not solely dependent on the characteristics of irrigation water but also on environmental factors, plant physiology, nutrients, and soil properties.64,65 This suggests that other factors may have critically affected the uptake of pharmaceuticals in our study, rather than the quality of the irrigation water. The high organic matter content of the soil (5.6 ± 0.08%) may have immobilized the micropollutants, thereby affecting their uptake by the tomato plants. This is achieved by controlling the bioavailability of the pharmaceuticals by strongly binding them to the soil particles and reducing their uptake potential by the roots of the tomato plants.66,67 Existing studies have shown the contribution of soil organic matter in facilitating the lower uptake of these substances by plants.64,68 Generally, significant quantities of micropollutants have been found in the biomass of crops grown on soil containing lower organic matter than soils having higher organic matter. The significance of the results is the support of the assertion that the bioavailability and bioaccumulation of micropollutants under TWW irrigation are not exclusively dependent on the irrigation water characteristics. Therefore, the evaluation of health risks arising from micropollutant accumulation should not be limited to the pharmaceutical characteristics of the TWW alone, but also consider the soil characteristics, since the soil properties strongly influence their uptake by plants.
The very low concentration of micropollutants in the fruits suggests that they may be less bioaccessible to the tomato fruits. As already mentioned, plant physiology also plays an important role in the uptake and translocation of pollutants. The aboveground parts of plants have a lower accumulation tendency compared to the roots, possibly due to low translocation potential. Among the aboveground parts, higher bioaccumulation or distribution occurs in the leaves than in the fruits due to translocation by the transpiration stream.67,69,70 Stomata, which play an important role in transpiration, are absent in the tomato fruit. Therefore, the fruits were unable to transpire water through the stomata to create a driving potential for water and solutes (pharmaceuticals) to flow into them in significant quantities.67 Our assertions are supported by previous studies, which found that fruits with stomata (cucumber) accumulated more micropollutants than fruits without stomata (tomato) and had higher levels of bioaccumulation in stems and leaves than the fruits.67,71 Since the objective of the study focuses on only the edible parts of crops, no evaluation of bioaccumulation of micropollutants in other parts of the tomato plants (roots, stems, leaves, and flowers) or soil was done.
No major or significant difference was observed in the risk of exposure to pharmaceuticals in consuming tomatoes irrigated by the two irrigation water streams. This result is similar to the uptake and bioaccumulation of heavy metals or PTEs in the tomato fruits as noted in the previous section. We are of the view that the bioavailability, uptake and bioaccumulation of these pollutants are strongly influenced by the soil properties, plant physiology and physicochemical properties of the micropollutants rather than the quality of the irrigation water. Therefore, in any TWW irrigation project, the effort to eliminate or reduce the risk of consumers being exposed to micropollutants should not only focus on the water quality but also on soil properties that could enhance the uptake of micropollutants. Such an approach is applicable on a global scale and not limited to a particular region.
3.6 Risk of exposure to ARGs
Zhang et al. ascribe that edible parts of plants represent an important means through which resistance genes are spread to humans.72 To evaluate the direct risk of ARG exposure via food ingestion, we performed PCR analyses of the DNA that was extracted from the edible part of the cabbage plant (leaves). Although the extracted DNA samples did not all test positive for all the ARGs tested, they all tested positive for the amplification of the 16S rRNA gene target. The PCR results revealed that five of the target genes (ermB, sul2, blaTEM, tetA and qnrS) were present in both the tap water irrigated cabbage and TWW irrigated cabbage (Table 7, Appendix). These genes were ubiquitous in the tap water, the treated effluent and the soil except ermB, which was absent in tap water and soil.Two (2) out of the nine (9) targeted ARGs were not detected in any of the sample matrices (irrigation water, soil, and crops/plant biomass) and these were tetW and blaZ. The ermB, blaTEM and tetA genes were present in all the treated effluent samples (5 samples), sul1 and sul2 were detected in four (4) samples, and qnrS in three (3) of the effluent samples. The distribution and abundance of most of these ARGs in TWW have already been reported in the literature. Marano et al. documented the presence of blaTEM and qnrS together with other ARGs in TWW effluent from different WWTPs in Israel.6 Teixeira et al. have also reported the detection of sul1 and ermB in the effluent of five WWTPs across Europe.29 However, a different distribution of ARGs was observed for the tap water. sul2 was ubiquitous in all the tap water samples (3 samples) while tetA and qnrS were detected in two of the samples. blaTEM was present in only one sample of the tap water while the other remaining ARGs were absent. The low prevalence of the target ARGs in tap water compared to the TWW is due to the highly efficient and rigorous treatment processes employed in the production of tap water due to public safety.
The soil environment harbours a large number of antibiotics and ARGs from irrigation water. Under the pressure of antibiotic selection, the prevalence of ARB could occur making the soil environment a repository of ARGs.73 However, in this study, a contrary observation was made regarding the irrigated soils. Except for sul2 which was present in TWW irrigated soil, all the remaining targeted ARGs were not detected in both tap water and TWW irrigated soils. However, this finding could also be consistent with the fact that the relative amount of DNA carrying ARG genes can be underrepresented due to soil biodiversity and fall below the detection limit of the used technique. Indeed, studies have shown that a change in the microbial community can affect the distribution and abundance and, therefore, detection of ARGs in soil.72,74
In contrast, the results suggest a bioaccumulation effect of ARGs in the cabbage leaves that might originate from the irrigation water and soil. In a similar study, Cerqueira et al. reported the presence of blaTEM and qnrS1 genes in lettuce irrigated with water comprising 90% TWW. They asserted that the soil was the main driver for ARG uptake into the lettuce and the quality of the irrigation water played a limited role.32 The presence of blaTEM, tetA and qnrS in the cabbage plant could be attributed to the irrigation water and the soil media since these ARGs were present in the soil prior to the irrigation exercise and were present in the irrigation water too. The sul2 gene was not initially detected in the soil prior to the irrigation exercise but was present in the tap water and TWW; therefore, the irrigation water is ascribed as the source of sul2 in the cabbage. The ermB gene was absent in the soil matrix and tap water but present in the TWW indicating that its presence in the effluent irrigated cabbage was probably due to the irrigation. The presence of ermB in the tap water irrigated cabbage is suspected to have been caused by contamination during sample preparation since the gene was not found in the soil or the tap water and is also xenobiotic to the cabbage plant. Qualitatively, no difference was observed in the bioaccumulation of the different ARGs in the tap-water irrigated cabbage and TWW-irrigated cabbage. In both cases, there was uptake of ARGs from the soil and irrigation water into the phyllosphere of the cabbage. ARGs may have accumulated in the rhizosphere soil after irrigation and then transferred to the root system where they were uptake and migrated to the leaves of the cabbage.72 In the leaf zone, endophytes may serve as hosts for the ARGs and subsequently confer resistance creating a potential risk of antibiotic resistance dissemination. Theoretically, the consumption of this cabbage has the potential for ARG dissemination to humans since the genes could be hosted by pathogenic bacteria and confer resistance in infective human diseases. The studies of Duan et al. and Song et al. have identified that human pathogenic bacteria can serve as hosts for ARGs.75,76 The potential risk of ARG dissemination or exposure in the present study is associated with the use of not only the TWW/effluent but also the tap water. The abundance of the targeted ARGs in the different sample matrices is outside the scope of this study and therefore not discussed. The results indicate that both tap water and TWW contain ARGs and that soil seems to possess the capability of making their abundance underrepresented and thus not detectable (Table 7, Appendix A). In contrast, plant biomass (possibly the plant microbiome) acts as an ARG bioaccumulator (Table 7, Appendix). To further elucidate this key aspect, additional quantitative studies are needed.
4 Conclusion
The study investigated and evaluated the health risks associated with the use of TWW for crop irrigation using tomatoes, carrots and cabbage as test crops. The comprehensive assessment of the potential risk arising from pathogens, heavy metals/PTEs, micropollutants or pharmaceuticals and ARGs in the irrigation water led to the following findings:(i) The use of the TWW did not result in the contamination of tomato fruits with pathogens.
(ii) Bioaccumulation and bioaccessibility of As, Cd and Pb in the test crops were insignificant.
(iii) Health quotient and health hazard assessment of Cu and Zn indicated that the irrigated crops are safe for adult consumption but may be unsafe for children.
(iv) Except for gabapentin (3 μg kg−1), the levels of bioaccumulated pharmaceuticals in the irrigated crops were below the detection limit.
(v)tetA, ermB, blaTEM, sul2, sul3, and qnrS were taken up and accumulated in TWW-irrigated cabbage plants as well as the control plants (tap water-irrigated cabbage). No difference was observed in the presence and distribution of the ARGs between the TWW and the tap water-irrigated cabbage biomasses.
The results of the study suggest that TWW could be a suitable source of water for irrigation; however, risk management strategies should be implemented to protect consumers and the environment. The health risk associated with the use of treated effluent or TWW for crop irrigation is pollutant specific and therefore to ensure safe use of TWW, different treatment processes aimed at removing the different contaminants should be employed to protect public health. The focus should not be solely dependent on the quality of the TWW but also on other factors such as soil characteristics which may contribute to the risk of exposure.
Data availability
The data supporting this article have been included in the manuscript. Any additional data will be submitted upon request.Author contributions
Solomon Ofori: conceptualization, methodology, investigation, formal analysis, writing – original draft, and writing – review and editing. Ylenia Di Leto: methodology, formal analysis, and writing – review and editing. Štěpánka Smrčková: methodology, formal analysis, and writing – review and editing. Marco Antonio Lopez Marin: methodology, formal analysis, visualization, and writing – review and editing. Giuseppe Gallo: methodology, writing – review and editing, supervision, and resources. Iveta Růžičková: methodology, writing – review and editing, supervision, resources, and funding acquisition. Jiří Wanner: methodology, writing – review and editing, supervision, resources, and funding acquisition.Conflicts of interest
There are no conflicts of interest to declare.Appendix
| Sample description | Erythromycin | Sulfonamide | Beta-lactamase | Tetracycline | |||||
|---|---|---|---|---|---|---|---|---|---|
| erm(B) | sul1 | sul2 | sul3 | bla TEM | blaZ | tet(A) | tet(W) | qnrS | |
| a Biomass_Tap: tap water irrigated cabbage; Biomass_SE: secondary effluent irrigated cabbage; Soil_Tap (1, 2, 3, and 4): tap water irrigated soil samples; Soil_SE (1, 2, 3, and 4): secondary effluent irrigated soil samples; Soil_BF: soil sample taken before the start of the irrigation exercise; Tap water (1, 2, and 3): samples of tap water used for irrigating the cabbage crops; Secondary effluent (1, 2, and 3): samples of secondary effluent used for irrigating the cabbage crops. | |||||||||
| Biomass_tap | x | x | x | x | x | x | |||
| Biomass_SE | x | x | x | x | x | x | |||
| Soil_tap 1 | |||||||||
| Soil_tap 2 | |||||||||
| Soil_tap 3 | |||||||||
| Soil_tap 4 | |||||||||
| Soil_SE 1 | |||||||||
| Soil_SE 2 | x | ||||||||
| Soil_SE 3 | |||||||||
| Soil_SE 4 | x | ||||||||
| Soil_BF | x | x | x | ||||||
| Tap water 1 | x | x | x | x | |||||
| Tap water 2 | x | x | x | ||||||
| Tap water 3 | x | ||||||||
| Secondary effluent 1 | x | x | x | x | x | ||||
| Secondary effluent 2 | x | x | x | x | x | x | |||
| Secondary effluent 3 | x | x | x | x | x | ||||
| Secondary effluent 4 | x | x | x | x | x | ||||
| Secondary effluent 5 | x | x | x | x | x | ||||
| Compounds name | Precursor ion | Product ion | Fragmentor voltage | Collision energy | Accelerator voltage | Ret. time | Polarity |
|---|---|---|---|---|---|---|---|
| 2-Hydroxy-IB = 2-hydroxyibuprofen, 3-hydroxy-CBZ = 3-hydroxycarbamazepine, 17β-E2 = 17β-estradiol, ACS-K = acesulfame potassium, CBZ-epo = carbamazepine-10,11-epoxide, E1 = estron, E3 = estriol, and EE2 = 17α-ethinylestradiol. | |||||||
| 2-Hydroxy-IB | 221.1 | 159.1 | 78 | 16 | 4 | 6.77 | Negative |
| 2-Hydroxy-IB | 221.1 | 177 | 78 | 4 | 4 | 6.77 | Negative |
| 3-Hydroxy-CBZ | 253.1 | 209.9 | 113 | 16 | 4 | 7.15 | Positive |
| 3-Hydroxy-CBZ | 253.1 | 207.9 | 113 | 20 | 4 | 7.15 | Positive |
| Paracetamol | 152 | 110.1 | 122 | 16 | 4 | 3.91 | Positive |
| Paracetamol | 152 | 93 | 122 | 24 | 4 | 3.91 | Positive |
| Paracetamol IS | 156 | 114 | 122 | 16 | 4 | 3.89 | Positive |
| Paracetamol IS | 156 | 97 | 122 | 24 | 4 | 3.89 | Positive |
| ACS-K | 162 | 82 | 89 | 12 | 4 | 2.26 | Negative |
| ACS-K | 162 | 78 | 89 | 40 | 4 | 2.26 | Negative |
| Aspartame | 295.1 | 235 | 129 | 8 | 4 | 5.95 | Positive |
| Aspartame | 295.1 | 179.9 | 129 | 8 | 4 | 5.95 | Positive |
| Caffeine | 195.5 | 138.1 | 209 | 20 | 4 | 5.17 | Positive |
| Caffeine | 195.5 | 110 | 209 | 24 | 4 | 5.17 | Positive |
| Caffeine IS | 198 | 140.1 | 209 | 24 | 4 | 5.17 | Positive |
| Caffeine IS | 198 | 112 | 209 | 28 | 4 | 5.17 | Positive |
| Carbamazepine | 237 | 194.1 | 143 | 16 | 4 | 7.76 | Positive |
| Carbamazepine | 237 | 193.1 | 143 | 36 | 4 | 7.76 | Positive |
| Carbamazepine IS | 243 | 200 | 143 | 20 | 4 | 7.76 | Positive |
| Carbamazepine IS | 243 | 199 | 143 | 36 | 4 | 7.76 | Positive |
| CBZ-epo | 253 | 210.1 | 107 | 8 | 4 | 6.92 | Positive |
| CBZ-epo | 253 | 180.1 | 107 | 28 | 4 | 6.92 | Positive |
| Chloramphenicol | 325 | 277 | 107 | 16 | 4 | 6.48 | Positive |
| Chloramphenicol | 325 | 275 | 107 | 16 | 4 | 6.48 | Positive |
| Diclofenac | 296 | 250 | 130 | 12 | 4 | 8.34 | Positive |
| Diclofenac | 296 | 213 | 130 | 36 | 4 | 8.34 | Positive |
| Diclofenac IS | 302 | 256 | 120 | 8 | 4 | 8.34 | Positive |
| Diclofenac IS | 302 | 220 | 120 | 40 | 4 | 8.34 | Positive |
| E1 | 269.1 | 145 | 134 | 36 | 4 | 8.64 | Negative |
| E1 | 269.1 | 143 | 134 | 60 | 4 | 8.64 | Negative |
| E1 IS | 272.1 | 148 | 134 | 44 | 4 | 8.64 | Negative |
| E1 IS | 272.1 | 146 | 134 | 68 | 4 | 8.64 | Negative |
| E3 | 287.17 | 171 | 167 | 40 | 4 | 7.27 | Negative |
| E3 | 287.17 | 145 | 167 | 44 | 4 | 7.27 | Negative |
| E3 IS | 290.15 | 174 | 167 | 40 | 4 | 7.27 | Negative |
| E3 IS | 290.15 | 148 | 167 | 44 | 4 | 7.27 | Negative |
| EE2 | 295.2 | 269 | 194 | 32 | 4 | 8.62 | Negative |
| EE2 | 295.2 | 145 | 194 | 56 | 4 | 8.62 | Negative |
| EE2 IS | 297.2 | 269 | 194 | 24 | 4 | 8.62 | Negative |
| EE2 IS | 297.2 | 145 | 194 | 44 | 4 | 8.62 | Negative |
| Fluoxetine | 310.1 | 148.1 | 101 | 0 | 4 | 7.94 | Positive |
| Fluoxetine | 310.1 | 44 | 101 | 8 | 4 | 7.94 | Positive |
| Gabapentin | 172.1 | 154 | 101 | 12 | 4 | 4.11 | Positive |
| Gabapentin | 172.1 | 137 | 101 | 16 | 4 | 4.11 | Positive |
| Gemfibrozil | 249.2 | 127 | 98 | 8 | 4 | 9.43 | Negative |
| Gemfibrozil | 249.2 | 121 | 98 | 12 | 4 | 9.43 | Negative |
| Ibuprofen | 205.12 | 161.1 | 83 | 0 | 4 | 8.79 | Negative |
| Ibuprofen | 205.1 | 159.1 | 83 | 0 | 4 | 8.79 | Negative |
| Ibuprofen IS | 211.1 | 167.1 | 83 | 0 | 4 | 8.79 | Negative |
| Naproxen | 229.1 | 185.1 | 90 | 4 | 4 | 7.59 | Negative |
| Naproxen | 229.1 | 170,7 | 90 | 28 | 4 | 7.59 | Negative |
| NHDC | 611.2 | 303 | 200 | 36 | 4 | 7.18 | Negative |
| NHDC | 611.2 | 125 | 200 | 56 | 4 | 7.18 | Negative |
| Neotame | 379.2 | 172.1 | 140 | 20 | 4 | 8.41 | Positive |
| Neotame | 379.2 | 85.2 | 140 | 40 | 4 | 8.41 | Positive |
| Nimesulide | 307 | 229.05 | 134 | 12 | 4 | 8.15 | Negative |
| Nimesulide | 307 | 122 | 134 | 44 | 4 | 8.15 | Negative |
| Saccharin | 182 | 106 | 149 | 16 | 4 | 3.55 | Negative |
| Saccharin | 182 | 62 | 149 | 32 | 4 | 3.55 | Negative |
| Salicylic acid | 137 | 93 | 83 | 16 | 4 | 4.10 | Negative |
| Salicylic acid | 137 | 65 | 83 | 36 | 4 | 4.10 | Negative |
| Salicylic acid IS | 143 | 99 | 83 | 16 | 4 | 4.10 | Negative |
| Salicylic acid IS | 143 | 71 | 83 | 36 | 4 | 4.10 | Negative |
| Sucralose | 397 | 361 | 161 | 8 | 4 | 5.61 | Negative |
| Sucralose | 397 | 359 | 161 | 8 | 4 | 5.61 | Negative |
| Sulfamethoxazole | 254 | 156 | 113 | 12 | 4 | 5.31 | Positive |
| Sulfamethoxazole | 254 | 92 | 113 | 24 | 4 | 5.31 | Positive |
| Sulfamethoxazole IS | 260.1 | 98.1 | 110 | 28 | 4 | 5.31 | Positive |
| Sulfamethoxazole IS | 260.1 | 70.1 | 110 | 56 | 4 | 5.31 | Positive |
| Testosterone | 289.2 | 108.9 | 134 | 24 | 4 | 8.80 | Positive |
| Testosterone | 289.2 | 97 | 134 | 32 | 4 | 8.80 | Positive |
| Tramadol | 264.2 | 58.1 | 110 | 12 | 4 | 5.71 | Positive |
| Tramadol | 364.2 | 30.1 | 110 | 64 | 4 | 5.71 | Positive |
| Trimethoprim | 291 | 261 | 170 | 24 | 4 | 5.26 | Positive |
| Trimethoprim | 291 | 230 | 170 | 20 | 4 | 5.26 | Positive |
| Warfarin | 309.1 | 251 | 107 | 16 | 4 | 7.81 | Positive |
| Warfarin | 309.1 | 163 | 107 | 8 | 4 | 7.81 | Positive |
Acknowledgements
This work was funded by the Horizon 2020 project: Achieving wider uptake of water-smart solutions (H2020-SC5-2019-2) (Grant Agreement ID: 869283) and supported by the University of Chemistry and Technology Internal Grant, grant number: 217 88 2205. This research was also supported by: the European Commission – NextGenerationEU, Project SUS-MIRRI.IT “Strengthening the MIRRI Italian Research Infrastructure for Sustainable Bioscience and Bioeconomy”, code no. IR0000005PO, CUP D13C22001390001; the European Commission – NextGenerationEU, National Recovery and Resilience Plan (NRRP), Mission 4, Component 2, Investment 1.4 “Strengthening of research structures and creation of R&D ‘national champions’ on some Key Enabling Technologies”- Call for tender no. 3138 of 16 December 2021, rectified by Decree no. 3175 of 18 December 2021 of Italian Ministry of University – CN5 “National Biodiversity Future Center”, NBFC – code no. CN00000033, CUP B73C22000790001. The support of the Prague Wastewater Treatment Company (PVS and PVK) is highly acknowledged.References
- X. Ruan, S. Ge, Z. Jiao, W. Zhan and Y. Wang, Bioaccumulation and risk assessment of potential toxic elements in the soil-vegetable system as influenced by historical wastewater irrigation, Agric. Water Manag., 2023, 279, 108197 CrossRef
.
- Z. Deh-Haghi, A. Bagheri, Z. Fotourehchi and C. A. Damalas, Farmers' acceptance and willingness to pay for using treated wastewater in crop irrigation: A survey in western Iran, Agric. Water Manag., 2020, 239, 106262, DOI:10.1016/j.agwat.2020.106262
.
- L. Yi, W. Jiao, X. Chen and W. Chen, An overview of reclaimed water reuse in China, J. Environ. Sci., 2011, 23(10), 1585–1593, DOI:10.1016/S1001-0742(10)60627-4
.
- S. Bedbabis, B. Ben Rouina, M. Boukhris and G. Ferrara, Effect of irrigation with treated wastewater on soil chemical properties and infiltration rate, J. Environ. Manage., 2014, 133, 45–50, DOI:10.1016/j.jenvman.2013.11.007
.
- A. Reznik, E. Feinerman, I. Finkelshtain, F. Fisher, A. Huber-Lee, B. Joyce and I. Kan, Economic implications of agricultural reuse of treated wastewater in Israel: a statewide long-term perspective, Ecol. Econ., 2017, 135, 222–233, DOI:10.1016/j.ecolecon.2017.01.013
.
- R. B. M. Marano, A. Zolti, E. Jurkevitch and E. Cytryn, Antibiotic resistance and class 1 integron gene dynamics along effluent, reclaimed wastewater irrigated soil, crop continua: elucidating potential risks and ecological constraints, Water Res., 2019, 164, 114906 CrossRef CAS PubMed
.
- Z. Deh-Haghi, A. Bagheri, C. A. Damalas and Z. Fotourehchi, Horticultural products irrigated with treated sewage: are they acceptable?, Environ. Sci. Pollut. Res., 2021, 28, 54057–54068, DOI:10.1007/s11356-021-14552-8
.
- P. Vergine, A. Lonigro, C. Salerno, P. Rubino, G. Berardi and A. Pollice, Nutrient recovery and crop yield enhancement in irrigation with reclaimed wastewater: a case study, Urban Water J., 2017, 14(3), 325–330 CrossRef CAS
.
- S. Ofori, A. Puškáčová, I. Růžičková and J. Wanner, Treated wastewater reuse for irrigation: Pros and cons, Sci. Total Environ., 2021, 760, 144026 CrossRef CAS
.
- D. Dupont, Water use restrictions or wastewater recycling? A Canadian willingness to pay study for reclaimed wastewater, Water Resour. Econ., 2013, 1, 61–74 CrossRef
.
- L. Yi, W. Jiao, X. Chen and W. Chen, An overview of reclaimed water reuse in China, J. Environ. Sci., 2011, 23(10), 1585–1593 CrossRef
.
- A. Cherfi, M. Achour, M. Cherfi, S. Otmani and A. Morsli, Health risk assessment of heavy metals through consumption of vegetables irrigated with reclaimed urban wastewater in Algeria, Process Saf. Environ. Prot., 2015, 98, 245–252 CrossRef CAS
.
- F. O. Gudda, M. G. Waigi, E. S. Odinga, B. Yang, L. Carter and Y. Gao, Antibiotic-contaminated wastewater irrigated vegetables pose resistance selection risks to the gut microbiome, Environ. Pollut., 2020, 264, 114752 CrossRef CAS
.
- A. A. Adam, L. N. A. Sackey and L. A. Ofori, Risk assessment of heavy metals concentration in cereals and legumes sold in the Tamale Aboabo market, Ghana, Heliyon, 2022, 8, e10162 CrossRef CAS PubMed
.
- H. M. Jalil, S. Rezapour, A. Nouri and N. Joshi, Assessing the ecological and health implications of soil heavy metals in vegetable irrigated with wastewater in calcareous environments, Agric. Water Manag., 2022, 272, 107848 CrossRef
.
- S. Rezapour, B. Atashpaz, S. S. Moghaddam and C. A. Damalas, Heavy metal bioavailability and accumulation in winter wheat (Triticum aestivum L.) irrigated with treated wastewater in calcareous soils, Sci. Total Environ., 2018, 656, 261–269 CrossRef
.
- L. N. A. Sackey, K. Markin, A. Kwarteng, I. M. Ayitey and P. Kayoung, Presence and levels of potential trace elements in lettuce and spring onion grown in Kumasi, Ghana, Kuwait J. Sci., 2023, 51, 100143 CrossRef
.
- X. Xu, J. Wang, H. Wu, R. Lu and J. Cui, Bioaccessibility and bioavailability evaluation of heavy metal(loid)s in ginger in vitro: Relevance to human health risk assessment, Sci. Total Environ., 2022, 857, 159582 CrossRef PubMed
.
- M. Jaishankar, T. Tseten, N. Anbalagan, B. B. Mathew and K. N. Beeregowda, Toxicity, mechanism and health effects of some heavy metals, Interdiscipl. Toxicol., 2014, 7(2), 60–72 CrossRef
.
- S. Khalid, M. Shahid, Natasha, I. Bibi, T. Sarwar, A. H. Shah and N. K. Niazi, A review of environmental contamination and health risk assessment of wastewater use for crop irrigation with a focus on low and high–income countries, Int. J. Environ. Res. Publ. Health, 2018, 15(5), 895 CrossRef PubMed
.
- G. Gatta, A. Libutti, L. Beneduce, A. Gagliardi, G. Disciglio, A. Lonigro and E. Tarantino, Reuse of treated municipal wastewater for globe artichoke irrigation: assessment of effects on morpho-quantitative parameters and microbial safety of yield, Sci. Hortic., 2016, 213, 55–65 CrossRef
.
- M. Mcheik, J. Toufaily, B. Haj Hassan, T. Hamieh, M. T. Abi Saab, Y. Rouphael, E. Ferracin, I. Bashabshah and L. Al Hadidi, Reuse of treated municipal wastewater in irrigation: a case study from Lebanon and Jordan, Water Environ. J., 2017, 31(4), 552–558 CrossRef CAS
.
- A. Libutti, G. Gatta, A. Gagliardi, P. Vergine, A. Pollice, L. Beneduce, G. Disciglio and E. Tarantino, Agro-industrial wastewater reuse for irrigation of a vegetable crop succession under Mediterranean conditions, Agric. Water Manag., 2018, 196, 1–14 CrossRef
.
- J. B. Sallach, Y. Zhang, L. Hodges, D. Snow, X. Li and S. Bartelt-Hunt, Concomitant uptake of antimicrobials and Salmonella in soil and into lettuce following wastewater irrigation, Environ. Pollut., 2015, 197, 269–277 CrossRef CAS
.
- M. A. Croxen, R. J. Law, R. Scholz, K. M. Keeney, M. Wlodarska and B. B. Finlay, Recent advances in understanding enteric pathogenic Escherichia coli, Clin. Microbiol. Rev., 2013, 26(4), 822–880 CrossRef CAS PubMed
.
- D. Fatta-Kassinos, I. K. Kalavrouziotis, P. H. Koukoulakis and M. I. Vasquez, The risks associated with wastewater reuse and xenobiotics in the agroecological environment, Sci. Total Environ., 2011, 409(19), 3555–3563 CrossRef CAS PubMed
.
- M. Basiuk, R. A. Brown, D. Cartwright, R. Davison and P. M. Wallis, Trace organic compounds in rivers, streams, and wastewater in southeastern Alberta, Canada, Inland Waters, 2017, 7(3), 283–296 CrossRef CAS
.
- V. R. Diaz-Sosa, M. Tapia-Salazar, J. Wanner and D. L. Cardenas-Chavez, Monitoring and ecotoxicity assessment of emerging contaminants in wastewater discharge in the City of Prague (Czech Republic), Water, 2020, 12, 1079 CrossRef CAS
.
- A. M. Teixeira, I. Vaz-Moreira, D. Calderon-Franco, D. Weissbrodt, S. Purkrtova, S. Gajdos, G. Dottorini, P. H. Nielsen, L. Khalifa, E. Cytryn, J. Bartacek and C. M. Manaia, Candidate biomarkers of antibiotic resistance for the monitoring of wastewater and the downstream environment, Water Res., 2023, 247, 120761 CrossRef CAS
.
- X. M. Han, H. W. Hu, X. Z. Shi, J. T. Wang, L. L. Han, D. Chen and J. Z. He, Impacts of reclaimed water irrigation on soil antibiotic resistome in urban parks of Victoria, Australia, Environ. Pollut., 2016, 211, 48–57 CrossRef CAS
.
- F. Cerqueira, V. Matamoros, J. Bayona and B. Piña, Antibiotic resistance genes distribution in microbiomes from the soil-plant-fruit continuum in commercial Lycopersicon esculentum fields under different agricultural practices, Sci. Total Environ., 2019, 652, 660–670 CrossRef PubMed
.
- F. Cerqueira, V. Matamoros, J. M. Bayona, T. U. Berendonk, G. Elsinga, L. M. Hornstra and B. Piña, Antibiotic resistance gene distribution in agricultural fields and crops. A soil-to-food analysis, Environ. Res., 2019, 177, 108 CrossRef
.
- F. Cerqueira, V. Matamoros, J. Bayona, G. Elsinga, L. M. Hornstra and B. Piña, Distribution of antibiotic resistance genes in soils and crops. A field study in legume plants (Vicia faba L.) grown under different watering regimes, Environ. Res., 2019, 170, 16–25 CrossRef CAS
.
- Y. Liu, A. L. Neal, X. Zhang, H. Fan, H. Liu and Z. Li, Cropping system exerts stronger influence on antibiotic resistance gene assemblages in greenhouse soils than reclaimed wastewater irrigation, J. Hazard. Mater., 2022, 425, 128046 CrossRef CAS
.
- F. Chen, J. Ma, M. A. Ashraf, A. Zafar, Z. I. Khan, K. Ahmad, M. S. Alwahibi and M. S. Elshikh, Ecological risk assessment of zinc metal in different varieties of wheat (Triticum aestivum L.) irrigated with wastewater regimes: Assessing the public health risk, Agric. Water Manag., 2022, 267, 107615 CrossRef
.
- A. Mosa, O. A. Hawamdeh, M. Rady and A. A. Taha, Ecotoxicological monitoring of potentially toxic elements contamination in Eucalyptus forest plantation subjected to long-term irrigation with recycled wastewater, Environ. Pollut., 2023, 329, 121739 CrossRef CAS PubMed
.
- A. Forslund, J. H. J. Ensink, B. Markussen, A. Battilani, G. Psarras, S. Gola, L. Sandei, T. Fletcher and A. Dalsgaard, Escherichia coli contamination and health aspects of soil and tomatoes (Solanum lycopersicum L.) subsurface drip irrigated with on-site treated domestic wastewater, Water Res., 2012, 46, 5917–5934 CrossRef CAS
.
- M. Farhadkhani, M. Nikaeen, G. Yadegarfar, M. Hatamzadeh, H. Pourmohammadbagher, Z. Sahbaei and H. R. Rahmani, Effects of irrigation with secondary treated wastewater on physicochemical and microbial properties of soil and produce safety in a semi-arid area, Water Res., 2018, 144, 356–364 CrossRef PubMed
.
- V. K. Tripathi, T. B. S. Rajput, N. Patel and L. Nain, Impact of municipal wastewater reuse through micro-irrigation system on the incidence of coliforms in selected vegetable crops, J. Environ. Manage., 2019, 251, 109532 CrossRef CAS PubMed
.
- Q. Yan, Z. Zhong, X. Li, Z. Cao, X. Zheng and G. Feng, Characterization of heavy metal, antibiotic pollution, and their resistance genes in paddy with secondary municipal-treated wastewater irrigation, Water Res., 2024, 252, 121208 CrossRef CAS
.
- S. Ofori, D. K. Abebrese, A. Klement, D. Provazník, I. Tomášková, I. Růžičková and J. Wanner, Impact of treated wastewater on plant growth: leaf fluorescence, reflectance, and biomass-based assessment, Water Sci. Technol., 2024, 89(7), 1647 CrossRef CAS PubMed
.
- S. Ofori, D. K. Abebrese, I. Růžičková and J. Wanner, Reuse of treated wastewater for crop irrigation: Water suitability, fertilization potential, and impact on selected soil physicochemical properties, Water, 2024, 16, 484 CrossRef CAS
.
- V. J. G. Houba, E. J. M. Temminghoff, G. A. Gaikhorst and W. van Vark, Soil analysis procedures using 0.01 M calcium chloride as extraction reagent, Commun. Soil Sci. Plant Anal., 2000, 31(9–10), 1299–1396 CrossRef CAS
.
-
M. R. Motsara and R. N. Roy, Guide to laboratory establishment for plant nutrient analysis, FAO Fertilizer and Plant Nutrition Bulletin 19, Food and Agriculture Organization of the United Nations, Rome, Italy, 2008 Search PubMed
.
- J. Hunt, Dilute hydrochloric acid extraction of plant material for routine cation analysis, Commun. Soil Sci. Plant Anal., 1982, 13(1), 49–55 CrossRef CAS
.
-
USEPA, Risk Assessment Guidance for Superfund Volume I, Human Health Evaluation Manual (Part A), Office of Emergency and Remedial Response U.S. Environmental Protection Agency, Washington, D.C, 1989 Search PubMed
.
- I. Lente, J. Ofosu-Anim, A. K. Brimah and S. Atiemo, Heavy metal pollution of vegetable crops irrigated with wastewater in Accra, Ghana, West Afr. J. Appl. Ecol., 2014, 22(1), 41–58 Search PubMed
.
- W. Kim, J. Lee, A. Kunhikrishnan and D. Kim, Dietary exposure estimates of trace elements in selected agricultural products grown in greenhouse and associated health risks in Korean population, J. Agric. Chem. Environ., 2013, 2, 35–41 Search PubMed
.
- ČSN 77. Jakost Vod–Stanovení Koliformních Bakterií v Nedesinfikovaných Vodách (Water Quality–Detection and Enumeration of Coliform Bacteria in Non Disinfected Waters). Office for Technical Standardization, Metrology and State Testing Prague, Czech Republic, [Online], available from: https://shop.normy.biz/detail/85345#zakladni-informace CAS.
- ČSN 77. Jakost Vod–Stanovení Termotolerantních Koliformních Bakterií a Escherichia coli (Water Quality—Detection and Enumeration of Thermotolerant Coliform Bacteria and Escherichia coli). Office for Technical Standardization, Metrology and State Testing: Prague, C., [Online], available from: https://www.tzb-info.cz/normy/csn-75-7835-2009-03.
- Decree 252/2004, Annex Number 6, available from: https://www.zakonyprolidi.cz/cs/2004-252.
- ČSN I(Jakost vod - Stanovení microcystinů - Metoda extrakce tuhou fází (SPE) a HPLC s UV detekcí. (Water quality - Determination of microcystins -Method using solid phase extraction (SPE) and high performance liquid chromatography (HPLC) with ultraviolet (UV) d, [Online], available from: https://www.technicke-normy-csn.cz/csn-iso-20179-757574-226477.html#.
- R. Islam, T. Sultana, M. M. Joe, J. Cho and T. Sa, Comparisons of direct extraction methods of microbial DNA from different paddy soils, Saudi J. Biol. Sci., 2012, 19, 337–342 CrossRef
.
- Y. Di Leto, F. C. Capri, A. Mineo, A. Cosenza, G. Gallo, R. Alduina and G. Mannina, Initial pH Conditions Shape the Microbial Community Structure of Sewage Sludge in Batch Fermentations for the Improvement of Volatile Fatty Acid Production, Microorganisms, 2022, 10, 2073 CrossRef CAS PubMed
.
- F. C. Capri, Y. Di Leto, A. Presentato, I. Mancuso, M. L. Scatassa and R. Alduina, Characterization of Staphylococcus Species Isolates from Sheep Milk with Subclinical Mastitis: Antibiotic Resistance, Enterotoxins, and Biofilm Production, Foodborne Pathog. Dis., 2024, 21(1), 10–18 CrossRef CAS
.
-
J. P. Gray, A. Suhali-Amacher and S. D. Ray, Metals and metal antagonists, in Side Effects of Drugs Annual, ed. Ray S. D., Elsevier, Ch. 19, 2017, pp. 197–208 Search PubMed
.
-
U. M. Agnew and T. L. Slesinger, Zinc Toxicity, 2022, https://www.ncbi.nlm.nih.gov/books/NBK554548/ Search PubMed
.
- H. R. Gebeyehu and L. D. Bayissa, Levels of heavy metals in soil and vegetables and associated health risks in Mojo area, Ethiopia, PLoS One, 2020, 15(1), e0227883 CrossRef CAS
.
- A. Guadie, A. Yesigat, S. Gatew, A. Worku, W. Liu, F. O. Ajibade and A. Wang, Evaluating the health risks of heavy metals from vegetables grown on soil irrigated with untreated and treated wastewater in Arba Minch, Ethiopia, Sci. Total Environ., 2012, 761, 143302 CrossRef PubMed
.
- P. Makkaew, M. Miller, H. J. Fallowfield and N. J. Cromar, Microbial risk in wastewater irrigated lettuce: Comparing Escherichia coli contamination from an experimental site with a laboratory approach, Water Sci. Technol., 2016, 74, 749–755 CrossRef CAS PubMed
.
- A. A. Adegoke, I. D. Amoah, T. A. Stenström, M. E. Verbyla and J. R. Mihelcic, Epidemiological evidence and health risks associated with agricultural reuse of partially treated and untreated wastewater: a review, Front. Public Health, 2018, 6, 337 CrossRef PubMed
.
- Commission E, Regulation (EU) 2020/741 of the European Parliament and of the Council of 25 May 2020 on minimum requirements for water reuse, Off. J. Eur. Union, 2020, L 177, 32–55 Search PubMed
.
-
WHO, Wastewater use in agriculture, in Guidelines for the Safe Use of Wastewater, Excreta and Greywater, World Health Organization, Geneva, Switzerland, Vol. 2, 2006 Search PubMed
.
- E. B. Mordechay, V. Mordehay, J. Tarchitzky and B. Chefetz, Pharmaceuticals in edible crops irrigated with reclaimed wastewater: Evidence from a large survey in Israel, J. Hazard. Mater., 2021, 416, 126184 CrossRef PubMed
.
- F. Gudda, E. S. Odinga, L. Tang, M. G. Waigi, J. Wang, D. Abdalmegeed and Y. Gao, Tetracyclines uptake from irrigation water by vegetables: Accumulation and antimicrobial resistance risks, Environ. Pollut., 2023, 338, 122696 CrossRef CAS
.
- S. Thiele, Adsorption of the antibiotic pharmaceutical compound sulfapyridine by a long-term differently fertilized loess Chernozem, J. Plant Nutr. Soil Sci., 2000, 163, 589–594 CrossRef CAS
.
- M. Goldstein, M. Shenker and B. Chefetz, Insights into the uptake processes of wastewater-borne pharmaceuticals by vegetables, Environ. Sci. Technol., 2014, 48, 5593–5600 CrossRef CAS PubMed
.
- Q. Fu, X. Wu, Q. Ye, F. Ernst and J. Gan, Biosolids inhibit bioavailability and plant uptake of triclosan and triclocarban, Water Res., 2016, 102, 117–124 CrossRef CAS PubMed
.
- M. Shenker, D. Harush, J. Ben-Ari and B. Chefetz, Uptake of carbamazepine by cucumber plants: a case study related to irrigation with reclaimed wastewater, Chemosphere, 2011, 82, 905–910 CrossRef CAS PubMed
.
- R. Kodesova, H. Švecová, A. Klement, M. Fér, A. Nikodem, G. Fedorova, O. Rieznyk, M. Kočárek, A. Sadchenko, A. Chroňáková and R. Grabic, Contamination of water, soil, and plants by micropollutants from reclaimed wastewater and sludge from a wastewater treatment plant, Sci. Total Environ., 2024, 907, 167965 CrossRef CAS
.
- C. Wu, A. L. Spongberg, J. D. Witter and B. B. M. Sridhar, Transfer of wastewater-associated pharmaceuticals and personal care products to crop plants from biosolids-treated soil, Ecotoxicol. Environ. Saf., 2012, 85, 104–109 CrossRef CAS PubMed
.
- Y. Zhang, J. Zhou, J. Wu, Q. Hua and C. Bao, Distribution and transfer of antibiotic resistance genes in different soil-plant systems, Environ. Sci. Pollut. Res., 2022, 29, 59159–59172 CrossRef CAS PubMed
.
- N. Wang, X. Guo, Z. Yan, W. Wang, B. Chen, F. Ge and B. Ye, A comprehensive analysis on spread and distribution characteristic of antibiotic resistance genes in livestock farms of Southeastern China, PLoS One, 2016, 11(7), e0156889 CrossRef
.
- W. Sun, X. Qian, J. Gu, X. J. Wang and M. L. Duan, Mechanism and effect of temperature on variations in antibiotic resistance genes during anaerobic digestion of dairy manure, Sci. Rep., 2016, 6, 30237 CrossRef CAS
.
- M. Duan, H. Li, J. Gu, X. Tuo, W. Sun, X. Qian and X. Wang, Effects of biochar on reducing the abundance of oxytetracycline, antibiotic resistance genes, and human pathogenic bacteria in soil and lettuce, Environ. Pollut., 2017, 224, 787–795 CrossRef CAS PubMed
.
- W. Song, X. Wang, J. Gu, S. Zhang, Y. Yin, Y. Li, X. Qian and W. Sun, Effects of different swine manure to wheat straw ratios on antibiotic resistance genes and the microbial community structure during anaerobic digestion, Bioresour. Technol., 2017, 231, 1–8 CrossRef CAS PubMed
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4va00274a |
| This journal is © The Royal Society of Chemistry 2025 |