Open Access Article
Open Access ArticleWastewater-induced microplastic biofouling in freshwater: role of particle size and flow velocity†
Gaurav
Bhardwaj
 ,
Malihe
Mohammadiun
,
Carlos Saul
Osorio Gonzalez
,
Satinder
Kaur Brar
,
Malihe
Mohammadiun
,
Carlos Saul
Osorio Gonzalez
,
Satinder
Kaur Brar
 and
Shooka
Karimpour
*
and
Shooka
Karimpour
*
Department of Civil Engineering, Lassonde School of Engineering, York University, North York, Toronto, M3J 1P3, Ontario, Canada. E-mail: Shooka.karimpour@lassonde.yorku.ca
First published on 28th November 2024
Abstract
Microplastics (MPs), discharged from wastewater treatment plants (WWTPs), are found abundantly in freshwater systems. Along with MPs, various microorganisms that evade WWTP disinfection may colonize these particles, leading to biofouling. This study assessed the performance of six bacterial strains isolated from wastewater and the factors influencing biofilm formation using synthetic freshwater and polyethylene (PE) microplastics as a model. The effect of two PE microplastic sizes (180–200 μm and 3–4 mm) and three flow velocities (0.238, 0.11, and 0.077 m s−1) were tested on the isolated strains' microbial growth and biofilm formation. Smaller MPs notably enhanced the growth rate. The treatment with small PE microplastics and a low flow velocity promoted the biofilm formation compared to a higher flow velocity where rapid microbial growth was observed but showed a lower biofilm formation after seven days of cultivation. These findings reveal how MP size and flow velocities influence biofilm development, advancing the understanding of MP-microbial interactions in freshwater aquatic environments.
Environmental significanceMicroplastics in freshwater systems, often carried by wastewater effluents, facilitate unique ecological challenges. Some opportunist microorganisms bypass wastewater treatment processes and survive in freshwater by forming biofilms on microplastics. This adaptation enhances their persistence and potential to transport pathogens, affecting water quality and ecosystem health. Our study reveals that smaller microplastics, due to their increased surface area, are particularly conducive to biofilm development, offering a robust platform for microbial colonization. However, higher flow rates, while promoting growth, also induce shear stresses that can disrupt these biofilms, releasing pollutants back into the environment. Understanding these interactions is crucial for developing effective measures to mitigate the ecological impacts of microplastic pollution. |
1. Introduction
Microplastics (MPs), defined as plastic particles smaller than 5 mm, have emerged as persistent contaminants across global aquatic environments. Wastewater treatment plants (WWTPs) serve as one of the primary sources of MPs and associated microbiota in freshwater environments. Due to their small size, MPs offer a substantial surface area for microbial colonization, promoting the formation of biofilms, often referred to as the plastisphere.1 These microbial communities show more resilience and protection within biofilms and to their planktonic counterparts. Nevertheless, this biofilm-mediated protection enables the immobilized microorganism to withstand disinfection processes in WWTPs, subsequently entering freshwater bodies with the effluent.2In this sense, the presence of biofouled MPs in freshwater serves as reservoirs for various contaminants, including pathogens and facilitates horizontal gene transfer, enhancing microbial resistance.3 Furthermore, these biofilm-coated plastics, resembling nutrient-rich foods, can be ingested by aquatic fauna, leading to pollutant bioaccumulation within the food chains, and increasing health risks to ecosystems and humans through seafood consumption.4 It has also been reported that the presence of biofilms alters the MPs' physicochemical properties, such as density, and adsorption capacity, which ultimately impacts the MPs' dispersion in freshwater environments.5
Biofilms on microplastics (MPs) in freshwater systems, particularly those influenced by wastewater (WW) effluents, are composed of complex microbial communities. These communities are embedded in an extracellular polymeric substance (EPS) matrix, primarily made up of polysaccharides and proteins.6 The development of biofilm on MPs starts with reversible adhesion through electrostatic, hydrophobic interactions, and van der Waals forces,7 progressing to irreversible adhesion via covalent, ionic, and hydrogen bonding,8 leading to mature biofilm formation characterized by EPS production and microcolony establishment,9 and concluding with dispersal.10 Among all the phases, the mature growth phase is crucial, promoting the exponential growth of the microbial community and the concomitant increase of EPS production. Despite its importance, this phase is underrepresented. Most research on MP biofilm relies on in situ incubation in natural environments. The extended incubation duration required in these studies makes continuous monitoring challenging, leading to the underrepresentation of the growth phase of microorganisms.11,12
MP properties significantly influence biofilm formation by affecting density, buoyancy, and interactions with microorganisms. Smaller MPs often present higher specific surface area, potentially enhancing biofilm formation by offering more space for microbial attachment.13 MP type affects biofilm composition, with PP showing higher microbial attachment than HDPE and LDPE.14 Initial density also influences biofilm development, with lighter MPs interacting more with plankton and heavier MPs with periphyton.15 However, it remains uncertain whether the observed biofilm variations across different MP types result from polymer composition or particle density differences, which represents a significant research gap. Besides, MP's intrinsic properties, surface characteristics like roughness, surface energy, and hydrophobicity also play roles, with rough, high-energy and hydrophobic surfaces promoting adhesion and microbial growth.16,17 However, this remains a research gap as the impact of these surface characteristics in freshwater environments influenced by wastewater, particularly during the growth phase of microorganisms, has not been extensively studied.
Environmental factors that affect MP biofilm formation include nutrient concentration, temperature, pH, dissolved oxygen, light, and flow velocity, each critically shaping biofilm characteristics.11,13,18 Nutrient-rich freshwater influenced by wastewater can enhance biofilm formation and microbial diversity.19 Flow velocity affects both the structure and microbial composition of biofilms, with varying rates altering biofilm dynamics and stability.20 These critical factors are underexplored, highlighting a crucial research gap that our study aims to address. Building on the identified research gaps, this work examines the influence of MPs' size on microbial growth and biofilm formation. It also investigates how flow velocity impacts these processes on MPs. This comprehensive study includes a detailed analysis of how various MP characteristics affect biofilm stability and microbial dynamics, shedding light on the ecological interactions within aquatic systems. This approach helps to better understand the mechanisms behind biofilm resilience or susceptibility to environmental conditions, contributing significantly to the broader field of microplastic pollution management.
2. Materials and methods
2.1. Microbial strain isolation, identification, and inoculum preparation
Bacterial strains were isolated from the effluent of the Keswick Water Resource Recovery Facility (WRRF) in Ontario, Canada. The collected wastewater effluent was serially diluted using phosphate buffer saline. The diluted samples were streaked onto Nutrient Broth (NB) agar plates and prepared with the following composition: 5 g L−1 peptone, 3 g L−1 beef extract, 5 g L−1 NaCl, and 15 g L−1 agar. The plates were incubated at 30 ± 1 °C for 24 hours. The inoculum was then cultured in liquid NB media under the same conditions (30 ± 1 °C, 200 rpm) for 24 hours. To identify the isolated strains, microbial DNA was extracted using a Quick-DNA Miniprep Plus Kit (Zymo Research) according to the supplier. The extracted DNA was used for Sanger sequencing using a 3730 DNA Analyzer Sequencing Standard, BigDye Terminator v1.1 (ThermoFisher Scientific, USA).2.2. Bacterial survival studies using synthetic freshwater as a model
Bacterial survival was studied using synthetic hard freshwater (mimic Lake Ontario) according to the United States Environmental Protection Agency (2002) protocol and supplemented with Suwannee River natural organic matter (NOM) from the International Humic Substances Society (IHSS) to simulate the organic complexity of natural freshwater systems (see ESI data†). Briefly, fresh hard water was prepared containing NaHCO3 (192 mg L−1), CaSO4·2H2O (120 mg L−1), MgSO4 (12 mg L−1), KCl (80 mg L−1), and a nutrient solution consisting of glucose (1000 mg L−1), ammonium chloride (100 mg L−1), and diammonium phosphate (10 mg L−1). An inoculum, seeded at 10% v/v with an initial OD600 nm of 0.1, was incubated at 30 °C and 200 rpm for 24 hours. Bacterial growth and glucose consumption were monitored every 3 hours. Frequent data collection was necessitated to capture the rapid initial microbial responses and metabolic changes in a controlled setting. All experiments were conducted in duplicate and analyzed using OriginPro 2024 software (OriginLab Corporation, Northampton, United States).2.3. Biofilm formation over MPs
To study the effect of biofilm formation on microplastics (MPs), Pseudomonas fluorescens and Comamonas thiooxydans were inoculated into 250 mL flasks containing 100 polyethylene (PE) microbeads of two distinct sizes—small (180–200 μm) and larger (3–4 mm)—purchased from Cospheric LLC (Somis, CA, USA), suspended in 50 mL of synthetic freshwater. The flasks were incubated at 30 °C and agitated at 200 rpm for 96 hours. Bacterial growth and glucose consumption were monitored every 12 hours. This interval was designed to accommodate the slower dynamics of microbial growth and biofilm development on microplastics, allowing gradual changes to be observed. To study the effect of flow velocity on biofilm formation on MPs, Pseudomonas fluorescens with small PE microbeads was chosen as a model. A pseudo-continuous flow bioreactor system was established, operating at three flow rates: 65, 50, and 35 mL min−1. These flow rates corresponded to flow velocities of 0.238, 0.11, and 0.077 m s−1 within a cylindrical flow channel respectively. This setup created distinct shear stress conditions to examine the influence of flow velocity on biofilm development (see ESI data†). These flow velocities allow for the examination of how incremental changes in shear stress influence biofilm formation on MPs. Furthermore, the pseudo system was operated as a fed-batch, replenishing nutrients every 96 hours. The system was operated at 30 °C for 10 days with bacterial growth and EPS production monitored every 48 h. Extended data collection periods were used to monitor long-term trends and the cumulative impact of flow velocities on biofilm stability and EPS production. All experiments were conducted in duplicate and analyzed using OriginPro 2024 software (OriginLab Corporation, Northampton, United States).2.4. Quality assurance and quality control (QA/QC)
To ensure the integrity of the experiments and to minimize the introduction of random MPs, rigorous QA/QC measures were implemented. Prior to experimentation, all PE microbeads were pre-washed by soaking in distilled water to remove any surface residues. The beads were then rinsed with 70% ethanol and air-dried under sterile conditions. All experiments were conducted within a laminar flow hood to minimize airborne contamination. Solutions and media were prepared using filtered, deionized water. Glassware and equipment were meticulously cleaned and sterilized before use. Laboratory surfaces were cleaned thoroughly, and researchers wore cotton lab coats and nitrile gloves to avoid introducing synthetic fibers or particles. Instrument calibration and standardization procedures were followed according to manufacturer guidelines.2.5. Analytical methods
Microbial growth was monitored by measuring the optical density (OD) at 600 nm using a Genesys 50 UV-visible spectrophotometer (Thermo Scientific, Toronto, Canada). Substrate consumption was measured using the total DNS-reducing sugar method.21 Protein content in extracellular polymeric substances (EPS) was quantified via the Lowry protein assay. About 0.2 mL of sample was mixed with 1 mL of Lowry reagent, followed by the addition of 0.1 mL of 1 N phenol reagent. The mixture was incubated for 30 minutes at room temperature, and the absorbance was measured at 750 nm.22 For biofilm morphological analysis, MP surfaces were observed using a Leica LAS EZ4 Microscope equipped with a camera at a magnification of 40×. Detailed biofilm morphology was further analyzed using a Thermo Fisher Quanta 3D scanning electron microscope (SEM).3. Results and discussion
3.1. Performance of isolated bacterial strains using synthetic hard freshwater
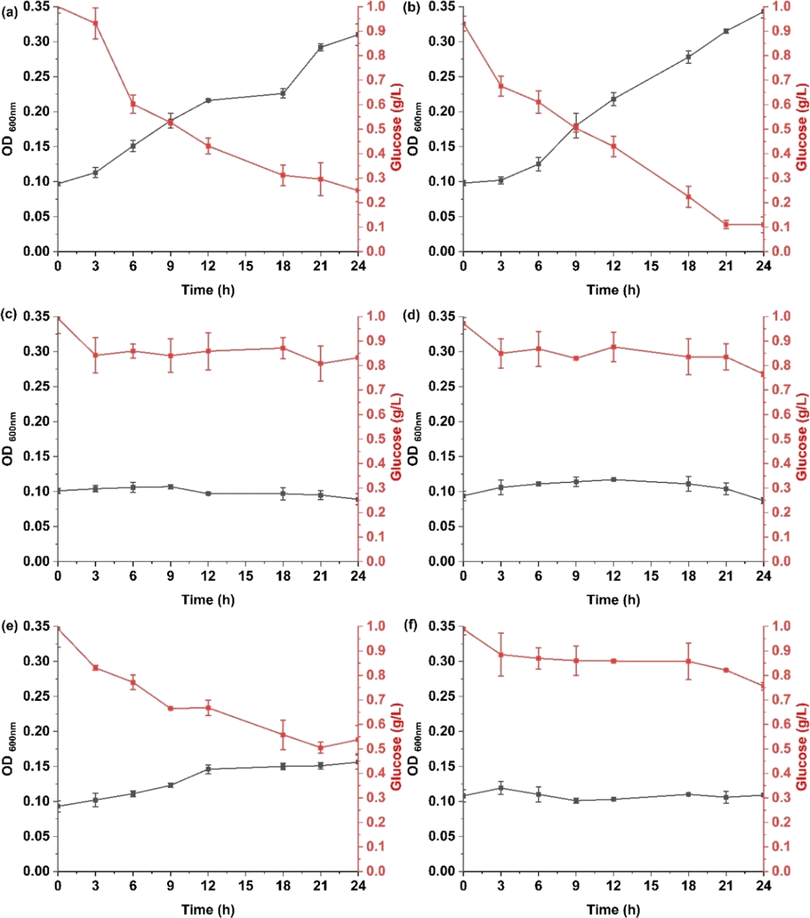
The screening for survival and metabolic competence of six bacterial strains isolated from wastewater effluent and growth in synthetic freshwater revealed notable differences in growth and glucose consumption. As shown in Fig. 1, WW1 and WW2 show superior microbial growth and glucose utilization profiles compared to other strains, with WW2 reaching an OD600 nm of 0.343, exhibiting an 88% consumption of the initial glucose concentration, while WW1 achieved a final OD600 nm of 0.310 and consumed 75% of the provided glucose. In contrast, other strains exhibited significantly lower growth and glucose consumption. Specifically, WW3, WW4, and WW6, which all started with an initial OD600 nm of 0.1, showed minimal increases, with final OD600 nm values slightly above the initial. These strains consumed less than 25% of the initial glucose, indicating poor adaptation to the synthetic freshwater environment and inefficient glucose metabolism. Consequently, only strains WW1 and WW2 were selected for subsequent MP biofilm experiments.3.2. Microplastic size as a determinant of microbial growth and biofilm development
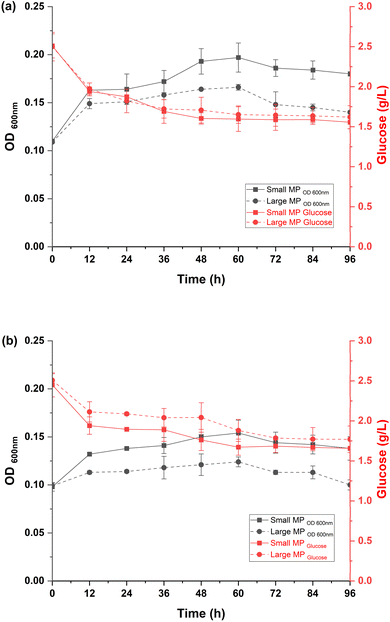
Of the six isolated bacteria, two of them (WW1 and WW2) showed better performance in synthetic hard fresh water, and to later identified as Pseudomonas fluorescens and Comamonas thiooxydans respectively. Fig. 2 shows the performance of P. fluorescens and C. thiooxydans in the presence of two different sizes of PE MPs. For P. fluorescens, growth was reduced to an OD600 nm of 0.197 with small MPs and 0.160 with large MPs, compared to 0.310 in the control, representing 1.57-fold and 1.94-fold decreases, respectively. Glucose consumption also dropped from 75% in the control to 40% with small MPs and 36% with large MPs. Similarly, C. thiooxydans showed growth reductions to an OD600 nm of 0.154 with small MPs and 0.124 with large MPs, compared to 0.343 in the control, indicating 2.23-fold and 2.77-fold decreases. Glucose consumption decreased from 90% in the control to 34% with smaller MPs and 30% with larger MPs.These results were indicative of C. thiooxydans being more sensitive to the presence of MPs than P. fluorescens, with a significant inhibitory effect on bacterial performance being demonstrated. Larger MPs were found to have a more pronounced impact, whereas smaller MPs, despite some inhibition, might support better microbial colonization and biofilm formation due to their higher surface area-to-volume ratio.23 This differential colonization pattern was also aligned with previous findings. Specifically, selective enrichment of Pseudomonas monteilii, Pseudomonas mendocina, and Pseudomonas syringae in smaller MPs biofilms was shown by Wu et al.24,25 Consequently, small PE microbeads paired with P. fluorescens have been chosen for further experiments to explore how flow velocities affect biofilm dynamics in continuous systems.
3.3. Flow velocity effect on biofilm development using small MPs and fresh hard water
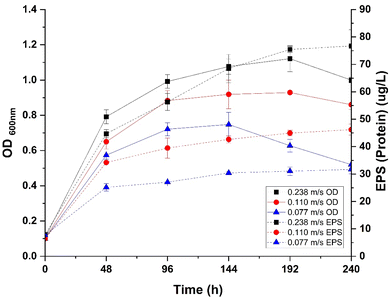
Fig. 3 illustrates the impact of varying flow velocities on biofilm development within a pseudo-continuous bioreactor system. In this system, the highest flow velocity (0.238 m s−1) was associated with a maximum OD600 nm of 1.127 at 144 hours, compared to lower flow velocities of 0.110 m s−1 and 0.077 m s−1, which exhibited maximum OD600 nm of 0.954 and 0.786, respectively. Additionally, protein content analysis indicates that EPS production is significantly influenced by flow velocity; a production of 76.61 μg L−1 was recorded at the highest flow velocity (0.238 m s−1), which is 1.66-fold higher than at 0.110 m s−1 (46.19 μg L−1) and 2.42-fold higher than at 0.077 m s−1 (31.67 μg L−1).The above results showed that higher flow velocities enhance both microbial growth and EPS production. Although enhancement in microbial growth was not found to be statistically significant (p-value = 0.1), whereas the increase in EPS production was statistically significant (p-value = 0.01). Additionally, an interesting result from the study was the positive correlation between microbial growth and EPS production, with a highly significant p-value (<0.0001).
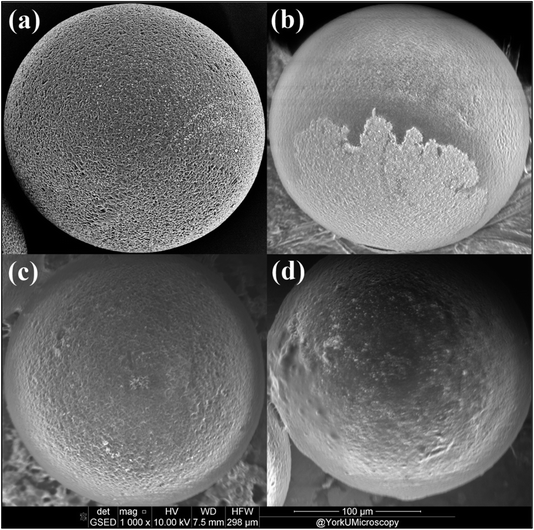
The results demonstrate that microbial growth and EPS production are enhanced by higher flow velocities, with a significant increase in EPS observed at these rates, likely due to improved nutrient and oxygen availability. Although this suggests the potential for more robust biofilm development, it is also shown that higher flow velocities impose greater shear stress, which could challenge the stable adherence of biofilms to microplastic surfaces.26 This phenomenon is supported by research in similar domains, where high flow velocities have been associated with increased biofilm erosion.27,28 The subsequent SEM images will explore how flow-induced stresses affect biofilm architecture on MPs, providing insights into the balance between growth facilitation and mechanical challenges in biofilm formation. In Fig. 4(b), patchy and less uniform biofilm coverage on MPs at the highest flow velocity (0.238 m s−1) is shown, suggesting that elevated shear forces hinder stable biofilm formation. In contrast, at the lowest flow velocity (0.077 m s−1), biofilms are observed to be more evenly distributed and coherent (Fig. 4(d)), indicative of more stable biofilm formation.
4. Conclusion
This study sheds light on the complex interactions between wastewater effluent, microplastics (MPs), and biofilm formation in freshwater environments. The survival strategies of opportunistic bacteria are highlighted, which pose significant challenges to ecosystem health and water quality. It is demonstrated that smaller MPs provide a more favorable surface for biofilm development due to their larger surface area-to-volume ratio. Increased flow rates, thus flow velocities are shown to boost microbial growth and EPS production, but they also generate shear stresses that disrupt stable microbial attachment, leading to biofilm erosion. These findings enhance the understanding of the ecological impacts of MPs, revealing how they serve as vectors for biofilm communities in aquatic systems.Further research is imperative to address several unresolved issues identified in this study. While the use of isolated strains provided valuable mechanistic insights, biofilms on MPs in natural systems are composed of complex microbial communities. Future studies should examine biofilm formation using mixed microbial consortia and a wider range of flow conditions incorporating scaling analyses to better relate laboratory findings to environmental scenarios. The long-term ecological impacts of biofilms on MPs, especially their role in harboring pathogens and influencing the transport and fate of pollutants, are key areas needing detailed examination. Additionally, the effects of MP weathering on biofilm formation are emphasized as requiring thorough investigation. This comprehensive approach is expected to deepen the understanding of microplastic pollution's ecological consequences and aid in developing more effective environmental protection measures.
Data availability
All data generated or analyzed during this study are included in the figures presented in this manuscript, which are self-explanatory and comprehensive. No ESI data files are associated with this submission. Upon publication, all relevant data will be contained within the article itself.Conflicts of interest
The authors report there are no competing interests to declare.Acknowledgements
This research was funded by the Natural Sciences and Engineering Research Council (Discovery Grant 23451, NSERC Alliance Option-2 Grants, ALLRP 571066 – 21, ALLRP-560377-20). We would also like to thank James and Joanne Love, Chair in Environmental Engineering, for their financial support.References
- E. R. Zettler, T. J. Mincer and L. A. Amaral-Zettler, Environ. Sci. Technol., 2013, 47, 7137–7146 CrossRef CAS PubMed.
- W. Boni, K. Parrish, S. Patil and N. L. Fahrenfeld, Water Environ. Res., 2021, 93, 334–342 CrossRef CAS PubMed.
- K. Abe, N. Nomura and S. Suzuki, FEMS Microbiol. Ecol., 2020, 96, fiaa031 CrossRef CAS PubMed.
- J. Moyal, P. H. Dave, M. Wu, S. Karimpour, S. K. Brar, H. Zhong and R. W. M. Kwong, Rev. Environ. Contam. Toxicol., 2023, 261, 8 CrossRef CAS.
- B. Zhang, X. Yang, L. Liu, L. Chen, J. Teng, X. Zhu, J. Zhao and Q. Wang, Sci. Total Environ., 2021, 770, 145303 CrossRef CAS PubMed.
- H.-C. Flemming and J. Wingender, Nat. Rev. Microbiol., 2010, 8, 623–633 CrossRef CAS PubMed.
- K. Hori and S. Matsumoto, Biochem. Eng. J., 2010, 48, 424–434 CrossRef CAS.
- M. H. Muhammad, A. L. Idris, X. Fan, Y. Guo, Y. Yu, X. Jin, J. Qiu, X. Guan and T. Huang, Front. Microbiol., 2020, 11, 928 CrossRef PubMed.
- K. Zhao, B. S. Tseng, B. Beckerman, F. Jin, M. L. Gibiansky, J. J. Harrison, E. Luijten, M. R. Parsek and G. C. L. Wong, Nature, 2013, 497, 388–391 CrossRef CAS PubMed.
- L. Hall-Stoodley, J. W. Costerton and P. Stoodley, Nat. Rev. Microbiol., 2004, 2, 95–108 CrossRef CAS PubMed.
- N. Wu, Y. Zhang, Z. Zhao, J. He, W. Li, J. Li, W. Xu, Y. Ma and Z. Niu, Sci. Total Environ., 2020, 708, 134876 CrossRef CAS PubMed.
- S. Oberbeckmann, B. Kreikemeyer and M. Labrenz, Front. Microbiol., 2017, 8, 2709 CrossRef PubMed.
- M. Kooi, E. H. van Nes, M. Scheffer and A. A. Koelmans, Environ. Sci. Technol., 2017, 51, 7963–7971 CrossRef CAS PubMed.
- S. Oberbeckmann, M. G. J. Loeder, G. Gerdts and A. M. Osborn, FEMS Microbiol. Ecol., 2014, 90(2), 478–492 CrossRef CAS PubMed.
- L. Miao, Y. Yu, T. M. Adyel, C. Wang, Z. Liu, S. Liu, L. Huang, G. You, M. Meng, H. Qu and J. Hou, J. Hazard. Mater., 2021, 403, 123577 CrossRef CAS PubMed.
- A. Kerr and M. J. Cowling, Philos. Mag., 2003, 83, 2779–2795 CrossRef CAS.
- M. Sudhakar, A. Trishul, M. Doble, K. Suresh Kumar, S. Syed Jahan, D. Inbakandan, R. R. Viduthalai, V. R. Umadevi, P. Sriyutha Murthy and R. Venkatesan, Polym. Degrad. Stab., 2007, 92, 1743–1752 CrossRef CAS.
- D. Kaiser, N. Kowalski and J. J. Waniek, Environ. Res. Lett., 2017, 12, 124003 CrossRef.
- S. He, M. Jia, Y. Xiang, B. Song, W. Xiong, J. Cao, H. Peng, Y. Yang, W. Wang, Z. Yang and G. Zeng, J. Hazard. Mater., 2022, 424, 127286 CrossRef CAS PubMed.
- J. P. Harrison, T. J. Hoellein, M. Sapp, A. S. Tagg, Y. Ju-Nam and J. J. Ojeda, in Freshwater Microplastics : Emerging Environmental Contaminants?, ed. M. Wagner and S. Lambert, Springer International Publishing, Cham, 2018, pp. 181–201 Search PubMed.
- G. L. Miller, Anal. Chem., 1959, 31, 426–428 CrossRef CAS.
- O. H. Lowry, N. J. Rosebrough, A. L. Farr and R. J. Randall, J. Biol. Chem., 1951, 193, 265–275 CrossRef CAS PubMed.
- A. A. Horton, A. Walton, D. J. Spurgeon, E. Lahive and C. Svendsen, Sci. Total Environ., 2017, 586, 127–141 CrossRef CAS PubMed.
- X. Wu, J. Pan, M. Li, Y. Li, M. Bartlam and Y. Wang, Water Res., 2019, 165, 114979 CrossRef CAS PubMed.
- C. Wu, K. Tanaka, Y. Tani, X. Bi, J. Liu and Q. Yu, Sci. Total Environ., 2022, 821, 153265 CrossRef CAS PubMed.
- I. Ieropoulos, J. Winfield and J. Greenman, Bioresour. Technol., 2010, 101, 3520–3525 CrossRef CAS PubMed.
- L. C. Gomes, J. M. R. Moreira, J. S. Teodósio, J. D. P. Araújo, J. M. Miranda, M. Simões, L. F. Melo and F. J. Mergulhão, Biofouling, 2014, 30, 535–546 CrossRef CAS PubMed.
- C. Picioreanu, M. C. van Loosdrecht and J. J. Heijnen, Biotechnol. Bioeng., 2001, 72, 205–218 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4va00303a |
| This journal is © The Royal Society of Chemistry 2025 |