Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Porosity and fluid pathway development during cadmium sequestration by calcium carbonate replacement†
Maude
Julia
 *a,
Christine V.
Putnis
ab,
Oliver
Plümper
c and
François
Renard
de
*a,
Christine V.
Putnis
ab,
Oliver
Plümper
c and
François
Renard
de
aInstitut für Mineralogie, Universität Münster, Corrensstrasse 24, 48149 Münster, Germany. E-mail: mjulia@uni-muenster.de
bSchool of Molecular and Life Sciences, Curtin University, Perth, 6845, Australia
cDepartment of Earth Sciences, Universiteit Utrecht, 3584 CB Utrecht, the Netherlands
dThe Njord Centre, Departments of Geosciences and Physics, University of Oslo, Oslo, Norway
eISTerre, Univ. Grenoble Alpes, Grenoble INP, Univ. Savoie Mont Blanc, CNRS, IRD, Univ. Gustave Eiffel, 38000 Grenoble, France
First published on 17th October 2024
Abstract
Cadmium contamination of ground water and soil has drastically increased in some areas through the last few decades and remediation strategies are currently being investigated. The coupled dissolution–precipitation of calcium carbonate in cadmium-containing solutions leads to the precipitation of a (Ca,Cd)CO3 phase of lower solubility, this process trapping cadmium from a solution into a solid phase. The present study analyses the reactions of two types of calcium carbonates (calcite as Carrara marble and aragonite) in cadmium solutions and compares the different reaction pathways and their respective efficiency. X-ray tomography scans of different Carrara marble and aragonite samples reacted in cadmium solutions for 16 to 64 days at 200 °C were acquired and analysed. The reaction in Carrara marble proceeds through a dissolution–precipitation reaction from the surface of the sample. The fluid moves through the porosity developed in the newly precipitated phase and along grain boundaries. Tomograms show that the porosity at the post-reaction time of imaging is mainly disconnected and that the reaction extent decreases with an increase in cadmium concentration of the solution. For aragonite, the main reaction pathway is opened by reaction-induced fracturing, which leads to a faster reaction than for the Carrara marble as the reaction pathways open faster towards the centre of the sample through successive hierarchical fracturing. The reaction rate for aragonite increases with time and cadmium concentration of the solution. Thus, the sequestration of cadmium from solution is potentially more efficient using aragonite due to the reaction-induced fracturing process taking place.
Environmental significanceHeavy metal contamination of soil and ground water caused by anthropogenic activities has increased in the last few decades and has become a real concern in some areas. Cadmium in one of these contaminant elements and its toxicity for humans through long term exposure has been shown to cause heavy renal and bone diseases. Our study focuses on the use of carbonate rocks as remediation materials as these are widely available and their reaction with cadmium-containing solutions can lead to the precipitation of less soluble phases containing cadmium through a coupled dissolution–precipitation mechanism. The study of the mechanisms of cadmium uptake by different carbonate materials will provide a base for the development of better remediation strategies. |
Introduction
Environmental pollution of surface waters has been steadily increasing in the years since industrialization mainly through anthropogenic activities, including mining, agriculture, industrial and pharmaceutical manufacturing processes. This leads to heavy metal water and soil pollution1–6 as well as excess phosphates7 from agricultural applications, causing eutrophication of waterways,8 and excess atmospheric CO2.9 Consequently, there is an increasing need to mitigate this contamination by sustainable methods using available and affordable materials. Many recent research activities are focusing attention on suitable methods to tackle toxic element contamination. Coupled dissolution–precipitation reactions have been studied as a potential means to remediate environmental pollution. Toxic elements such as Pb,10–12 Se,13–15 As,14,16 Sb,14,17 Cr,18 and Cd19–26 have all been studied to suggest potential decontamination strategies using dissolution–precipitation processes that involve minerals. Amongst the examples of pollution already cited, increasing soil and water cadmium (Cd) pollution has been a reason for concern in some regions.4,5 Anthropogenic cadmium pollution originates from activities such as non-ferrous metal mining, the intensive use of phosphate fertilizers or the disposal of municipal waste.27 Long term exposure to cadmium can lead to serious health problems such as osteomalacia and renal tubular failure.28–30 This has promoted the need to find remediation strategies to sequester cadmium from the soil and surface water.31–33Coupled dissolution–precipitation reactions allow the precipitation of a stable mineral phase, containing the target toxic element, through the dissolution of a parent mineral with a higher solubility in a contaminated solution. During a replacement reaction, if the difference in molar volumes of the parent and final phases, as well as their relative solubilities allows a reaction to proceed, this reaction can lead to the complete pseudomorphic replacement of the parent mineral.34 Additionally, for a pseudomorphic replacement to be complete, the new phase must provide a pathway for the fluid to reach the reaction interface with the parent material such as connected porosity, grain boundaries or fracture networks. Some coupled dissolution–precipitation reactions have been observed to lead to a complete pseudomorphic replacement of the parent material.35,36
Carbonates, such as limestone, dolomite, and marble, are common rocks in the crust of the Earth and their relative solubility and crystal structure allow for reactions with multi-component aqueous solutions to result in the formation of a new more stable (less soluble) phase. Therefore, reactions with carbonates present a viable possibility of achieving some acceptable degree of environmental remediation.10,11,13,16–18,37
The present study focuses on the replacement reactions between carbonates and solutions containing dissolved cadmium. Due to the existence of an ideal solid solution between calcite (CaCO3) and otavite (CdCO3),38,39 coupled dissolution–precipitation reactions have been investigated between CaCO3 and cadmium-containing solutions as a potential cadmium trapping strategy. As otavite has a slightly lower molar volume than both calcite and aragonite21 a potential replacement of the CaCO3 sample could be reached. Experiments have shown that the surfaces of calcite single crystals are passivated by cadmium in solution due to the epitaxial growth of a CdCO3 layer on the crystal surface.22,24 This is due to the similar crystallographic structure and lattice parameters of CaCO3 and CdCO3 as well as the lower solubility of CdCO3 compared to CaCO3. Our previous study21 showed that by using polycrystalline calcite (such as marble) or a polymorph of calcite (such as aragonite) a partial replacement of CaCO3 by a (Ca,Cd)CO3 solid solution could be achieved. The presence of grain boundaries and random orientation of the calcite grains in the case of Carrara marble have allowed the initial material to dissolve and be replaced with a porous (Ca,Cd)CO3 solid solution probably by hindering the epitaxial growth of otavite onto the calcite surface22 and facilitating access of the fluid into the sample. As the new precipitating phase has a lower molar volume and identical crystallographic structure to calcite, the replacement is pseudomorphic and does not cause fracturing of the sample. The replacement proceeded more efficiently from the surface than from the grain boundaries in the Carrara marble samples. In the case of aragonite during the reaction with a cadmium-containing solution, the change of crystallographic structure and subsequent interfacial stresses induced fracturing of the samples during the replacement reaction, allowing fluid penetration and replacement inside the sample. Reaction-induced fracturing has been observed and studied previously, showing that it could in some cases sustain a replacement reaction or inhibit it.40–44 However, all observations were made on two-dimensional images of sample sections and the progression of the reaction with time and solution concentration could not be quantified. More information about the reaction extent for both materials as well as their dominant fluid pathways is required to determine which material and type of reaction are more efficient for cadmium-sequestration from solution. This high pressure and temperature reaction could provide a potential method for water decontamination in industrial settings as well as a starting point to extend research on experimental conditions closer to an environmental setting.
In order to study the extent of the reaction for both aragonite and Carrara marble (calcite) we have reproduced some of the reactions from Julia et al.21 and analysed the resulting samples using 3D X-ray tomography imaging. Both aragonite and Carrara marble samples were reacted in cadmium solutions for different durations and solution concentrations and scanned by X-ray microtomography to obtain 3D X-ray adsorption tomograms. These tomograms were then compared to assess the extent of the reaction, development of porosity and potential fluid pathways, depending on the sample type, reaction time and solution composition.
Methods
Sample preparation
Cubes of Carrara marble (Italy), with dimensions of 3 × 3 × 3 mm3, consisting of compacted calcite grains, with an average size of approximately 300 μm, with randomly oriented grain boundaries, were cut with a saw from a single marble block in the absence of water, following the method of Julia et al.21 Crystals of aragonite (Molina de Aragón, Spain) were broken into 2–3 mm wide pieces. The purity of the starting materials was assessed by X-ray diffraction (the XRD spectra of the starting materials are available as ESI Fig. S1 and S2†).Solutions with cadmium concentrations of 0.4 M and 2 M were prepared with technical grade CdCl2 (99%, Fisher Scientific) dissolved in deionized water (resistivity > 18 mΩ cm). The pH of the solutions was measured with an Inolab pH/ION Level 2 pH meter and calculated with PHREEQC.45
The samples were reacted at 200 °C for 16, 32 or 64 days in a 0.4 or 2 M CdCl2 solution. 3 mL Teflon sample holders were filled with 2 mL of solution, with the sample and sealed in brass cylinders. The autogenous pressure reached in the sample holders during the reaction can be estimated to be equal to the vapor pressure, so 16 bars. After reaction the samples were cooled in a stream of compressed air, dried and imaged by X-ray microtomography.
X-ray microtomography acquisition
The acquisition of the X-ray microtomography scans for the Carrara marble samples after different durations of reactions was conducted at the beamline BM05 at the European Synchrotron Radiation Facility (ESRF) in Grenoble, France. Each Carrara marble sample was scanned at the beamline BM05 at ESRF, using a pink beam (e.g., white beam with two filters of 6.67 mm of aluminium and 20 mm of SiO2). For each volume, 2999 radiographs were acquired over a rotation of 360 degrees. The duration of each scan was 4 minutes. The volumes were reconstructed using phase contrast as in Mirone et al. (2014).46 The spatial resolution of the 3D tomograms is between two and three times the voxel size.The aragonite samples were scanned at the EPOS-NL MINT facility, Utrecht University, the Netherlands, with a Zeiss Xradia 610 Versa X-ray tomography microscope system equipped with a 160 kV X-ray source. A full volume of each sample was acquired with the voxel size necessary to attain full visualization of the sample. Four higher resolution scans were acquired in specific areas of some of the samples. The spatial resolution of the 3D tomograms is between three and five times the voxel size. The specifications of the different scans are provided in Table 1.
| Sample type (X-ray imaging) | Reaction conditions | Sample name | Scan type | Voxel size (μm) | Additional sample prepared for SEM |
|---|---|---|---|---|---|
| Carrara marble (ESRF, beamline BM05) | 16 days – 0.4 M CdCl2 | Carrara – 01 | Full sample | 2.3 | Yes |
| Zoom | 0.36 | ||||
| 32 days – 0.4 M CdCl2 | Carrara – 02 | Full sample | 2.3 | Yes | |
| Zoom | 0.36 | ||||
| 64 days – 0.4 M CdCl2 | Carrara – 03 | Full sample | 2.3 | Yes | |
| Zoom | 0.36 | ||||
| 16 days – 2 M CdCl2 | Carrara – 04 | Full sample | 2.3 | Yes | |
| Zoom | 0.36 | ||||
| 32 days – 2 M CdCl2 | Carrara – 05 | Full sample | 2.3 | Yes | |
| Zoom | 0.36 | ||||
| 64 days – 2 M CdCl2 | Carrara – 06 | Full sample | 2.3 | Yes | |
| Zoom | 0.36 | ||||
| Aragonite (Zeiss Xradia 610 Versa) | 16 days – 0.4 M CdCl2 | Aragonite – 01 | Full sample | 5.5 | No |
| Zoom | 0.43 | ||||
| 32 days – 0.4 M CdCl2 | Aragonite – 02 | Full sample | 5.5 | Yes | |
| Zoom | 0.8 | ||||
| 64 days – 0.4 M CdCl2 | Aragonite – 03 | Full sample | 5.5 | Yes | |
| Zoom | 0.43 | ||||
| Zoom | 0.43 | ||||
| 16 days – 2 M CdCl2 | Aragonite – 04 | Full sample | 6.7 | No | |
| 32 days – 2 M CdCl2 | Aragonite – 05 | Full sample | 6.7 | Yes | |
| 64 days – 2 M CdCl2 | Aragonite – 06 | Full sample | 6.7 | No |
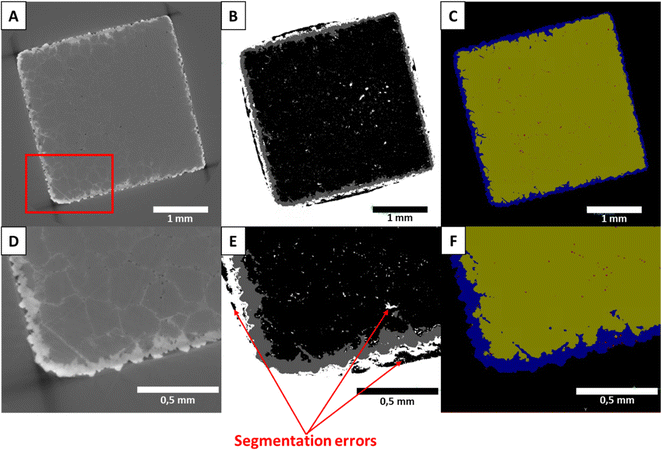
Data segmentation and analysis
The segmentation of the reacted Carrara marble cube dataset was conducted with the software Ilastik 1.3.3.47 The software was trained on 100 images to analyse a stack of 1000 images of each tomogram and separate the following phases: the background, the solid solution (the replaced phase of the samples), the remaining calcite, and the pores. Each of the 100 training images was manually segmented until the phase separation was completed before feeding the remaining images to the software for automatic segmentation. The top and bottom face of the samples were excluded to ensure a consistent segmentation by feeding the software with similar images. An example of the segmentation outcome is given in Fig. 1.The segmented Carrara marble datasets were then analysed using the software Thermo Scientific Avizo 3D 2023.1. Potential areas of phase misattribution were manually corrected and the volume of the respective phases was extracted as well as the porosity connectivity. In addition, the pore volume repartition was obtained using the software Dragonfly during re-examination of the dataset. After extraction of the volumes of the different segmented phases, the results were extrapolated to represent the full cube, instead of the analysed slice. This assumes that the reaction has the same behaviour on all faces of the cube.
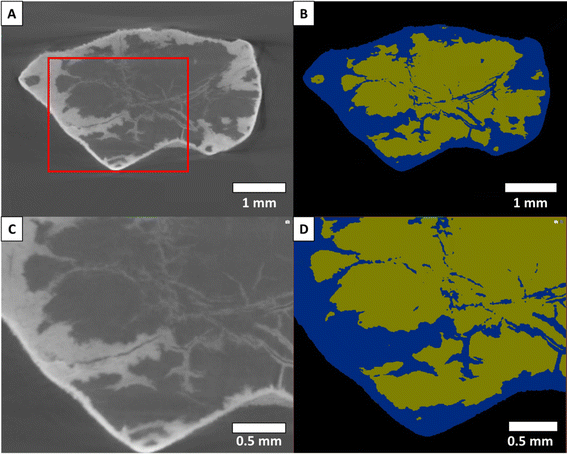
The reacted aragonite samples were segmented by grey-scale separation with the software Avizo 3D. The porosity could not be separated due to the combination of the pores small size and the limited resolution of the images. Only three phases were identified: the background, the solid solution (reacted sample) and the remaining aragonite.
Efficiency of the segmentation
As visible in Fig. 1(B) and (E), some areas of the Carrara marble samples segmented with Ilastik 1.3.3 are attributed to the wrong phase due to some shadow effects and closeness of grey levels present in the original dataset. Most of these misattributions consisted of some background attributed as calcite on the sides of the samples and spots of background falsely detected inside the samples. These artefacts were manually corrected during analysis and reattributed to the correct phase. A comparison of the different steps of the segmentation is shown in Fig. 1. The segmentation showed other limitations such as the non-separation of the crystals of CdCl2 precipitated on the surface of the sample during quenching of the reaction from the rest of the solid solution created by the replacement of the original material (SEM images of a reacted Carrara marble sample with highlighted CdCl2 crystals are available as ESI Fig. S3†). Moreover, the mineral replacement observed along the grain boundaries could not be segmented due to the low difference of grey level between the replaced phase and the original material combined with the thinness of the replacement along the grain boundaries. After thorough examination of the sample scans and their segmentation, it was concluded that only the thin (<5 μm) replacement layer along the grain boundaries was unsegmented and no larger replaced area had been omitted. Both these segmentation limitations were consistent within the dataset and deemed not to hinder the comparison of the samples.For the aragonite dataset, the crystals of CdCl2 precipitated on the surface (SEM image of an aragonite sample with highlighted CdCl2 crystals is available as ESI Fig. S4†) could not be separated from the rest of the replaced area as their colours on the grey level scale are overlapping and no specific shape/delimitation mark could provide a good ground for other segmentation methods such as watershed segmentation or the Ilastik 1.3.3 software. The porosity present in the samples could also not be segmented to a separate phase. Most of the porosity was segmented with the replaced phase, as shown in Fig. 2.
Scanning electron microscopy and elemental analysis
Additional aragonite and Carrara marble samples were reacted under similar conditions to those analysed by X-ray microtomography and were embedded in epoxy resin and cut through the centre. They were then carbon coated and observed by scanning electron microscopy (SEM) and energy-dispersive spectroscopy analysis. The reaction conditions used for these samples are shown in Table 1.Results
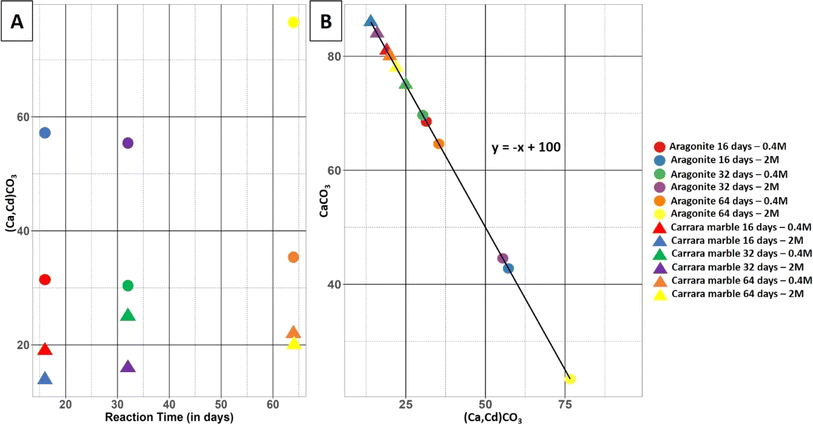
Extent of the reaction
The volumes of the replaced phase and remaining original material were extracted for all samples and are plotted against each other in Fig. 3.The data show that the replacement of the samples during the reaction is more advanced at equal time and solution concentration for the aragonite samples than for the Carrara marble samples (Fig. 3). The Carrara marble dataset shows that the reaction keeps progressing with time for both solutions tested. A slowing of the reaction is visible when the solution concentration is increased. The aragonite samples show little reaction progression between samples reacted for 16 and 32 days and a larger gap between samples reacted for 32 and 64 days. An increase in the solution concentration leads to a higher replacement rate and widens the gap between the samples reacted for 32 and 64 days. The replacement can be divided in two categories: the replacement progressing inside the sample through reaction-induced fracturing and the replacement layer on the sample surface. The thickness of the replaced layer along the sample surface was measured for samples Aragonite-01 to Aragonite-05 using the X-ray microtomography scans, as displayed in ESI Fig. S5† with the measurement value and its standard deviation for each sample. Sample Aragonite-06 presented too many fractures and a too advanced replacement to separate areas where the replacement progressed from fractures starting from the outer surface. One hundred measurements were taken from three cross-sections on each sample, carefully selecting areas as far as possible from fractures to avoid reaction interference. An example image of the thickness measurement of the surface replacement in an aragonite sample is provided in Fig. S6.† A slight increase in the layer thickness could be observed with increasing time of reaction for the samples reacted in 0.4 M CdCl2 solution as well as with an increase in cadmium concentration in the solution. This increase of thickness with reaction time was not consistent for the samples reacted in a 2 M CdCl2 solution, however the third sample of this set could not be used for comparison and sample specificities could have influenced the surface reaction.
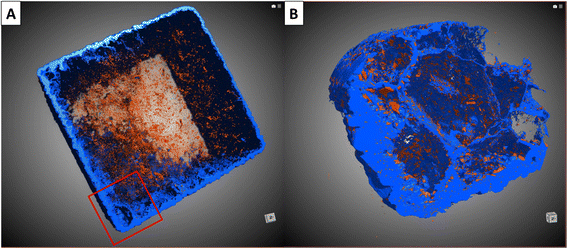
Porosity and connectivity
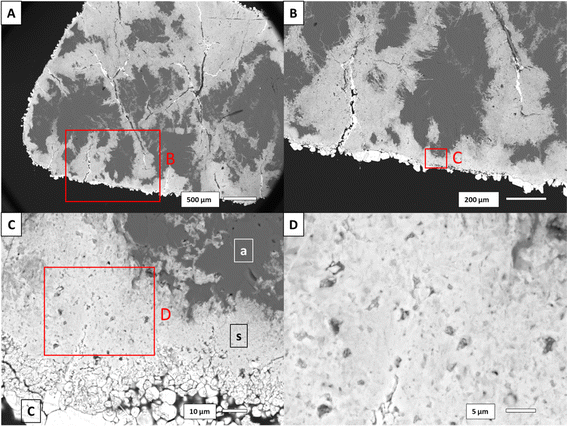
The porosity volume was extracted from the full scans of the Carrara marble dataset. A three-dimensional rendering of the porosity and the replaced area is shown in Fig. 4. The segmented pores represent less than 1% of the volume of each sample with no specific trend related to the reaction time and/or the solution concentration. The minimum pore size extracted for each sample was 10 μm3 and was limited by the segmentation method efficiency to detect small pores. The majority of the pores had a volume of the order of 10–100 μm3 (ESI Fig. S7† shows the pore size distribution for each Carrara marble sample). The pore connectivity was analysed from both the full scans and the zoom scans. Mostly the porosity was disconnected at the spatial resolution of the images and at the time when the scans were acquired (1–2 months after the end of the reaction).The porosity of the replaced phase in the aragonite dataset could not be segmented from the rest of the material. SEM images of a reacted aragonite sample sectioned through the middle were used to gather information about the porosity in the replaced phase. Fig. 5 shows the morphology of the replacement along newly formed fractures and a thin layer of replacement on the sides of the samples. The zoom in Fig. 5(B) shows that the thin layer of replacement along the sample surface is porous, although not being the area with the most replacement. The size of the pores visible by SEM in the aragonite sample goes down to ∼0.5 μm. SEM images of the porosity in both Carrara marble and aragonite samples reacted under similar conditions is available in the ESI as Fig. S8† as visual comparison.
Discussion
This study compares the extent and mechanism of the coupled dissolution–precipitation reaction of two different forms of CaCO3, Carrara marble consisting of compact calcite grains and single aragonite crystals, in cadmium-containing solutions leading to the precipitation of (Ca,Cd)CO3 solid solutions for different partial pseudomorphs of CaCO3. The extent of the reaction was more advanced for the aragonite samples than for the Carrara marble samples, for the same reaction conditions (Fig. 3). This difference may be explained by the different types of pathways used by the fluid to reach the unreacted material for the two types of samples and the small difference of solubility between calcite, – log KC = 11.47 at 200 °C, and aragonite, – log KA = 11.28 at 200 °C.48In the case of Carrara marble, it has already been shown that fluid can penetrate along the grain boundaries and through newly formed connected porosity in the replaced areas.21,35,49,50 The respective molar volumes of calcite (36.93 cm3 mol−1) and otavite (33.97 cm3 mol−1)21 are quite close and show that the replacement of calcite by otavite or a (Ca,Cd)CO3 solid solution is not optimal for the creation of porosity. The analysis of the porosity inside the reacted Carrara marble samples, after segmentation of X-ray tomograms, does not show any connectivity at the resolution of the tomograms acquired in the present study. However, the advancement of the reaction through time observed in Fig. 3 suggests that fluid pathways allowed the reaction to progress. It is possible that the cooling process of the samples before imaging caused a reequilibration of the pore space leading to the disappearance of pore connectivity through the minimising of surface area. Textural reequilibration during a replacement reaction is driven by a reduction of surface energy and can result in a loss of pore connectivity.36 X-ray tomography was carried out several weeks post experiments giving time for loss of pore connectivity. Moreover, the porosity analysed is the result of the segmentation of the scans and porosity with a size smaller than the resolution of the segmentation (<0.5 μm) could not be imaged, causing a loss of information concerning nanopores. Smaller pores have probably been present and have provided a pathway for the fluid towards the centre of the sample. Furthermore it has been shown that precipitation could be inhibited in nanopores compared to larger pores,51,52 which would allow the pores, if interconnected, to provide an open fluid pathway during the reaction before their reequilibration.
The porosity of the aragonite dataset could not be analysed, however information about reaction pathways could still be deduced, implying interconnected pathways must have existed. The analysis of the replacement layer on the sample surface, which occurred without help of nearby fracturing, shows that the porosity in this layer is probably connected as the thickness of this layer increased through time (Fig. S5†), which requires a fluid pathway to be opened. The respective molar volumes of aragonite (34.15 cm3 mol−1) and otavite (33.97 cm3 mol−1)21 are extremely close and not optimal for porosity creation. Although the porosity seems connected, it was not the principal fluid pathway used by the reaction to proceed through the aragonite sample. The replacement of aragonite by a (Ca,Cd)CO3 solid solution leads to reaction-induced fracturing of the material as visible in Fig. 5(A) where fractures cross the sample in a hierarchical manner despite their absence from the starting material. These fractures could have been caused by stresses at the reaction interface with the precipitation of the new phase with a different crystallographic orientation as there was no increase in molar volume which could have created internal stress in the aragonite crystals. Another explanation for the presence of reaction-induced fracturing has been highlighted by Monasterio-Guillot et al. (2021),53 who suggest that the pressure exerted by crystallization of a mineral at the surface of another mineral can lead to a build up of stress sufficient to induce fracturing of the material. It was observed that the precipitation of Na-phillipsite in etch pit edges and cracks of their host material's surface created enough stress to cause fracturing. It is possible that the presence of defects on our aragonite samples, caused during their preparation, could potentially have acted as favourable precipitation sites for otavite crystals. In the case where the supersaturation reached at the surface of our samples was high enough, the precipitation of otavite would have led to the accumulation of stress and fracturing of the aragonite crystals. These fractures opened new fluid pathways and promoted higher reaction rates, which explains why more replacement is observed inside the sample compared to the more limited replacement happening on the sample surface. After the initial surface reaction and appearance of the primary fractures from the sample surface, the aragonite reacts further with the solution and is replaced by the solid solution phase, leading to the formation of secondary fractures that keep providing new pathways for the fluid. This fracturing network, in addition to the porosity present in the newly precipitated phase, enables the fluid to reach the centre of the samples.40,44 This reaction-induced fracturing-driven replacement process also explains the behaviour of the reaction progress in the aragonite samples displayed in Fig. 3. The reaction advances faster with time, which is the case for a transport-driven process and is due to the decrease of the distance between unreacted material and bulk fluid when fractures appear.40
The replacement of CaCO3 by (Ca,Cd)CO3 is limited by the transport of cadmium from the bulk fluid to the reaction interface. As observed by Julia et al.21 in the Carrara marble samples reacted in cadmium solutions, the cadmium concentration in the (Ca,Cd)CO3 solid solution was lower close to the reaction interface and in the grain boundaries than in the bulk of the reacted volume. This observation has been explained by the kinetic limitations on the equilibration of a solution inside the restricted volume of the sample porosity with the bulk solution, leading to a reduction of cadmium in the solution at the reaction interface. This process could explain the slower reaction rate for Carrara marble compared to aragonite. The fluid needs to proceed through the porosity and grain boundaries in the Carrara marble cubes. This restricted space, also associated with the possibility of cadmium complexes attaching to the pore surfaces, could simultaneously slow down fluid transport and hence the dissolution reaction,54 and would slow down the equilibration of the fluid inside the sample with the bulk fluid. Effectively this is a kinetically controlled reaction. Moreover, the porosity evolves during the reaction and it is possible that a partial disconnection of the pores takes place even before the cooling process and analysis of the samples, reducing the fluid mobility inside the sample. On the other hand, the aragonite samples underwent reaction-induced fracturing, leading to the creation of new and wider fluid pathways that could be replenished from the bulk fluid quickly after their formation. The fluid at the reaction interface would then have a composition closer to the bulk fluid than in the case of Carrara marble, avoiding evolved equilibration and the slowing down of the reaction. Moreover calcite and otavite present an excellent epitaxy22 and the precipitation of a partially pore free otavite layer on some parts of the calcite surface at the reaction interface could contribute to the slow reaction rate observed.
X-ray tomography as an analytical tool has many advantages, enabling the observation of the whole sample instead of extrapolating results from two-dimensional slices and introducing a time dimension in the analysis. The reaction quantification in this study is made easier and more precise using three-dimensional tomograms as the samples can present specificities and irregularities, which can impact extrapolation from two-dimensional slices. This is especially important for the aragonite samples as the replacement proceeds mainly through reaction-induced fracturing, a process which is hierarchical and irregular throughout the sample. In this case the analysis of the microtomography scans is a more precise and reliable way to extract information about the evolution of the reaction with time and solution concentration. Moreover, the analysis of the microtomography scans of the Carrara marble dataset was necessary to gather information about the porosity volume and connectivity. This analysis allowed the interpretation of a probable closure of the porosity after termination of the reaction and showed that the amount and size of the porosity were similar in all samples. However, the analysis of X-ray microtomography scans has some limitations, mainly due to the segmentation method used, with the omission of nanoporosity (with a diameter lower than 100 nm) in the segmentation as well as the difficulty to extract porosity information in the aragonite dataset because of the limited resolution of the scan and sample specificities, such as grey level difference between the phases or interferences during imaging. Nanoporosity can have a strong impact on the permeability and reactivity of a material as constrained volume modifies the dissolution/precipitation behaviour of minerals.49 Some recent studies have developed new techniques to extract information on porosity distribution and connectivity using two-dimensional images of the samples and using these to model the porosity,55,56 which could be of interest for further studies of the CaCO3 to (Ca,Cd)CO3 replacement in cadmium-solutions. X-ray microtomography also provides important time-related information on the process, such as the acceleration of aragonite replacement with time, which is consistent with a reaction-induced fracturing replacement pathway. Moreover, although not possible in the case of this study, other studies observing reactions in minerals through time used dynamic imaging of their samples by imaging the same sample in situ at discrete time steps throughout the reaction.57,58 By doing so, studies can be emancipated from the variations due to the specificities of natural samples, such as grain size variation, inclusions, and defects, and hence observe the evolution of a reaction with greater precision.
Conclusion
The present study enables a comparison of the efficiency of the replacement of CaCO3 by (Cd,Ca)CO3 solid solutions for Carrara marble and aragonite. Observations showed that aragonite reacts faster in cadmium-containing solutions than Carrara marble due to the reaction-driven fracturing process occurring in the samples that provides new and efficient pathways for fluid infiltration and subsequent reaction. Both datasets showed that the newly formed phase contained porosity. Overall, the porosity in the replaced phase was shown to provide a pathway for the fluid in both aragonite and Carrara marble samples as reaction progress could be observed. However, the aragonite dataset showed that reaction-induced fracturing could create a more efficient fluid pathway and could increase reaction rates with time due to the multiplication of the fracture network through the progression of the reaction. This study shows that CaCO3 as aragonite can act as an effective sink for cadmium-contaminated fluids and consequently be beneficial for water decontamination. However both aragonite and Carrara marble were able to effectively sequester cadmium. The reactions presented in this paper require high temperature and pressure, and so are applicable to industrial decontamination. Under environmental low temperature and pressure conditions, we suggest that as calcite and aragonite solubilities decrease with temperature, the reaction could also provide an efficient decontamination strategy in natural settings. The impact of the reaction-driven fracturing in aragonite could be modified by the pressure and temperature changes and thus, similar reactions should be investigated at lower temperature and pressure in order to assess their efficiency for environmental remediation.Data availability
Additional data such as X-ray diffraction spectra of starting materials, Back-scattered electron images of reacted samples, images of measurement methods applied on the aragonite tomographs and a figure showing the pore size distribution in the Carrara marble samples are available in the ESI.† The tomographs of both datasets are available on the repository ScienceDB: Maude Julia, Christine V. Putnis, François Renard, et al. Tomographs of Carrara marble samples reacted in cadmium chloride solution at 200 °C[DS/OL]. V1. Science Data Bank, 1[2024-08-15]. https://cstr.cn/31253.11.sciencedb.11705. CSTR:31253.11.sciencedb.11705. Maude Julia, Christine V. Putnis, Oliver Plümper, et al. Tomographs of aragonite samples reacted in cadmium chloride solution at 200 °C[DS/OL]. V1. Science Data Bank, 2024[2024-08-15]. https://cstr.cn/31253.11.sciencedb.11915. CSTR:31253.11.sciencedb.11915.Conflicts of interest
The authors have no conflicts of interest to declare.Acknowledgements
We thank Elodie Boller at the European Synchrotron Radiation Facility for the acquisition of the X-ray tomograms of the Carrara marble samples. We also thank Yuntao Ji for the acquisition of X-ray tomograms of the aragonite samples at Utrecht University. Additionally we thank Maik Trogisch in the preparation workshop of the Institut für Mineralogie, University of Münster, for his careful sample preparation enabling tomography and SEM analyses. This project received funding from the European Union's Horizon 2020 research and innovation programme under the Marie Skłodowska-Curie initial training network (FluidNET) grant agreement No. 956127. This project also received extra funding from the H2020 EXCITE project (grant agreement no. 101005611) for Transnational Access conducted at the MINT facility at Utrecht University for the aragonite dataset X-ray tomogram acquisition.References
- S. V. Mohan, P. Nithila and S. J. Reddy, J. Environ. Sci. Health, Part A: Environ. Sci. Eng. Toxic Hazard. Subst. Control, 1996, 31, 283–289 CrossRef.
- O. Morton-Bermea, E. Hernández-Álvarez, G. González-Hernández, F. Romero, R. Lozano and L. E. Beramendi-Orosco, J. Geochem. Explor., 2009, 101, 218–224 CrossRef CAS.
- A. S. Mohammed, A. Kapri and R. Goel, in Biomanagement of Metal-Contaminated Soils, ed. M. S. Khan, A. Zaidi, R. Goel and J. Musarrat, Springer Netherlands, Dordrecht, 2011, vol. 20, pp. 1–28 Search PubMed.
- Z. Li, Z. Ma, T. J. Van Der Kuijp, Z. Yuan and L. Huang, Sci. Total Environ., 2014, 468–469, 843–853 CrossRef CAS.
- M. A. Khan, S. Khan, A. Khan and M. Alam, Sci. Total Environ., 2017, 601–602, 1591–1605 CrossRef CAS.
- Q. Yang, Z. Li, X. Lu, Q. Duan, L. Huang and J. Bi, Sci. Total Environ., 2018, 642, 690–700 CrossRef CAS PubMed.
- Phosphorus in Agriculture: 100 % Zero, ed. E. Schnug and L. J. De Kok, Springer Netherlands, Dordrecht, 2016 Search PubMed.
- G. M. Filippelli, Elements, 2008, 4, 89–95 CrossRef CAS.
- S. R. Gislason and E. H. Oelkers, Science, 2014, 344, 373–374 CrossRef CAS PubMed.
- F. Di Lorenzo, G. Cametti, D. Vanhecke and S. V. Churakov, Cryst. Growth Des., 2020, 20, 6157–6169 CrossRef CAS.
- A. Godelitsas, J. M. Astilleros, K. Hallam, S. Harissopoulos and A. Putnis, Environ. Sci. Technol., 2003, 37, 3351–3360 CrossRef CAS PubMed.
- R. J. Reeder, G. M. Lamble and P. A. Northrup, Am. Mineral., 1999, 84, 1049–1060 CrossRef CAS.
- C. V. Putnis, F. Renard, H. E. King, G. Montes-Hernandez and E. Ruiz-Agudo, Environ. Sci. Technol., 2013, 47, 13469–13476 CrossRef CAS PubMed.
- F. Renard, A. Røyne and C. V. Putnis, Geosci. Front., 2019, 10, 17–27 CrossRef CAS.
- F. Renard, G. Montes-Hernandez, E. Ruiz-Agudo and C. V. Putnis, Chem. Geol., 2013, 340, 151–161 CrossRef CAS.
- F. Renard, C. V. Putnis, G. Montes-Hernandez, E. Ruiz-Agudo, J. Hovelmann and G. Sarret, Geochim. Cosmochim. Acta, 2015, 159, 61–79 CrossRef CAS.
- F. Renard, C. V. Putnis, G. Montes-Hernandez, H. E. King, G. D. Breedveld and G. Okkenhaug, Environ. Sci. Technol., 2018, 52, 107–113 CrossRef CAS PubMed.
- M. G. Guren, C. V. Putnis, G. Montes-Hernandez, H. E. King and F. Renard, Chem. Geol., 2020, 552, 119770 CrossRef CAS.
- V. G. R. Chada, D. B. Hausner, D. R. Strongin, A. A. Rouff and R. J. Reeder, J. Colloid Interface Sci., 2005, 288, 350–360 CrossRef CAS PubMed.
- J. A. Gómez del Río, P. J. Morando and D. S. Cicerone, J. Environ. Manage., 2004, 71, 169–177 CrossRef.
- M. Julia, C. V. Putnis, H. E. King and F. Renard, Chem. Geol., 2023, 621, 121364 CrossRef CAS.
- C. Pérez-Garrido, L. Fernández-Díaz, C. M. Pina and M. Prieto, Surf. Sci., 2007, 601, 5499–5509 CrossRef.
- S. L. Riechers, K. M. Rosso and S. N. Kerisit, J. Phys. Chem. C, 2017, 121, 5012–5019 CrossRef CAS.
- S. L. Riechers and S. N. Kerisit, Cryst. Growth Des., 2018, 18, 159–170 CrossRef CAS.
- J. M. Zachara, C. E. Cowan and C. T. Resch, Geochim. Cosmochim. Acta, 1991, 55, 1549–1562 CrossRef CAS.
- S. J. Köhler, P. Cubillas, J. D. Rodríguez-Blanco, C. Bauer and M. Prieto, Environ. Sci. Technol., 2007, 41, 112–118 CrossRef PubMed.
- A. Kubier, R. T. Wilkin and T. Pichler, Appl. Geochem., 2019, 108, 104388 CrossRef CAS.
- L. Järup and A. Åkesson, Toxicol. Appl. Pharmacol., 2009, 238, 201–208 CrossRef.
- K. Aoshima, Soil Sci. Plant Nutr., 2016, 62, 319–326 CrossRef CAS.
- G. F. Nordberg, A. Åkesson, K. Nogawa and M. Nordberg, in Handbook on the Toxicology of Metals, Elsevier, 2022, pp. 141–196 Search PubMed.
- World Health Organization, Exposure to Cadmium: A Major Public Health Concern, 2010, WHO/CED/PHE/EPE/19.4.3 Search PubMed.
- M. Uchimiya, ACS Sustainable Chem. Eng., 2014, 2, 2019–2027 CrossRef CAS.
- L. Lei, X. Cui, C. Li, M. Dong, R. Huang, Y. Li, Y. Li, Z. Li and J. Wu, Chemosphere, 2022, 286, 131684 CrossRef CAS.
- K. Pollok, C. V. Putnis and A. Putnis, Am. J. Sci., 2011, 311, 211–236 CrossRef CAS.
- E. T. Pedrosa, C. V. Putnis and A. Putnis, Chem. Geol., 2016, 425, 1–11 CrossRef CAS.
- C. V. Putnis, K. Tsukamoto and Y. Nishimura, Am. Mineral., 2005, 90, 1909–1912 CrossRef CAS.
- S. J. Freij, A. Putnis and J. M. Astilleros, J. Cryst. Growth, 2004, 267, 288–300 CrossRef CAS.
- E. Königsberger, R. Hausner and H. Gamsjäger, Geochim. Cosmochim. Acta, 1991, 55, 3505–3514 CrossRef.
- Q. Wang and N. H. de Leeuw, Mineral. mag., 2008, 72, 525–529 CrossRef CAS.
- B. Jamtveit, C. V. Putnis and A. Malthe-Sørenssen, Contrib. Mineral. Petrol., 2009, 157, 127–133 CrossRef CAS.
- B. Jamtveit and Ø. Hammer, Geochem. perspect., 2012, 1, 418–432 Search PubMed.
- O. Plümper, A. Røyne, A. Magrasó and B. Jamtveit, Geology, 2012, 40, 1103–1106 CrossRef.
- F. Renard, J. Geophys. Res.: Solid Earth, 2021, 126, e2020JB021451 CrossRef CAS.
- C. Perdikouri, S. Piazolo, A. Kasioptas, B. C. Schmidt and A. Putnis, Eur. J. Mineral., 2013, 25, 123–136 CrossRef CAS.
- D. L. Parkhurst and C. A. J. Appelo, User's Guide to PHREEQC (Version 2) : a Computer Program for Speciation, Batch-Reaction, One-Dimensional Transport, and Inverse Geochemical Calculations, 1999 Search PubMed.
- A. Mirone, E. Brun, E. Gouillart, P. Tafforeau and J. Kieffer, Nucl. Instrum. Methods Phys. Res., B, 2014, 324, 41–48 CrossRef CAS.
- S. Berg, D. Kutra, T. Kroeger, C. N. Straehle, B. X. Kausler, C. Haubold, M. Schiegg, J. Ales, T. Beier, M. Rudy, K. Eren, J. I. Cervantes, B. Xu, F. Beuttenmueller, A. Wolny, C. Zhang, U. Koethe, F. A. Hamprecht and A. Kreshuk, Nat. Methods, 2019, 16, 1226–1232 CrossRef CAS.
- C. L. Christ, P. B. Hostetler and R. M. Siebert, J. Res. U.S. Geol. Surv., 1974, 2, 175–184 CAS.
- L. H. Filiberto, C. V. Putnis and M. Julia, Contrib. Mineral. Petrol., 2023, 178, 53 CrossRef CAS.
- L. Jonas, T. John, H. E. King, T. Geisler and A. Putnis, Earth Planet. Sci. Lett., 2014, 386, 64–74 CrossRef CAS.
- B. Jamtveit, M. Kobchenko, H. Austrheim, A. Malthe-Sørenssen, A. Røyne and H. Svensen, J. Geophys. Res., 2011, 116, B12204 CrossRef.
- A. G. Stack, A. Fernandez-Martinez, L. F. Allard, J. L. Bañuelos, G. Rother, L. M. Anovitz, D. R. Cole and G. A. Waychunas, Environ. Sci. Technol., 2014, 48, 6177–6183 CrossRef CAS PubMed.
- L. Monasterio-Guillot, A. Fernandez-Martinez, E. Ruiz-Agudo and C. Rodriguez-Navarro, Geochim. Cosmochim. Acta, 2021, 304, 258–280 CrossRef CAS.
- W. Stumm, Colloids Surf., A, 1997, 120, 143–166 CrossRef CAS.
- H. Amiri, H. Vogel and O. Plümper, J. Geophys. Res.: Mach. Learn. Comput., 2024, 1, e2024JH000178 Search PubMed.
- A. Chogani and O. Plümper, Contrib. Mineral. Petrol., 2023, 178, 78 CrossRef CAS PubMed.
- F. Marone, C. M. Schlepütz, S. Marti, F. Fusseis, A. Velásquez-Parra, M. Griffa, J. Jiménez-Martínez, K. J. Dobson and M. Stampanoni, Front. Earth Sci., 2020, 7, 346 CrossRef.
- C. Noiriel and F. Renard, C. R. Geosci., 2022, 354, 255–280 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4va00316k |
| This journal is © The Royal Society of Chemistry 2025 |