Open Access Article
Open Access ArticleGeochemical speciation, pollution assessment, and source identification of heavy metals in sediment cores of the Cau River basin, Hai Duong province, Vietnam†
Ba Lich Pham‡
 *a,
Huy Thong Vuab,
Van Linh Nguyena,
Thi Kim Thuong Nguyen
*a,
Huy Thong Vuab,
Van Linh Nguyena,
Thi Kim Thuong Nguyen a,
Anh Duc Trinh
a,
Anh Duc Trinh c and
Thi Thao Ta
c and
Thi Thao Ta *a
*a
aDepartment of Analytical Chemistry, Faculty of Chemistry, VNU University of Science, 19 Le Thanh Tong Street, Hanoi 100000, Vietnam. E-mail: phambalichls@gmail.com; tathithao@hus.edu.vn
bFundamental Sciences Department, University of Fire Fighting and Prevention, Hanoi 100000, Vietnam
cNuclear Training Center, Vietnam Atomic Energy Institute, 140 Nguyen Tuan, Thanh Xuan, Hanoi 100000, Vietnam
First published on 17th February 2025
Abstract
Heavy metal contamination in sediment has caused severe threats to the aquatic ecosystem and public health worldwide. Networks of rivers and their tributaries serve as dynamic habitats for these potentially harmful metals through aqueous–sedimentary equilibrium shifts. Hence, determining the distinct chemical forms of a given heavy metal in sediment is crucial for evaluating its bio-lability and toxicity. This study demonstrates the geochemical speciation using a sequential extraction procedure to fractionate individual phases (exchangeable, carbonate, Fe–Mn oxide, organic, and residual) of nine heavy metals (Cu, Pb, Cd, Zn, Fe, Co, Ni, Mn, and Cr) in the sediment of a river system in Hai Duong, a deltaic province in Vietnam. A quantitative assessment of environmental risk factors (e.g., contamination factor and risk assessment code) and the pseudo-partitioning coefficient between pore water and sediment was conducted to define the pollution levels of heavy metals and their contaminated areas. Furthermore, multivariate analyses facilitate a profound comprehension of the contributions to pollution. Analyses of the extracts from the sequential extraction procedure were performed by inductively coupled plasma-mass spectrometry. The results of sedimentary heavy metal speciation indicate that the critical risks of Cd (15.8–38.4%) and Mn (16.3–53.8%) to the aquatic ecosystem are due to their higher retrieval from the exchangeable fraction. Additionally, an appreciable percentage of Co (26.3–58.0%), Mn (16.8–66.3%), Ni (16.0–53.1%), Pb (6.75–69.7%), and Zn (4.42–45.8%) in the carbonate fraction highlights a strong tendency for co-precipitation or ion exchange of these metals with carbonate minerals. Whilst colloids of Fe–Mn oxides act as efficient scavengers for metals such as Fe, Mn, Zn, and Pb, organic matter forms primarily function in trapping Cu, Pb, Fe, Cr, Co, and Ni. Our findings in the ecological risk evaluations and multivariate analyses indicate that Cr, Ni, and Fe are ascribed to natural lithogenic provenances. In contrast, anthropogenic inputs induce Cd, Mn, Cu, and Pb high-environmental risks.
Environmental significanceHeavy metals are critically hazardous pollutants that pose a significant threat to public health due to their accumulation in the food chain. Deciphering their transport mechanisms between the water phase and sediment in river systems is crucial not only for tracing their emission sources but also for devising remediation strategies and sustainable development measures to protect aquatic ecosystems. This study conducted a comprehensive and methodical investigation of their pollution levels in a municipal watercourse system in Hai Duong, part of Vietnam's Northern Key Economic Zone. The findings indicate that high-risk elements predominantly liberate from urban runoff, sewage drainage, and industrial wastewater discharge, while low-risk metals are primarily ascribed to lithogenic origins. These observations will provide valuable references for local authorities to implement prompt supervision and solutions for managing toxic metals on the surface waters of Hai Duong province. |
1. Introduction
Nowadays, the pollution of heavy metals (HMs) in the aquatic environment is a global concern due to their potent effects on the food chain, particularly humans. HMs are naturally found on Earth but in stable and benign forms. However, anthropogenic activities tend to accumulate them in bio-available forms.1 The increase of HMs in labile forms is attributed to natural sources and anthropogenic activities such as industrial development, mining and refining, agricultural drainage, domestic discharges, and atmospheric deposition.2 Consequently, their genotoxicity and carcinogenicity, persistence, and abiotic degradation in the ecological system pose a serious risk to human health. Specifically, the respiratory, nervous, reproductive, and digestive systems are particularly susceptible to HMs and may even display apoptosis/necrosis pathways.3,4HMs transported into aquatic systems are mainly incorporated into sediments through adsorption, flocculation, ion exchange, precipitation, and complexation in the water column.5,6 Sediments serve as the archive of HMs and therefore become the storage of potentially hazardous metals.7 HMs entering natural water become part of the water column–sediment system, and their distribution is controlled by a dynamic equilibrium of numerous physico-chemical processes. HM dissolution occurs as a function of environmental conditions (e.g., temperature, pH, redox potential, electrical conductivity, and organic ligand contents), which usually increase contamination of aquatic systems.8 The potential for environmental pollution can be diminished if these metals are locked in sediments.9 However, HMs are not permanently trapped in sediment due to exchange processes of metals with carbonates, oxides, sulfides, organometallic compounds, and ions in the crystal lattice of minerals such as Fe(III) stored in ferrihydrite. Providing only data on the metal abundance in samples, total HM concentrations could not be the basis for assessing the toxicity and potential risks of metal pollutants.10,11 Different forms of a given metal have different impacts on the ecosystem. Therefore, it is necessary to conduct metal speciation analysis in sediments to estimate the actual impacts and the mobility of metals via downstream transport, deposition, and release to biota under environmental condition changes.12 Various studies have recently investigated and reported contaminated conditions with distinctive spatial and temporal distributions and ecotoxicological impacts of HMs in sediments along rivers/lakes across different regions, e.g., Brazil,13 Greece,14 Turkey,1 Bangladesh,15 India,16,17 Pakistan,18–21 Egypt,22,23 Iran,24–26 China,27–34 etc.
Speciation refers to a description of the chemical forms of metal–ligand complexes in the aqueous phase. In the study of geochemical speciation in sediment, the total content of metals is partitioned into five different specific fractions, distinguishing binding phases in the descending order of mobility. Sinks for water-soluble or exchangeable metals fall into the phase 1 category (F1). Geochemical phase 2 presents the tendency to precipitate in the lattice of secondary minerals like carbonates, sulfides, phosphates, or oxides (weakly absorbed-F2). Metals occluded in amorphous materials such as Fe–Mn oxy-hydroxide are classified into phase 3 (reducible-F3). Lastly, metals complexed with organic matter (humin, humic and fulvic acids containing various functional groups: hydroxyl –OH, carbonyl C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, carboxyl –COOH) (oxidizable-F4) or chemisorbed on the lattice of primary and secondary minerals such as silicates (residual-F5) respectively constitute the final two categories.35,36 Amongst different methods to elucidate the so-called “solid/particulate speciation”, sequential extraction procedures (SEPs) were developed to more accurately separate labile fractions (inorganic and organic) from residual phases and to help quantify the biolability of different pollutants and trace HMs associated with soils and sediments.37 Sequential extraction techniques are able to provide the origin of metal input, digenetic transformation within the sediments, and the reactivity of HM species of both natural and anthropogenic attribution.12
O, carboxyl –COOH) (oxidizable-F4) or chemisorbed on the lattice of primary and secondary minerals such as silicates (residual-F5) respectively constitute the final two categories.35,36 Amongst different methods to elucidate the so-called “solid/particulate speciation”, sequential extraction procedures (SEPs) were developed to more accurately separate labile fractions (inorganic and organic) from residual phases and to help quantify the biolability of different pollutants and trace HMs associated with soils and sediments.37 Sequential extraction techniques are able to provide the origin of metal input, digenetic transformation within the sediments, and the reactivity of HM species of both natural and anthropogenic attribution.12
Depending upon the required sensitivity, versatility, sample matrix, and applications, various analytical techniques are available for analyzing trace levels of HMs. These include graphite furnace atomic absorption spectroscopy (GFAAS),38 cold vapor atomic absorption spectroscopy (CV-AAS),39,40 X-ray fluorescence (XRF) spectroscopy,41–43 anodic stripping voltammetry (ASV),44,45 inductively coupled plasma optical emission spectroscopy (ICP-OES),46,47 inductively coupled plasma-mass spectrometry (ICP-MS),15,48 and high-performance liquid chromatography (HPLC) coupled with inductively coupled plasma-mass spectrometry (ICP-MS).49,50 Nevertheless, specialized techniques are particularly suited for different capabilities, such as XRF and ICP-OES for trace detection (ppm–ppb levels). Some are effective for ultra-trace (ppt) single-element analysis such as GFAAS and CV-AAS, which is specific and sensitive for mercury (Hg) only (ppm, ppb, and ppt signify parts per million, parts per billion, and parts per trillion, respectively). Techniques like ASV, ICP-OES, and XRF require expertise to address matrix interference in complex samples. Amongst these, ICP-MS is considered the gold standard due to its unparalleled sensitivity and versatility, making it an ideal and well-suited choice for simultaneous multi-element analysis at ultra-trace levels. The coupling of HPLC with ICP-MS is excellent for speciation in complex matrices (e.g., organic and inorganic arsenic species50) but entails intricate and advanced instrumentation and is relatively more time-consuming compared to direct methods. Thus, employing SEP alongside ICP-MS for trace metal speciation in sediment provides valuable insights into the chemical forms and potential deficiencies of HMs in sediment.
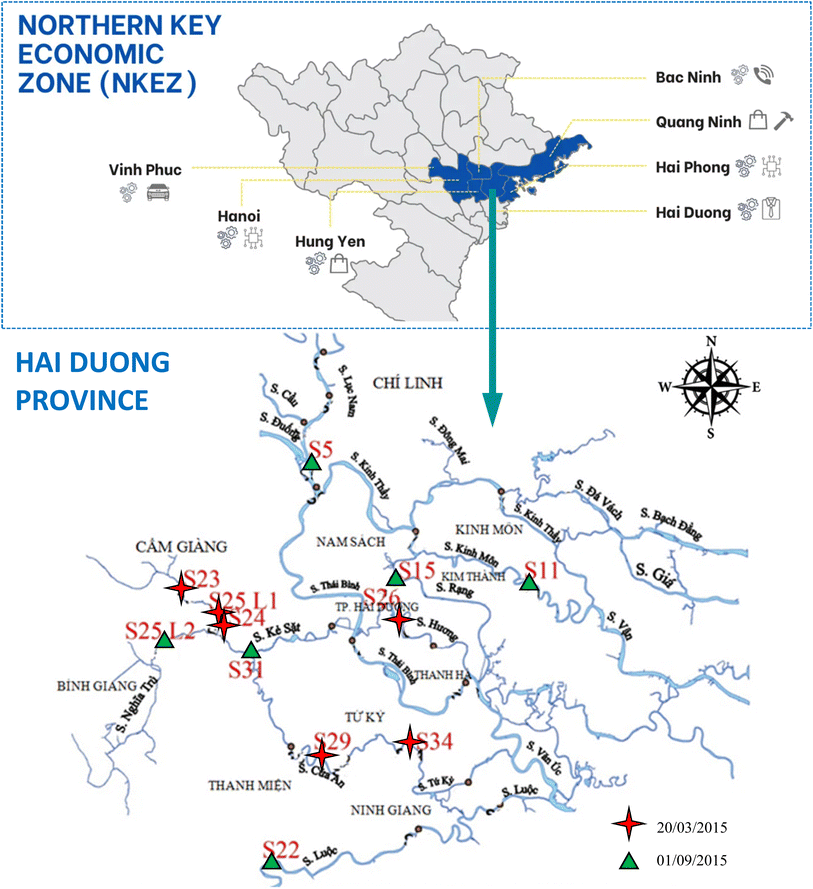
Hai Duong is a northeastern province located in the Northern Key Economic Zone (NKEZ) that has experienced rapid industrial and urban development in Vietnam (Fig. 1). Specifically, Hai Duong has a large population (2.567 million in 2019) and a number of industrial zones such as Lai Vu, Nam Sach, Tan Truong, Trang Due, etc. In addition, due to the intensive agriculture in Hai Duong, there are numerous local waterways for irrigation and drainage. According to the Vietnamese Department of Natural Resources and Environment (DNRE), Hai Duong has recently been impacted significantly by pollution from industrial zones.51,52 Therefore, wastewater and pollutants are mainly released into sewage and river systems. There are a few publications about pollution circumstances in Hai Duong that assessed the quality of surface water and the nutrient pollution source using multivariate statistical techniques53 and interpreted the anthropogenic impacts through the spatio-temporal variation of stable isotopes of NO3−.54 In these previous studies, it was mentioned that the pollution in the Hai Duong river systems resulted primarily from an accumulation of anthropogenic pollution. However, none of these studies have examined and demonstrated the pollution of HM species at different depths of sediment cores. In addition, it is essential to systematically assess the quality of sediment by using specific techniques and pollution indices in this area.
In this research, we aim to fill this gap by investigating the geochemical speciation, environmental risk assessment, and the contributions to pollution of HMs (Cu, Pb, Cd, Zn, Fe, Co, Ni, Mn, and Cr) using SEP for HM fractionation and ICP-MS for elemental analysis in sediment cores collected from six study sites in the river systems of Hai Duong province. We present analyses for (1) qualitatively comparing the concentrations of HM species at different sediment depths, (2) rationalizing the distribution pattern of HMs amongst the geochemical phases, (3) quantitatively assessing the sediment quality by estimating the environmental risk of each metal along the sediment depth via the contamination factors (Cf) and risk assessment code (RAC), and (4) predicting the apportionment of pollution sources and polluted areas by using multivariate statistical analyses. We illustrate the first-ever demonstration of cross-referencing data from the DNRE to address the long-standing central question of fluxes of HMs in sediments. These results provide a valuable indication for further studies in planning remedy strategies in polluted areas.
2. Materials and methods
2.1. Study area
The study area of our interest was selected from the river system in Hai Duong province, which is shown in detail in Table 1. The river system in Hai Duong province is located approximately between 20°42′–21°03′N and 106°09′–106°33′E (Fig. 1). Geographically, Hai Duong has a dense network of rivers with two major river systems: the Thai Binh and Bac Hung Hai River systems. Crossing Hai Duong province, the Thai Binh River is roughly 73 km long and receives 30–40 billion m3 of water per year. The Thai Binh River has three tributaries (the Luc Nam, Cau, and Duong rivers) and is of pivotal importance for mineral, cement, and building materials transportation. However, the Bac Hung Hai River, which was built in 1958, is 200 km long, including the Sat, Cam Giang, Thau, Do Day, Dinh Dao, Tu Ky, and Cau Xe rivers, as well as the canal and dike systems that serve as agricultural irrigation for the major cities in Bac Ninh, Hung Yen, and Hai Duong provinces. Therefore, these rivers have been subject to industrial release and agricultural and domestic sewage from Hai Duong province, which have made a discernible contribution to the deterioration of water and sediments.| Sampling site no. | Sampling site | Geographic coordinates | Description (from the bank: from the bridge) |
|---|---|---|---|
| S23 | Cam Giang bridge | N: 20°58′3.96′′ E: 106°10′4.34′′ | (8 m: 30 m) |
| S24 | Ghe bridge, Cam Giang | N: 20°56′14.88′′ E: 106°12′39.26′′ | (5 m: 30 m) |
| S25L1 | Cay bridge, Binh Giang | N: 20°54′′16.08′′ E: 106°13′53.20′′ | (6 m: 60 m) |
| S26 | Cat bridge, Hai Duong | N: 20°55′50.98′′ E: 106°19′41.75′′ | 20 m from the bank |
| S29 | Neo bridge, Thanh Mien | N: 20°46′55.61′′ E: 106°14′35.79′′ | (7 m: 70 m) |
| S34 | Van bridge, Tu Ky | N: 20°48′57.02′′ E: 106°24′6.98′′ | (7 m: 60 m) |
| S5 | Pha Lai bridge, Chi Linh | N: 21°6′10.53′′ E: 106°17′51.84′′ | Near the Pha Lai thermal power company |
| S11 | Phu Thai, Kim Thanh | N: 20°57′48.70′′ E: 106°31′51.77′′ | 500 m from the Van River |
| S15 | CCN Lai Vu, Nam Sach | N: 20°59′38.24′′ E: 106°24′37.19′′ | Near the Lai Vu industrial zone |
| S22 | Tien Phong, Thanh Mien | N: 20°42′1.12′′ E: 106°15′9.65′′ | Ship berthing sites |
| S25L2 | Ke Sat bridge, Ke Sat | N: 20°54′54.25′′ E: 106° 8′57.66′′ | 5 m from the bank |
| S31 | Hiep bridge, Ninh Giang | N: 20°45′50.36′′ E: 106°17′13.91′′ | 70 m from the bridge |
The authors opted for the sampling sites according to the reference data of the Center of Environmental Monitoring and Analysis, Vietnamese Department of Natural Resources and Environment (DNRE) – Hai Duong province about polluted areas and industrial zones from 2009 to 2014.52 Samples were collected from the twelve studied sites in the northern part of the Hai Duong province during two batches. The first batch of samples, including only pore water samples, was collected at S23, S24, S25L1, S26, S29, and S34 sites (star symbols) during the spring time (20th March 2015), while the second batch of samples, including pore water and sediment core samples were collected during the autumn time (September 1st, 2015) at S5, S11, S15, S22, S25L2 and S31 sites (triangle symbols).
2.2. Sample collection and sample pre-preparation
The field technique for collecting pore water samples is dialysis. The sampling device is called a “peeper”, which is based on the single-sided design of Hesslein55 and made of poly(methyl methacrylate) (PMMA), indicated in Fig. S1.† The dimensions of the peeper are 66 cm × 16.5 cm × 2.5 cm. The peeper sampler uses a 0.2 μm polyethersulfone (PES) exchange membrane to cover the perforated stackers (vials). After three weeks, equilibrium conditions were reached, and the peepers were harvested from sampling locations and cleaned with deionised water. A pore water sample was collected by inserting a clean tip and plastic pipet through the PES membrane from three consecutive stackers of 3 cm length on the peeper. Samples were pre-acidified with 65% HNO3 solution to pH ≤ 2, stored in an ice box, and brought to the laboratory for analysis of total HM content.Sediment cores were collected at six sampling locations in the second campaign on September 1st, 2015, using a locally fabricated core sampler or sediment corer equipped with 40 cm long steel tubes with a diameter of 8 cm (see Fig. S2†). The device was placed perpendicular to the sediment surface on sites and compressed to about 40 cm so that the sediment occupied all of the device's space. The retrieved core was cut into four sections with 10 cm for each. Raw sediments destined for sequential extraction were dried in air and ground into fine powder using an agate mortar. A sieve of 63 μm mesh size was utilized to discard the oversize grains; then, samples were packed in polyethylene air-tight bags and stored in a refrigerator at 2 °C.
2.3. Sequential extraction of heavy metals in sediment
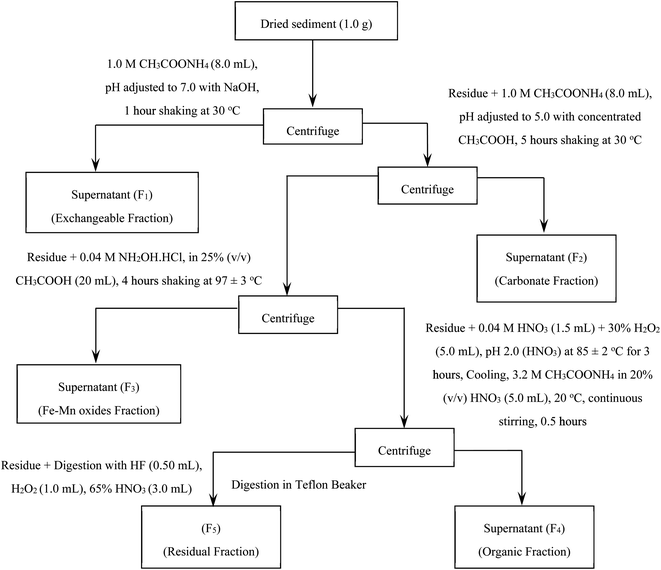
All reagents were of analytical reagent grade (certified purity > 99.9%) unless otherwise stated. Double deionized water (Milli-Q Millipore water with a resistivity of 18.2 MΩ cm) was used for all dilutions. Acetic acid CH3COOH (glacial, 100% Fisher Scientific), hydroxyl-ammonium chloride NH2OH·HCl (ACROS Organics), hydrogen peroxide H2O2 (30% Fisher Scientific), and ammonium acetate CH3COONH4 and nitric acid HNO3 (65%, Suprapur Merck, Germany) were of super pure quality (99.950–99.9959%). To mitigate ambiguity in terminology, “element(s)” will henceforth be used interchangeably to denote potentially toxic “HM(s)”, unless explicitly defined otherwise.In order to determine the amounts and geochemical phases of the HMs (Cu, Pb, Cd, Zn, Fe, Co, Ni, Mn, and Cr), a modified five-step sequential extraction procedure (SEP) was adopted.35 The SEP performance is represented in Fig. 2. Prior to the SEP, the samples were air-dried, ground, and purged with nitrogen (99.999% purity). After each extraction step, the separation of extract from the solid residue was proceeded by centrifugation at 4000 revolutions per minute (rpm) for 25 minutes. The collected fractions were subjected to trace element analysis.
2.4. Elemental analysis of heavy metals and method validation
After the SEP, all extracted solutions were kept in polyethylene (PE) containers and stored in a freezer until direct concentration measurements. The determination of HM (Cu, Pb, Cd, Zn, Fe, Co, Ni, Mn, and Cr) concentrations was performed using an ELAN 9000 ICP-MS system (PerkinElmer Sciex, Penlivia, Canada) at the Faculty of Chemistry, VNU University of Science, Hanoi, Vietnam. To mitigate isobaric interference, we carefully chose the isotopes of the investigated elements (53Cr, 55Mn, 57Fe, 59Co, 60Ni, 63Cu, 66Zn, 111Cd, and 208Pb) to minimize the mass overlapping phenomena. Analytical standard solutions were prepared using nitric acid (HNO3) as a medium, which exerted minimal impact on mass interference with the elements under investigation. Therefore, nitric acid was selected as the matrix for sample solutions in ICP-MS measurements for this study. Nevertheless, our choice of sample matrix and element isotopes did not completely resolve and overcome the mass interference phenomenon for Cd and Pb. Mathematical correction equations for these two HM elements were applied (Table 2).53| Parameters | Conditions |
|---|---|
| ICP-MS system | Elan 9000 (PerkinElmer) |
| Optimized RF power | 1200 W |
| Optimized lens voltage | 8.00 V |
| Optimized nebulizer gas flow rate | 1.05 L min−1 |
| Ar plasma gas flow | 15.0 L min−1 |
| Auxiliary gas flow | 2.00 L min−1 |
| Injection rate | 26 cycles per min |
| Rinsing rate | 48 cycles per min |
| Stabilization rate | 0.10 cycles per s |
| Rinsing time | 120 s |
| Stabilization time | 30 s |
| Rejection parameter q (RPq) | 0.45 |
| Sampling depth | 5.10 mm |
| Replicates per sample | 3 |
| Integration time | 5.80 s |
| Working vacuum pressure | 1.2–1.3 × 10−5 Torr |
| Standby vacuum pressure | 2.0–3.0 × 10−6 Torr |
| Isobaric correction equations | |||
|---|---|---|---|
| Isotope | Isotope ratio | Interference | Correction equation |
| 111Cd | 12.8 | 95Mo16O+; 94Zr16OH+; 39K216O2H+ | I(111Cd) = I(m/z 111) − 1.07 × [I(108MoO) − 0.712 × I(106Pd)] |
| 208Pb | 52.4 | 192Pt16O+ | I(208Pb) = I(m/z 208) + 1.00 × I(206Pb) + 1.00 × I(207Pb) |
All glassware (Pyrex) and containers were soaked evenly in 2.00% HNO3 for 24 hours and then rinsed prior to use. Standard metal solutions (103 mg L−1) were either purchased from Merck or prepared in the laboratory from pure metals to establish the calibration curve and determine the detection limit (LOD) and quantitation limit (LOQ) in the ICP-MS method validation procedure. The instrumental LOD and LOQ were evaluated as 3 and 10 times the standard deviation of blank solutions/the intercept divided by the slope of the calibration curve, respectively. To evaluate the LOQ in μg g−1 (method LOQ), it is calculated by dividing the instrumental LOQ by the sample mass (g) and then multiplying by the dilution volume (L) used for the decomposition.
The calibration curves for Pb, Mn, Ni, Zn, Co, Cu, Cd, and Cr were constructed with four points at concentrations of 4.00 ppb, 20.0 ppb, 100 ppb, and 200 ppb. The calibration curve for Fe was solely based on concentrations of 8.00 ppb, 40.0 ppb, 200 ppb, and 400 ppb. All calibration curves achieved a standard deviation, resulting in R > 0.999. The LODs for nine elements (Cr, Mn, Fe, Ni, Co, Cu, Zn, Cd, and Pb), expressed in parts per trillion (ppt or 10−12 g mL−1), were 1.80, 11.4, 20.1, 16.7, 17.8, 15.0, 11.4, 12.3, and 4.20, respectively. Their method LOQs in 10−5 μg g−1 were 4.50, 28.5, 50.2, 41.8, 44.5, 37.5, 28.5, 30.8, and 10.5, respectively.
To validate the accuracy of our method, we assessed bias by obtaining reference values through two approaches: certified reference material (CRM) and spiking studies. The CRM used in this study was MESS-3, a marine sediment material with certified values from the National Research Council of Canada. Our analyses showed that the relative bias (RB) and relative standard deviation (RSD) values in both approaches were below 15.0%, complying with FDA guidelines or SANTE/SANCO guidelines (<20.0%). When analyzing the MESS-3 reference sample, the maximum RB value was 4.15%, and the maximum of RSD was 7.98%. For the standard addition method, the highest RB was 5.45%, and the highest RSD was 2.56%. Hence, it can be concluded that this analytical method achieved acceptable precision and trueness, meeting the accuracy requirements for simultaneous analyses of HM content in sediment cores.
The concentrations of five geochemical components (exchangeable fraction, carbonate fraction, Fe–Mn oxide fraction, organic fraction, and residual fraction) in the sediment cores determined by ICP-MS were converted to percentage terms of the five selective fractions F1, F2, F3, F4, and F5 using eqn (1).
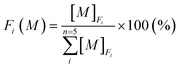 | (1) |
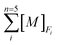 is the total concentration of element M from five specific fractions. The normalization condition follows eqn (2).
is the total concentration of element M from five specific fractions. The normalization condition follows eqn (2).
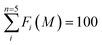 | (2) |
The fractionation of each metal is indicated in the distribution column profile (Sections 3.1 and 3.2) at different sampling sites at different depths of sediments.
2.5. Environmental risk assessment of heavy metals
In order to estimate the level of HM pollution in sediment quantitatively, the contamination factors (Cf) of elements and risk assessment code (RAC) in the sediment core samples are required.
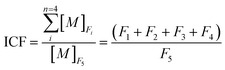 | (3) |
| GCF = ∑ICF | (4) |
It is well established in the literature that the retention time for trace metals in sediments is measured as a time span needed to reduce the element concentration by half.58 ICF values can be used as a relative measure of metal retention in sediments. The lower the ICF values, the higher the relative metal retention.
Bioavailability is a measure of the degree and rate of absorption of a substance by living systems and is the primary measure of toxicity.59 An increase in adsorptive or “environmentally available” fractions, exchangeable and/or carbonate-bound fractions, which display a dynamic equilibrium between the aqueous phase and sediment of weakly bonded metals, could increase the bioavailable components.57,60 Since the acid-soluble fractions (F1 and F2) are directly proportional to bioaccessibility, the risk assessment code is defined as the sum of F1 and F2 fractions on the scale of percentage (eqn (5)). Thus, RAC (%) is a crucial factor for estimating the effect of anthropogenic activities such as agricultural runoff and tourism on the environment. The high RAC values have been interpreted as evidence of relatively high lability and biological availability of HMs in sediment.
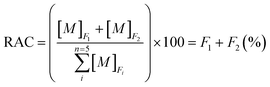 | (5) |
 | (6) |
2.6. Multivariate statistical analysis
Numerous factors control the sources of HMs, including natural erosion, aqueous degradation, and anthropogenic inputs such as pollutants. Therefore, multivariate statistical analyses were performed to evaluate the dynamics of HMs and clarify the pollution contributions of bioavailable HMs in sediment cores. By applying Minitab 16 statistical software, principal component analysis (PCA) and cluster analysis (CA) were conducted. The other calculations were carried out using OriginPro 2018.3. Results and discussion
3.1. Fractionation of heavy metals in sediment cores
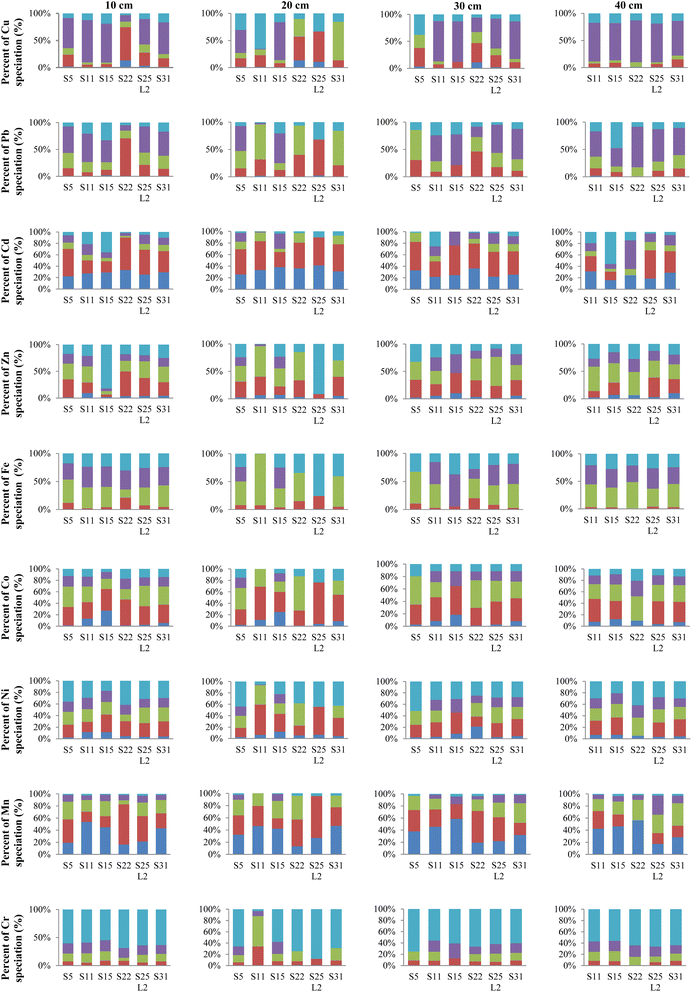
The fractionation of HMs between the sediment phases is shown in Fig. 3. The speciation results express a high Cd, Mn, Co, and Ni content in the exchangeable fraction (phase F1), which theoretically harms aquatic biota. In contrast, the exchangeable fractions of Cr and Fe are trivial (<2.00% of the total). Specifically, Cd mostly underscores high concentrations in all samples at all examined sites, whereas the high proportion of Mn concentrations is only recognized at S11, S15, and S31. The percentage of HMs in sediment in the exchangeable phase descends in the following order: Cd, Mn ≫ Ni, Co > Zn > Cu, Pb > Fe, Cr. The descending order of binding ability with the carbonate phase (phase F2) is generally Cd > Co > Mn > Ni > Zn > Pb > Cu, Fe, Cr. From the range of percentages of HMs in the carbonate phase, Co (26.3–58.0%), Mn (16.8–66.3%), Ni (16.0–53.1%), Zn (4.42–45.8%), and Pb (6.75–69.7%) represent the majority portion in the carbonate phase, as these metals possess a particularly high affinity towards carbonate CO32− and are prone to co-precipitation with carbonate minerals such as calcite CaCO3 and dolomite MgCO3·CaCO3. Carbonate-bound Mn exhibits the second-highest abundance among the non-lithogenic fractions. A higher content of Mn in carbonate-bound forms is most likely since Mn with a similar ionic radius to Ca facilitates Ca substitution in the carbonate phase.62 The lower percentage of Cr (approximately less than 10.0%) associated with the carbonate fraction in the sediment cores is ascribed to the inability of Cr3+ to precipitate or complex with carbonates.12 Among the non-lithogenic fractions, the Fe–Mn (oxyhydr)oxide reducible phase (phase F3) is the leading fraction of most metals except Cu and Cd. This can be attributed to HMs' adsorption, flocculation, and co-precipitation with the colloids of Fe–Mn (oxyhydr)oxides such as FeOOH and MnOOH.66 Furthermore, organic matter (phase F4) bound Cu, Pb, Fe, Cr, Co, and Ni seem to be the second most dominant fraction among the non-geogenic portions. A smaller fraction of Mn, Ni, and Zn in the organic species compared to other metals is probably a result of competition between the Fe–Mn-organic complex and hydrous Fe–Mn oxide forms.24 Results of fractionation analysis suggest that the residual fraction (phase F5) is primarily dominated by Cr (54.9–68.6%), Ni (17.1–43.9%), and Fe (15.5–37.4%) distribution in the sediment core. The metal concentration in the residual fraction pertains to the contribution of natural sources. A higher concentration percentage in this fraction indicates a lower pollution level and the geological derivation of the metals in sediments of the studied region since the residual solid should principally constitute primary and secondary minerals. These minerals may tightly hold those metals within their crystal structure even if they are under the conditions typically encountered in nature over a reasonable time span.35 Therefore, the high percentages of Cr, Ni, and Fe in the residual fraction demonstrate that they are relatively stable and have weak bioavailability and toxicity.Overall, the total concentration in the five fractions of HMs from the six sampling sites is similar, highlighting a decrease from the surface of the cores to deeper layers, especially for Cu from 26.9 μg g−1 at 10 cm to 4.82 μg g−1 at 40 cm at the S22 site. Surface enrichment may arise from the recent enrichment of metals in the water column, which has been interpreted as pollution in recent years. This is because the pollutants are always absorbed into the top sediment first and then sink into the deeper layers by the chemical exchange process.67 Higher contents of Pb, Cd, Ni, Cu, and Zn in the top layer of sediment at S22, S11, and S15 reveal that these three sites experienced more negative impacts from pollution than the other sites. This pollution can be traced to various anthropogenic activities such as sand exploitation (site S22) or industrial wastewater effluent (Lai Vu industrial zone near S15). The amount of metal species derived from those activities is dominantly in non-residual fractions. The difference becomes smaller at greater depths. This propensity is explained as the HMs in the non-residual fractions are mainly caused by pollution in the top sediments. At greater depths, the trace of recent pollution is less, and the species distribution depends on the geology and hydrology of sediment.
3.2. Variation of metal species distribution with depth
Regardless of sampling sites and depths, the phase distribution (based on % metal speciation) of the studied metals in sediment core samples generally followed the orders given below.Cd: exchangeable ≈ carbonate > residual ≈ organic > Fe–Mn oxide.
Mn: exchangeable ≈ carbonate > Fe–Mn oxide > organic > residual.
Fe: Fe–Mn oxide > organic > residual > carbonate > exchangeable.
Zn: carbonate ≈ Fe–Mn oxide > residual > organic > exchangeable.
Co: carbonate > Fe–Mn oxide > organic ≈ residual > exchangeable.
Cu: organic > residual > carbonate > Fe–Mn oxide > exchangeable.
Pb: organic > carbonate > Fe–Mn oxide > residual > exchangeable.
Ni: residual > carbonate > Fe–Mn oxide > organic > exchangeable.
Cr: residual > organic ≈ Fe–Mn oxide > carbonate > exchangeable.
Based on their similar association with the different phases shown above, the distribution patterns of HM species are divided into four specific groups. The first classification consists of a group bound to labile phases (exchangeable and carbonate) with the presence of Cd and Mn (Section 3.2.1). The second group is associated with Fe–Mn oxides and comprises Co, Zn, and Fe (Section 3.2.2). Cu and Pb (Section 3.2.3) are predominant in the third group associated with organic matter. Lastly, Cr and Ni (Section 3.2.4) are identified to be mainly present in the group bound to residual components.
3.3. Risk assessment of heavy metals in sediments
| Site | Depth | ICF | GCF | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Cu | Pb | Cd | Zn | Fe | Co | Ni | Mn | Cr | |||
| S5 | 10 cm | 10.7 | 13.0 | 16.1 | 4.83 | 4.67 | 7.02 | 1.78 | 54.7 | 0.652 | 113 |
| 20 cm | 2.26 | 13.0 | 35.7 | 3.13 | 3.22 | 5.52 | 1.28 | 38.6 | 0.509 | 103 | |
| 30 cm | 1.64 | 5.98 | 59.2 | 2.11 | 2.06 | 4.12 | 0.945 | 32.1 | 0.329 | 108 | |
| S11 | 10 cm | 6.97 | 3.86 | 3.65 | 3.75 | 3.21 | 6.38 | 2.42 | 35.4 | 0.695 | 66.4 |
| 30 cm | 7.06 | 3.12 | 2.92 | 3.09 | 5.47 | 7.65 | 2.10 | 83.4 | 0.791 | 116 | |
| 40 cm | 4.75 | 4.89 | 4.13 | 2.71 | 3.81 | 7.73 | 2.38 | 59.3 | 0.749 | 90.5 | |
| S15 | 10 cm | 4.23 | 2.04 | 1.79 | 0.221 | 3.32 | 17.4 | 4.82 | 63.0 | 0.817 | 97.7 |
| 20 cm | 5.12 | 3.85 | 24.2 | 3.31 | 3.03 | 12.0 | 3.49 | 84.5 | 0.720 | 140 | |
| 30 cm | 6.77 | 3.44 | — | 4.36 | 1.67 | 7.56 | 2.26 | 22.2 | 0.638 | 48.9 | |
| 40 cm | 4.56 | 1.10 | 0.792 | 5.62 | 2.61 | 9.40 | 3.81 | 28.1 | 0.781 | 56.8 | |
| S22 | 10 cm | 27.8 | 20.4 | 53.7 | 4.53 | 2.30 | 4.88 | 1.42 | 50.1 | 0.457 | 166 |
| 20 cm | 8.50 | 14.6 | 31.2 | 5.78 | 1.91 | 6.74 | 1.62 | 24.1 | 0.340 | 94.8 | |
| 30 cm | 15.7 | 11.2 | 54.4 | 7.13 | 2.60 | 7.39 | 3.03 | 26.3 | 0.498 | 128 | |
| 40 cm | 6.65 | 10.8 | 5.83 | 2.65 | 3.70 | 3.79 | 1.39 | 41.7 | 0.557 | 77.1 | |
| S25L2 | 10 cm | 8.55 | 12.8 | 19.7 | 4.04 | 2.85 | 5.75 | 2.20 | 31.2 | 0.560 | 87.7 |
| 20 cm | 1.99 | 2.14 | 8.65 | 0.0943 | 0.319 | 3.20 | 1.25 | 21.5 | 0.135 | 39.3 | |
| 30 cm | 11.3 | 19.7 | 34.6 | 10.7 | 3.86 | 7.70 | 2.55 | 48.2 | 0.614 | 139 | |
| 40 cm | 4.30 | 6.70 | 36.3 | 6.87 | 2.79 | 7.92 | 2.61 | 33.1 | 0.505 | 101 | |
| S31 | 10 cm | 4.96 | 4.97 | 9.04 | 3.03 | 3.13 | 6.08 | 2.38 | 48.8 | 0.574 | 83.0 |
| 20 cm | 5.45 | 5.33 | 13.0 | 2.33 | 1.47 | 3.93 | 1.35 | 26.4 | 0.444 | 59.7 | |
| 30 cm | 6.72 | 7.05 | 11.4 | 4.39 | 4.40 | 7.76 | 2.65 | 57.0 | 0.653 | 102 | |
| 40 cm | 6.17 | 8.50 | 18.0 | 4.11 | 3.09 | 6.55 | 2.35 | 31.4 | 0.566 | 80.7 | |
| Mean values | 10 cm | 10.5 | 9.51 | 17.3 | 3.40 | 3.25 | 7.92 | 2.50 | 47.2 | 0.626 | 102 |
| 20 cm | 4.66 | 7.78 | 22.6 | 2.93 | 1.99 | 6.27 | 1.80 | 39.0 | 0.430 | 87.4 | |
| 30 cm | 8.20 | 8.40 | 32.5 | 5.30 | 3.34 | 7.03 | 2.26 | 44.8 | 0.587 | 107 | |
| 40 cm | 5.29 | 6.40 | 13.0 | 4.39 | 3.20 | 7.08 | 2.51 | 38.7 | 0.632 | 81.2 | |
The GCF tendency generally decreases with depth, although the GCF values are unusual due to the lack of data from S11 at a depth of 20 cm and S5 at a depth of 40 cm. In general, sediments from S5, S22, and S15 in the shallower layers (10 and 20 cm) have the highest ICF and GCF values, meaning that these sampling sites possess the most potential environmental risk. Nonetheless, the S25L2 and S11 sites present more potential ecological risks in the deeper layers than the S5, S22, and S15 sites. Specifically, the trend of the GCF at different depths is expressed in Table 4. Our results identify sites located near the sewage drainage system of the Pha Lai thermal power company (S5) and Lai Vu (S15) and Phu Thai (S11) industrial zones as having high potential environmental risks related to toxic HMs.
| Depth | Average GCF |
|---|---|
| 10 cm | S22 > S5 > S15 > S25L2 > S31 > S11 |
| 20 cm | S15 > S5 > S22 > S31 > S25L2 |
| 30 cm | S25L2 > S22 > S11 > S31 > S5 > S15 |
| 40 cm | S25L2 > S11 > S31 > S22 > S15 |
| Category | Risk | Percentage of acid-soluble fractions (exchangeable and carbonate) (%) |
|---|---|---|
| 1 | No risk (N) | <1 |
| 2 | Low risk (L) | 1–10 |
| 3 | Medium risk (M) | 11–30 |
| 4 | High risk (H) | 31–50 |
| 5 | Very high risk (V) | >50 |
From the selected samples, there were no elements at the no-risk level (<1.00%). The RAC gave values below 10.0% for Cr and Fe, suggesting the high stability of Cr and Fe in the sediments.
From RAC analysis, the mobility was considerably higher in Cu, Pb, and Zn. This means that Cu, Pb, and Zn (medium-risk category) could be noticeable in the near future. However, Cu and Pb in the top sediments also unveil a very high risk at the S22, S5, S25L2, and S11 sites. In category 4, Co and Ni populate the high-risk group. Co and Ni were bound either in the exchangeable or in the carbonate fraction (11.0–30.0%) in most of the sites, which reflects that Co and Ni can easily enter the food chain and pose detrimental impacts on the ecosystem.
In general, this assessment (Table 6) emphasizes that Cd and Mn posed a very high risk (RAC more than 50.0%) at all sampling sites and depths. Because of the toxicity and availability of Cd, it is considered a highly hazardous metal, whereas Mn and Zn are less toxic and essential elements also. There are several sources of Cd in aquatic systems, including runoff containing phosphate fertilizers from agricultural areas near the river. These phosphate fertilizers, applied to agricultural farms, most probably contain Cd.90 The presence of Cd could also be a result of road traffic, which has been described as a vital source of Cd emission.91 Therefore, instant supervision and significant remediation must be performed for Cd mobilization as soon as possible.
| Site | Depth | Cu | Pb | Cd | Zn | Fe | Co | Ni | Mn | Cr |
|---|---|---|---|---|---|---|---|---|---|---|
| S5 | 10 cm | 23.8 | 15.2 | 70.3 | 35.2 | 11.8 | 33.5 | 24.2 | 57.8 | 7.52 |
| 20 cm | 17.2 | 15.4 | 69.2 | 31.3 | 7.84 | 29.1 | 18.5 | 64.0 | 5.66 | |
| 30 cm | 37.7 | 30.7 | 82.4 | 34.6 | 10.7 | 35.0 | 24.3 | 73.2 | 8.89 | |
| S11 | 10 cm | 5.54 | 7.67 | 50.2 | 29.2 | 2.05 | 42.0 | 29.1 | 70.5 | 5.09 |
| 20 cm | 22.8 | 31.6 | 83.6 | 40.5 | 7.73 | 69.0 | 59.4 | 79.5 | 33.9 | |
| 30 cm | 7.36 | 9.34 | 48.4 | 26.5 | 2.99 | 46.6 | 28.7 | 74.2 | 8.63 | |
| 40 cm | 7.39 | 15.7 | 57.9 | 14.0 | 3.11 | 47.5 | 31.5 | 71.5 | 8.37 | |
| S15 | 10 cm | 6.49 | 12.1 | 48.6 | 6.83 | 4.33 | 64.9 | 41.8 | 63.0 | 8.81 |
| 20 cm | 8.13 | 12.6 | 64.3 | 22.6 | 3.82 | 59.7 | 43.1 | 59.1 | 7.62 | |
| 30 cm | 11.6 | 21.3 | 75.9 | 47.0 | 5.37 | 64.6 | 45.7 | 83.0 | 12.5 | |
| 40 cm | 8.83 | 8.67 | 30.8 | 29.4 | 2.72 | 44.1 | 37.1 | 65.9 | 7.72 | |
| S22 | 10 cm | 74.6 | 70.6 | 90.1 | 49.6 | 21.1 | 46.6 | 30.4 | 82.5 | 8.27 |
| 20 cm | 57.1 | 40.1 | 80.4 | 33.7 | 15.1 | 27.4 | 22.6 | 57.2 | 7.54 | |
| 30 cm | 46.9 | 46.2 | 79.4 | 33.8 | 20.0 | 29.6 | 38.6 | 71.7 | 7.25 | |
| 40 cm | 1.25 | 0.0400 | 24.6 | 6.74 | 0.0815 | 9.49 | 5.25 | 56.4 | 0.572 | |
| S25L2 | 10 cm | 27.7 | 21.3 | 68.9 | 37.4 | 7.45 | 34.9 | 27.0 | 63.2 | 5.50 |
| 20 cm | 66.6 | 68.2 | 89.6 | 8.62 | 24.2 | 76.2 | 55.6 | 95.6 | 11.9 | |
| 30 cm | 24.3 | 17.7 | 65.0 | 23.4 | 8.41 | 39.7 | 27.2 | 61.4 | 6.51 | |
| 40 cm | 6.63 | 11.0 | 67.9 | 38.7 | 4.05 | 43.6 | 28.4 | 35.3 | 6.05 | |
| S31 | 10 cm | 16.9 | 13.9 | 66.5 | 29.7 | 4.57 | 37.6 | 29.9 | 67.9 | 7.51 |
| 20 cm | 13.3 | 21.1 | 77.6 | 40.1 | 5.07 | 55.0 | 36.2 | 77.4 | 8.91 | |
| 30 cm | 11.4 | 10.9 | 66.0 | 34.1 | 2.84 | 45.1 | 34.5 | 52.1 | 8.91 | |
| 40 cm | 15.0 | 15.3 | 66.5 | 35.9 | 3.06 | 42.3 | 34.0 | 47.3 | 8.22 | |
| Average | 22.5 | 22.5 | 66.3 | 30.0 | 7.76 | 44.5 | 32.8 | 66.5 | 8.77 | |
These sites are located in industrial zones, such as Phu Thai (S11), and the pollution of this area may be imputed to industrial sewage drainage. However, fishery and sand exploitation chiefly mediate the proportion of HMs in S25L2. S5 and S31 are related to the sites affected by municipal sewage from the crowded residential area of Ninh Giang district (S31) and the coal firing process of the Pha Lai thermal power company.
Overall, the results of this study suggest that the mobility and bioavailability of the nine HMs probably decline in the following order: Mn > Cd ≫ Co > Ni > Zn > Pb ≈ Cu ≫ Cr > Fe.
| Depth | Site | Cu | Pb | Cd | Zn | Fe | Co | Ni | Mn | Cr |
|---|---|---|---|---|---|---|---|---|---|---|
| 10 cm | S5 | 6.00 | 0.379 | 1.78 | 6.16 | 0.903 | 0.866 | 2.63 | 0.578 | 0.595 |
| S11 | 10.6 | 1.58 | 4.30 | 20.1 | 0.376 | 0.378 | 2.85 | 0.608 | 0.822 | |
| S15 | 5.55 | 2.03 | 5.90 | 1.03 | 0.117 | 0.162 | 5.99 | 0.984 | 0.745 | |
| S22 | 0.484 | 0.257 | 0.165 | 2.12 | 0.605 | 0.123 | 0.665 | 0.973 | 0.0229 | |
| S25L2 | 1.04 | 0.327 | 0.679 | 0.744 | 0.611 | 0.526 | 0.561 | 2.67 | 0.0189 | |
| S31 | 3.04 | 1.68 | 7.37 | 4.26 | 0.246 | 0.689 | 1.68 | 1.76 | 0.213 | |
| Mean | 4.45 | 1.04 | 3.37 | 5.74 | 0.476 | 0.457 | 2.40 | 1.26 | 0.403 | |
| 20 cm | S5 | 5.86 | 0.655 | 1.85 | 9.81 | 1.32 | 1.05 | 2.16 | 0.973 | 0.408 |
| S11 | 26.9 | 2.44 | 2.16 | 15.1 | 1.22 | 0.668 | 3.74 | 1.24 | 1.83 | |
| S15 | 8.60 | 1.17 | 1.63 | 6.06 | 0.300 | 0.479 | 6.80 | 1.00 | 0.822 | |
| S22 | 0.694 | 0.177 | 0.608 | 1.54 | 0.377 | 0.239 | 1.04 | 2.16 | 0.187 | |
| S25L2 | 7.33 | 1.30 | 0.337 | 0.442 | 1.00 | 0.796 | 1.96 | 3.13 | 0.0878 | |
| S31 | 6.22 | 2.75 | 9.20 | 8.77 | 0.160 | 0.286 | 2.18 | 0.269 | 0.249 | |
| Mean | 9.26 | 1.42 | 2.63 | 6.95 | 0.729 | 0.586 | 2.98 | 1.46 | 0.597 | |
| 30 cm | S5 | 18.5 | 0.990 | 2.93 | 13.9 | 3.11 | 1.18 | 3.36 | 2.01 | 0.790 |
| S11 | 11.5 | 0.717 | 2.01 | 7.38 | 0.922 | 0.733 | 2.18 | 1.10 | 0.389 | |
| S15 | 7.21 | 7.01 | 25.1 | 8.99 | 0.258 | 0.382 | 12.6 | 1.59 | 1.15 | |
| S22 | 0.372 | 0.0425 | 0.152 | 1.23 | 0.0326 | 0.211 | 0.795 | 1.75 | 0.114 | |
| S25L2 | 2.93 | 0.270 | 1.24 | 3.06 | 0.140 | 0.230 | 1.54 | 0.475 | 0.420 | |
| S31 | 3.81 | 0.823 | 2.83 | 3.57 | 0.145 | 0.251 | 1.05 | 0.459 | 0.235 | |
| Mean | 7.39 | 1.64 | 5.71 | 6.36 | 0.767 | 0.498 | 3.59 | 1.23 | 0.515 | |
| Reference | 0.316 | 0.0251 | 0.501 | 0.0794 | — | 0.794 | 0.126 | — | 0.0126 | |
From the values of Table 7, the distribution coefficient Kd values are significant compared with modified reference values92,93 for Cu, Cd, Ni, and Zn at all depths from 10 to 30 cm sediment. Cu, Zn, and Ni with very high Kd values imply that they are bonded weakly to the sediment layer, support metal outspreading into the aquatic environment, and pose an adverse impact. This movement tendency toward the water phase of Cu, Zn, and Cd is quite similar and reasonable with five-fractioning extraction analysis about the high proportion of Cu, Pb, Cd, and Mn in labile/acid soluble phases (exchangeable and carbonate phases), which are easier to transfer to the water phase. On the other hand, Cr, Fe, and Co have much lower Kd values than the others. They have been partitioned in sediment for a longer time due to a higher affinity with the sediment phase than the water phase. The calculated Kd values and the distribution of HMs in two phases, water and sediment, in this study are consistent with the studies of Daoquan Xu et al.62 and Li-Jyur Tsai et al.61 Between different sampling sites, almost all HMs at different depths demonstrate high distribution coefficients Kd at S11, S5, and S15 sites, which are near or even in the drainage and sewage system of Hai Duong industrial zones (Lai Vu and Phu Thai) and Pha Lai thermal power station. Furthermore, some display high values at S31 – Hiep bridge, Ninh Giang, specifically with Cd, Zn, and Co.
| Location | Cu | Pb | Cd | Zn | Fe | Co | Ni | Mn | Cr | References |
|---|---|---|---|---|---|---|---|---|---|---|
| Vietnam | ||||||||||
| Cau River | 7.63 | 13.5 | 0.140 | 19.6 | 4157 | 2.60 | 3.70 | 272 | 5.05 | This study |
| Bach Dang River | 33.9 | 48.1 | 0.560 | 82.9 | 29![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 080 080 |
— | 26.5 | 549 | 67.4 | Anh et al.94 |
| Cam River | 82.0 | 92.0 | — | 178 | — | 17.0 | 38.0 | 827 | 90.0 | Ho et al.95 |
| Ha Long Bay | 14.5 | 30.4 | 0.080 | 50.9 | — | — | — | — | — | Dang Hoai et al.96 |
| Ba Lat Estuary | 40.4 | 56.2 | 0.390 | 98.6 | 32![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 986 986 |
13.0 | 35.1 | 766 | 75.7 | Anh et al.97 |
| Thai Binh coast | 41.4 | — | 0.260 | 93.1 | — | — | 27.4 | — | 39.6 | Duong et al.98 |
| Van Don-Tra Co coast | 55.3 | 21.8 | 3.90 | 165 | — | 21.7 | 28.5 | — | 134 | Nguyen Nhu et al.99 |
| Mekong River | — | 2.08 | 0.100 | 99.0 | 163 | 4.95 | 8.30 | 172 | — | Nguyen et al.100 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||||||||
| Other countries | ||||||||||
| Pearl River, China | 36.0 | 46.8 | — | 137 | — | — | 37.2 | — | 82.3 | Ma et al.101 |
| Payra River, Bangladesh | 34.1 | 14.6 | 0.100 | 66.4 | — | 15.7 | 40.3 | 434 | 44.6 | Sultana et al.15 |
| Gohar Rood River, Iran | 59.5 | 30.0 | 0.100 | 213 | 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 660 660 |
34.0 | 58.2 | 1802 | 175 | Ashayeri et al.24 |
| Astore River, Pakistan | 13.2 | 22.6 | 0.660 | 36.6 | 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 382 382 |
24.0 | 23.6 | 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 650 650 |
24.3 | Ali et al.102 |
| Kunhar River, Pakistan | 14.9 | 12.7 | 2.10 | — | 31![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 600 |
17.6 | 46.5 | — | 17.4 | Muhammad et al.103 |
| Hooghly River, India | 16.7 | 10.7 | 0.200 | 46.9 | 23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 322 322 |
— | 19.8 | 482 | 31.8 | Mondal et al.104 |
| Pazarsuyu Stream, Turkey | 114 | 72.1 | 0.700 | 138 | 42![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 024 024 |
20.6 | 23.3 | 1137 | 60.4 | Ustaoğlu et al.105 |
| Nile River, Egypt | 21.5 | 13.9 | 2.70 | 76.4 | 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
12.1 | 48.6 | 660 | 26.4 | Al-Afify et al.106 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||||||||
| SQGs | ||||||||||
| TEL | 18.7 | 30.2 | 0.680 | 124 | — | — | 15.9 | — | 52.3 | MacDonald et al.107 |
| PEL | 108 | 112 | 4.21 | 271 | — | — | 42.8 | — | 160 | MacDonald et al.108 |
| LEL | 16.0 | 31.0 | 0.600 | 120 | 2000 | — | 16.0 | 460 | 26.0 | MacDonald et al.108 |
| SEL | 110 | 250 | 10.0 | 820 | 4000 | — | 75.0 | 1100 | 110 | MacDonald et al.108 |
| ERL | 34.0 | 46.7 | 1.20 | 150 | — | — | 20.9 | — | 81.0 | Long et al.109 |
| ERM | 270 | 218 | 9.60 | 410 | — | — | 51.6 | — | 370 | Long et al.109 |
Based on the comparison in Table 8, some key points can be surmised: (1) the concentrations of most metals (Cu, Pb, Zn, Fe, Co, Ni, Mn, and Cr) in the Cau River basin are lower than those found in the other locations near Hai Duong province, but manifold higher than the maximum contents of Pb, Fe, and Mn in the Mekong River. Nevertheless, the concentration of Cd is higher than that in Ha Long Bay, the Mekong River, and some international rivers in Bangladesh and Iran. This can be attributed solely to human influence. (2) The values of Cu, Pb, Zn, and Mn in the sediment in the Cau River basin are generally on par with those in the Payra River, Astore River, Kunhar River, and Hooghly River. Meanwhile, Cr, Ni, Fe, and Co have lower concentrations than in other rivers. (3) Almost all HMs are in the lower range of each SQG, especially Cr. This means that the impacts of these metals are moderate and have occasional adverse biological effects on the ecosystem. Nonetheless, Cu still poses a high risk for aquatic organisms. Despite a few differences between each SQG, Cr and Ni are relatively non-labile metals and pose minimal contamination risk to the ecosystem. All in all, Hai Duong province, with its rapid industrialization and urbanization, has experienced pollution caused by anthropogenic activities, similar to that of other developing countries around the world.
3.4. Multivariate statistical analysis for source identification of heavy metals in sediments
| Variable | Component | ||
|---|---|---|---|
| PC1 | PC2 | PC3 | |
| a Bold: these values represent relatively high loadings (absolute values > 0.4) on the components. | |||
| Cu | 0.439 | 0.268 | −0.277 |
| Pb | 0.423 | 0.353 | −0.190 |
| Cd | 0.488 | −0.063 | −0.134 |
| Zn | 0.072 | 0.227 | −0.330 |
| Fe | −0.078 | 0.492 | −0.020 |
| Co | 0.217 | 0.192 | 0.734 |
| Ni | −0.123 | 0.515 | 0.355 |
| Mn | 0.476 | −0.133 | 0.261 |
| Cr | −0.301 | 0.431 | −0.165 |
| Eigenvalues | 3.18 | 2.34 | 1.24 |
| Proportion of variance | 0.354 | 0.260 | 0.139 |
| Cumulative proportion | 0.354 | 0.614 | 0.753 |
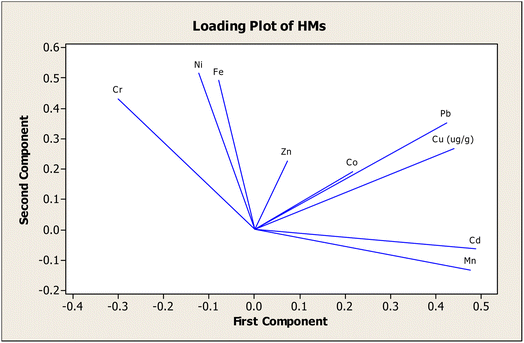
The results indicate that three principal components were extracted from the loadings of nine variables (Cu, Pb, Cd, Zn, Fe, Co, Ni, Mn, and Cr) in sediments of six sampling stations. The concentrations of the nine variables were reduced to three PCs (PC1, PC2, and PC3) whose eigenvalues are greater than 1.00 and cumulative variance is 75.3% (Table 9). Thus, this reduction is statistically significant and sufficient to describe and explain data structure information. The loadings on PC1 and PC2 are depicted in Fig. 4.
Principal component 1 (PC1), corresponding to 35.4% of the total variance, is distinguished by the presence of Cu, Pb, Cd, and Mn with positive loading values (>0.40). In this PC, Cd and Mn make up the largest proportion in the labile fraction (exchangeable and carbonate), whilst organic matter (oxidizable fraction) is presumed to be the major sink of Cu and Pb. In general, one or a combination of non-residual fractions (acid-soluble, organic matter, and Fe–Mn oxide) is the key storage for depositing these metals. Despite the high proportion of Mn in the labile fraction, Mn is still referred to as a lithogenic element.110 This is due to the fact that Mn is detected in the parent material of sediments. From CF and RAC analyses, it is deduced that these metals are specific contaminant elements from urban runoff, automobile emissions, sewage sludge, and industrial wastewater from Hai Duong's industrial zones in the examined areas. Consequently, these metals are principally supposed to originate from anthropogenic activities, which are in line with the findings of Sundaray et al.12 Based on the above reasonings, PC1 may be regarded as the “Anthropogenic Pollutant Source”.111
Principal component 2 (PC2) covers 26.0% of the total variance and is dominated by Cr, Fe, and Ni with >0.40 loadings. From the fractionation of these metals, the residual phase occupies a prominent ratio amongst the five phases. In contrast with metals in PC1, Cr and Fe exhibit a predisposition to attach longer to the sediment and reveal minor toxicity and less harmful exposure to the environment. The results of CF of Ni are consistent with this proposition, but the RAC and Kd studies otherwise signify Ni as a medium or high-risk element. In addition, Fe is reckoned to be present in the chemical composition of sedimentary rocks in the forms of hematite (α-Fe2O3), goethite (α-FeO(OH)), magnetite (FeO·Fe2O3), limonite (FeO(OH)·nH2O), and siderite (FeCO3).71 It is inferred that these metals are likely derived from natural parent rock materials, and PC2, therefore, may be ascribed as the “Lithogenic Pollutant Source”.12
Principal component 3 (PC3) constitutes 13.9% of the total variance and is differentiated by a high positive loading (>0.70) of Co. In the case of Co and Zn, relatively high levels in the carbonate fraction and the Fe–Mn oxide fraction, respectively, are ascertained in all samples. Taking these observations into consideration, PC3 may reflect a combination of source contributions and can be named the “Synergistic Pollutant Source”. This component likely emanates from human activities releasing HMs into contiguous environments or the geochemical activities of nature.
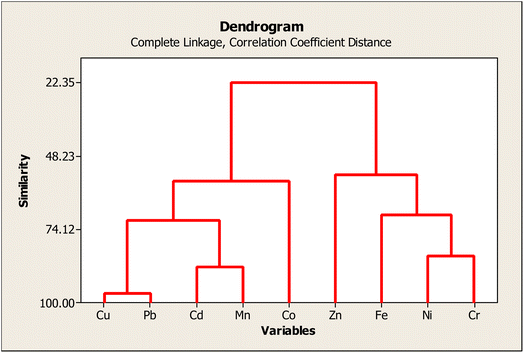
Cluster (I) consists of Cu, Pb, Cd, Mn, and Co, highlighted by the highest percentage bound to the non-residual structure. The contents of these metals are comparable with their corresponding background values, emphasizing that these metals are mainly derived from apparent anthropogenic inputs. However, the metals in the cluster (II) occupy a higher proportion of the residual fraction, especially Cr, implying natural geological sources. Two sub-clusters formed by Co and Zn at a 70.0% similarity level can be individuated. This formation seems to be synonymous with PCA performance.
3.5. Summary of results of fractionation, risk assessment, and multivariate analysis
Geochemical speciation data illustrate four defined groups based on the majority proportion in phases: labile-bound group (Cd and Mn), Fe–Mn oxides-bound group (Co, Zn, and Fe), organic matter-bound group (Cu and Pb), and residual-bound group (Cr and Ni). First, the qualitative rationales for HMs that dominated in each speciation phase of sediment are taken into account. HMs established in non-residual phases are liable to have weak affinities with sediment, especially the labile-bound group, thus being more mobile. Then, by quantitatively investigating the pollution indices and the transfer of HMs between water and sediment phases via the Kd definition, it is obvious to recapitulate that Cd, Mn, Cu, Pb, Zn, and Co may easily manifest severe effects on aquatic ecosystems with headwaters contaminated by industrial zones and manufacturing firms (S5, S11, S15, and S22). Statistically, Cd, Mn, Cu, and Pb are mostly contributed by anthropogenic provenances. Nevertheless, Cr, Ni, and Fe apparently disclose the lower mobility to equilibrate with the water phase and are highlighted by lithogenic sources. The environmental risk assessments of HMs demonstrate harmony with their sediment speciation. These analyses also support our earlier statement that HMs accumulate near the surface of the sediments.4. Conclusions
In this research, a modified five-step SEP and ICP-MS method is presented for geochemical speciation analysis of HM species (Cu, Pb, Cd, Zn, Fe, Co, Ni, Mn, and Cr) in sediment core samples of the Hai Duong river system. The quality of sediment and identification of HM contaminated areas were quantitatively assessed and systematically achieved using environmental risk indices and multivariate statistical analyses. We found that most HMs are dominantly deposited in the top layer of the sediment cores. While Zn and Co showed a high affinity to the carbonate phase and were classified into a “synergistic component”, Cr, Ni, and Fe manifested strong adhesion towards primary inert mineral phases and were categorized as the “lithogenic component”. In the “anthropogenic component”, Mn, Cd, Pb, and Cu may be removed from these sediments at a faster mobility rate than the others and then introduce their widespread potential threat to the neighboring habitat. Our results allocated the contaminated areas of toxic HM pollutants, indicating that they are in close proximity to the sewage drainage system of the Pha Lai thermal power company (S5) and the waste disposal of Lai Vu (S15), Phu Thai (S11) industrial enterprises. Overall, Cd posed a very high risk vulnerability to the surroundings at some polluted stations in the studied area. The remediation and annual monitoring for this metal are recommended. In addition, the adoption of ArcGIS for pollution distribution in density analysis will be further investigated in the next study.Data availability
The data used to support the findings of this study have been included in the ESI.†Author contributions
Ba Lich Pham: conceptualization, first draft preparation, data treatment, paper writing, editing, and review; Huy Thong Vu and Van Linh Nguyen: chemical analyses and data collection; Thi Kim Thuong Nguyen and Anh Duc Trinh: proofreading and editing; Thi Thao Ta: conceptualization, editing, review, and supervision. All authors have approved the final version of the manuscript for publication and agreed to be held accountable for the work performed therein.Conflicts of interest
The authors declare that there are no conflicts of interest regarding this paper.Acknowledgements
The authors wish to thank Kenneth Crossley for constructive comments on English grammar and clarity.References
- Ş. Fural, S. Kükrer, İ. Cürebal and D. Aykır, Spatial distribution, environmental risk assessment, and source identification of potentially toxic metals in Atikhisar dam, Turkey, Environ. Monit. Assess., 2021, 193, 1–16 CrossRef PubMed.
- A. Wali, G. Colinet and M. Ksibi, Speciation of Heavy Metals by Modified BCR Sequential Extraction in Soils Contaminated by Phosphogypsum in Sfax, Tunisia, Environmental Research, Engineering and Management, 2014, 4, 14–26 Search PubMed.
- J.-J. Kim, Y.-S. Kim and V. Kumar, Heavy metal toxicity: an update of chelating therapeutic strategies, J. Trace Elem. Med. Biol., 2019, 54, 226–231 CrossRef CAS PubMed.
- P. B. Tchounwou, C. G. Yedjou, A. K. Patlolla and D. J. Sutton, Heavy metal toxicity and the environment, Exper. Suppl., 2012, 101, 133–164 Search PubMed.
- T. Wang, J. Pan and X. Liu, Characterization of heavy metal contamination in the soil and sediment of the Three Gorges Reservoir, China, J. Environ. Sci. Health, Part A, 2017, 52, 201–209 CrossRef CAS PubMed.
- M. A. Okbah, M. I. El-Gammal, M. S. Ibrahim and Y. A. A. Waheshi, Geochemical speciation of trace metals in sediments of the northern Nile Delta Lake by sequential extraction technique, Chem. Ecol., 2020, 36, 236–255 CrossRef CAS.
- B. Chen, R. He, P. Cai, G. Huang and F. Wang, Geochemical Speciation, Risk Assessment, and Sources Identification of Heavy Metals in Mangrove Surface Sediments from the Nanliu River Estuary of the Beibu Gulf, China, Sustainability, 2022, 14, 1–20 Search PubMed.
- H. C. Liu and C. F. You, in Sediment Watch: Monitoring, Ecological Risk Assessment and Environmental Management, NOVA Science Publishers, 2018, pp. 227–249 Search PubMed.
- M. S. Bhuyan, S. M. B. Haider, G. Meraj, M. A. Bakar, M. T. Islam, M. Kunda, M. A. B. Siddique, M. M. Ali, S. Mustary, I. A. Mojumder and M. A. Bhat, Assessment of Heavy Metal Contamination in Beach Sediments of Eastern St. Martin's Island, Bangladesh: Implications for Environmental and Human Health Risks, Water, 2023, 15, 2494 CrossRef CAS.
- S. Bećiragić, J. Huremović, T. Muhić-Šarac, M. Memić, A. Selović and S. Žero, Metal Levels in Surface Soils after Different Extraction Procedures, Bull. Chem. Technol. Bosnia Herzegovina, 2013, 41, 1–5 Search PubMed.
- Y. Qiao, Y. Yang, J. Gu and J. Zhao, Distribution and geochemical speciation of heavy metals in sediments from coastal area suffered rapid urbanization, a case study of Shantou Bay, China, Mar. Pollut. Bull., 2013, 68, 140–146 CrossRef CAS PubMed.
- S. K. Sundaray, B. B. Nayak, S. Lin and D. Bhatta, Geochemical speciation and risk assessment of heavy metals in the river estuarine sediments - a case study: Mahanadi basin, India, J. Hazard. Mater., 2011, 186, 1837–1846 CrossRef CAS PubMed.
- T. T. L. Santos, J. L. S. Mounier and R. V. Marins, Trace metal partitioning in the parnaíba delta in dry season, equatorial coast of Brazil, Environ. Pollut., 2024, 345, 123500 CrossRef CAS PubMed.
- E. Prifti, H. Kaberi, V. Paraskevopoulou, P. Michalopoulos, C. Zeri, S. Iliakis, M. Dassenakis and M. Scoullos, Vertical Distribution and Chemical Fractionation of Heavy Metals in Dated Sediment Cores from the Saronikos Gulf, Greece, Journal of Marine Science and Engineering, 2022, 10, 376–400 CrossRef.
- S. Sultana, N. Sultana, M. Moniruzzaman, M. R. Dastagir and M. K. Hossain, Unmasking heavy metal contamination: tracing, risk estimating and source fingerprinting from coastal sediments of the Payra River in Bangladesh, Mar. Pollut. Bull., 2025, 211, 117455 CrossRef CAS PubMed.
- A. Chowdhury, A. Naz and S. K. Maiti, Distribution, speciation, and bioaccumulation of potentially toxic elements in the grey mangroves at Indian Sundarbans, in relation to vessel movements, Mar. Environ. Res., 2023, 189, 106042 CrossRef CAS PubMed.
- K. K. Jayasooryan, E. V. Ramasamy, P. K. Chandini and M. Mohan, Fractionation and accumulation of selected metals in a tropical estuary, south-west coast of India, Environ. Monit. Assess., 2021, 193, 220 CrossRef CAS PubMed.
- S. Muhammad, Evaluation of heavy metals in water and sediments, pollution, and risk indices of Naltar Lakes, Pakistan, Environ. Sci. Pollut. Res., 2023, 30, 28217–28226 CrossRef CAS PubMed.
- S. Muhammad, A. Zeb, M. R. Shaik and M. E. Assal, Spatial distribution of potentially toxic elements pollution and ecotoxicological risk of sediments in the high-altitude lakes ecosystem, Physics and Chemistry of the Earth, Parts A/B/C, 2024, 135, 103655 CrossRef.
- A. U. Haq, S. Muhammad and A. Ahmad, Spatial Distribution, Risk Assessment, and Provenance of Heavy Metals Contamination in the Sediments of the Gilgit River Basin, Northern Pakistan, Soil Sediment Contam., 2024, 33, 1365–1381 CrossRef.
- S. Muhammad and I. Ullah, Spatial and temporal distribution of heavy metals pollution and risk indices in surface sediments of Gomal Zam Dam Basin, Pakistan, Environ. Monit. Assess., 2023, 195, 1155 CrossRef CAS PubMed.
- A. Mosalem, M. Redwan, A. A. Abdel Moneim and S. Rizk, Distribution, speciation, and assessment of heavy metals in sediments from Wadi Asal, Red Sea, Egypt, Environ. Monit. Assess., 2024, 196, 215 CrossRef CAS PubMed.
- N. F. Soliman, A. M. Younis and E. Elkady, Chemical speciation and comprehensive risk assessment of metals in sediments from Nabq protectorate, the Red Sea using individual and synergistic indices, Mar. Pollut. Bull., 2024, 201, 116219 CrossRef CAS PubMed.
- S. Y. Ashayeri, B. Keshavarzi, F. Moore, A. Ahmadi and P. S. Hooda, Risk assessment, geochemical speciation, and source apportionment of heavy metals in sediments of an urban river draining into a coastal wetland, Mar. Pollut. Bull., 2023, 186, 114389 CrossRef CAS PubMed.
- S. Rezapour, F. Asadzadeh, A. Nouri, H. Khodaverdiloo and M. Heidari, Distribution, source apportionment, and risk analysis of heavy metals in river sediments of the Urmia Lake basin, Sci. Rep., 2022, 12, 17455 CrossRef CAS PubMed.
- F. Rezaei, M. Rastegari Mehr, A. Shakeri, E. Sacchi, K. Borna and O. Lahijani, Predicting bioavailability of potentially toxic elements (PTEs) in sediment using various machine learning (ML) models: a case study in Mahabad Dam and River-Iran, J. Environ. Manage., 2024, 366, 121788 CrossRef CAS PubMed.
- Y. Yuan, B. Liu and H. Liu, Spatial distribution and source identification for heavy metals in surface sediments of East Dongting Lake, China, Sci. Rep., 2022, 12, 7940 CrossRef CAS PubMed.
- Y.-J. Tu, P.-C. Luo, Y.-L. Li, J. Liu, T.-T. Sun, G.-J. Li and Y.-P. Duan, Seasonal heavy metal speciation in sediment and source tracking via Cu isotopic composition in Huangpu River, Shanghai, China, Ecotoxicol. Environ. Saf., 2023, 260, 115068 CrossRef CAS PubMed.
- W. Sun, K. Yang, R. Li, T. Chen, L. Xia, X. Sun and Z. Wang, Distribution characteristics and ecological risk assessment of heavy metals in sediments of Shahe reservoir, Sci. Rep., 2022, 12, 16239 CrossRef CAS PubMed.
- Z. Huang, C. Liu, X. Zhao, J. Dong and B. Zheng, Risk assessment of heavy metals in the surface sediment at the drinking water source of the Xiangjiang River in South China, Environ. Sci. Eur., 2020, 32, 23 CrossRef CAS.
- W. Que, L. Yi, Y. Wu and Q. Li, Analysis of heavy metals in sediments with different particle sizes and influencing factors in a mining area in Hunan Province, Sci. Rep., 2024, 14, 20318 CrossRef CAS PubMed.
- Y. Cao, Y. Li, L. Jia, Q. Wang, T. Niu, Q. Yang, Q. Wang, X. Zeng, R. Wang and L. Yue, Long-term and combined heavy-metal contamination forms a unique microbiome and resistome: a case study in a Yellow River tributary sediments, Environ. Res., 2024, 252, 118861 CrossRef CAS PubMed.
- H. Hu, F. Guo, X. Chen, Y. Wang, J. Liu and H. Cheng, Tin and lead compounds in fish and crustacean from aquaculture ponds in Zhejiang Province of East China: accumulation and health risk assessment, Aquaculture, 2025, 595, 741586 CrossRef CAS.
- Q. Chen, L. Wu, C. Zhou, G. Liu and L. Yao, A study of environmental pollution and risk of heavy metals in the bottom water and sediment of the Chaohu Lake, China, Environ. Sci. Pollut. Res., 2024, 31, 19658–19673 CrossRef CAS PubMed.
- A. Hass and P. Fine, Sequential Selective Extraction Procedures for the Study of Heavy Metals in Soils, Sediments, and Waste Materials—A Critical Review, Crit. Rev. Environ. Sci. Technol., 2010, 40, 365–399 CrossRef CAS.
- Ś. Ryszard and T. Marzena, Chemical Fractionation in Environmental Studies of Potentially Toxic Particulate-Bound Elements in Urban Air: A Critical Review, Toxics, 2022, 10, 1–29 Search PubMed.
- C. Gleyzes, S. Tellier and M. Astruc, Fractionation studies of trace elements in contaminated soils and sediments: a review of sequential extraction procedures, TrAC, Trends Anal. Chem., 2002, 21, 451–467 CrossRef CAS.
- D. J. Butcher, Recent advances in graphite furnace atomic absorption spectrometry: a review of fundamentals and applications, Appl. Spectrosc. Rev., 2024, 59, 247–275 CrossRef CAS.
- M. M. López Guerrero, M. T. Siles Cordero, E. Vereda Alonso, A. García de Torres and J. M. Cano Pavón, Cold vapour generation electrothermal atomic absorption spectrometry and solid phase extraction based on a new nanosorbent for sensitive Hg determination in environmental samples (sea water and river water), Microchem. J., 2017, 132, 274–279 CrossRef.
- N. Manousi and A. N. Anthemidis, A flow-batch lab-in-syringe foam microextraction platform for the simultaneous preconcentration and in situ membraneless gas-liquid separation of mercury prior to cold vapor atomic absorption spectrometry, Anal. Chim. Acta, 2024, 1290, 342208 CrossRef CAS PubMed.
- M. Patra, S. N. Upadhyay and S. K. Dubey, Synchrotron induced X-ray fluorescence spectroscopy reveals heavy metal translocation in sludge amended soil–plant systems: assessment of ecological and health risks, Environ. Geochem. Health, 2024, 46, 399 CrossRef CAS PubMed.
- E. Marguí, D. Eichert, J. Jablan, F. Bilo, L. E. Depero, A. Pejović-Milić, A. Gross, H. Stosnach, A. Kubala-Kukuś, D. Banaś and L. Borgese, An overview of the applications of total reflection X-ray fluorescence spectrometry in food, cosmetics, and pharmaceutical research, J. Anal. At. Spectrom., 2024, 39, 1700–1719 RSC.
- Q. Zhang, F. Li and W. Yang, A deep spectral prediction network to quantitatively determine heavy metal elements in soil by X-ray fluorescence, J. Anal. At. Spectrom., 2024, 39, 478–490 RSC.
- T. T. K. Nguyen, H. T. Luu, L. D. Vu, T. T. Ta and G. T. H. Le, Determination of Total Mercury in Solid Samples by Anodic Stripping Voltammetry, J. Chem., 2021, 2021, 8888879 Search PubMed.
- S. Laschi, P. S. Sfragano, F. Tadini-Buoninsegni, N. Guigues and I. Palchetti, Development of a flow system for decentralized electrochemical analysis of heavy metals using screen-printed electrodes: the importance of sensor stability, Analyst, 2024, 149, 4239–4249 RSC.
- J. Zhong, Z. Wang, Y. Chen, W. Huan, M. Shi, L. Lei, X. Yu and L. Chen, Determination of trace heavy metal elements in litterfall by inductively coupled plasma optical emission spectrometry after extraction using choline chloride-based deep eutectic solvents, RSC Adv., 2024, 14, 22497–22503 RSC.
- Erdiwansyah, A. Gani, H. Desvita, Mahidin, Bahagia, R. Mamat and S. M. Rosdi, Investigation of heavy metal concentrations for biocoke by using ICP-OES, Results Eng., 2025, 25, 103717 CrossRef CAS.
- C. S. Provete, B. M. Dalfior, R. Mantovaneli, M. T. W. D. Carneiro and G. P. Brandão, Comparison of the Performance of ICP-MS, CV-ICP-OES, and TDA AAS in Determining Mercury in Marine Sediment Samples, ACS Omega, 2024, 9, 49229–49238 CrossRef CAS PubMed.
- F. Guo, P. Zeng, J. Liu, H. Hu, W. Zhu, Y. Wang and H. Cheng, Simultaneous preconcentration and quantification of ultra-trace tin and lead species in seawater by online SPE coupled with HPLC-ICP-MS, Anal. Chim. Acta, 2024, 1294, 342294 CrossRef CAS PubMed.
- M. H. Nguyen, T. D. Pham, T. L. Nguyen, H. A. Vu, T. T. Ta, M. B. Tu, T. H. Y. Nguyen and D. B. Chu, Speciation Analysis of Arsenic Compounds by HPLC-ICP-MS: Application for Human Serum and Urine, J. Anal. Methods Chem., 2018, 2018, 9462019 Search PubMed.
- T. T. Ta, D. A. Trinh and N. T. Do, Nitrogen flow assessment in rapidly urbanizing Hai Duong province, downstream of Cau River Basin, Vietnam, J. Mater. Cycles Waste Manage., 2018, 20, 533–542 CrossRef CAS.
- Hai Duong Department of Natural Resources and Environment, Report on Water Quality Monitoring during the period 2009–2014 in Hai Duong Province, Department of Natural Resources and Environment (DNRE), Vietnam, 2014 Search PubMed.
- S. H. Le, A. D. Trinh, H. T. Vu and T. T. Ta, Assessment of surface water quality and nutrient pollution source using multivariate statistical technique: a case study of Cau River Basin in Hai Duong province, The 4th Analytica Vietnam Conference, 2015, 25, 71–79 Search PubMed.
- T. T. Ta, S. H. Le, H. Q. Trinh, T. N. M. Luu and A. D. Trinh, Interpretation of anthropogenic impacts (agriculture and urbanization) on tropical deltaic river network through the spatio-temporal variation of stable (N, O) isotopes of NO3(−), Isot. Environ. Health Stud., 2016, 52, 487–497 CrossRef CAS PubMed.
- R. H. Hesslein, An in situ sampler for close interval pore water studies, Limnol. Oceanogr., 1976, 21, 912–914 CrossRef CAS.
- M. A. M. Abdallah, Chemical speciation and contamination assessment of Pb and V by sequential extraction in surface sediment off Nile Delta, Egypt, Arabian J. Chem., 2017, 10, 68–75 CrossRef CAS.
- M. Tytła, Assessment of Heavy Metal Pollution and Potential Ecological Risk in Sewage Sludge from Municipal Wastewater Treatment Plant Located in the Most Industrialized Region in Poland—Case Study, International Journal of Environmental Research and Public Heath, 2019, 16, 24–30 Search PubMed.
- A. Kabata-Pendias, Trace elements in soils and plants, Taylor & Francis Group, CRC Press, Boca Raton, 4th edn, 2010 Search PubMed.
- D. Sah, P. K. Verma, M. K. Kandikonda and A. Lakhani, Chemical fractionation, bioavailability, and health risks of heavy metals in fine particulate matter at a site in the Indo-Gangetic Plain, India, Environ. Sci. Pollut. Res. Int., 2019, 26, 19749–19762 CrossRef CAS PubMed.
- J. Singh, A. S. Kalamdhad and B.-K. Lee, Reduction of eco-toxicity risk of heavy metals in the rotary drum composting of water hyacinth: waste lime application and mechanisms, Environ. Eng. Res., 2015, 20, 212–222 CrossRef.
- L.-J. Tsai, K. C. Yu, J.-S. Huang and S.-T. Ho, Distribution of heavy metals in contaminated river sediment, J. Environ. Sci. Health, Part A, 2002, 37, 1421–1439 CrossRef PubMed.
- D. Xu, Y. Wang, R. Zhang, J. Guo, W. Zhang and K. Yu, Distribution, speciation, environmental risk, and source identification of heavy metals in surface sediments from the karst aquatic environment of the Lijiang River, Southwest China, Environ. Sci. Pollut. Res., 2016, 23, 9122–9133 CrossRef CAS PubMed.
- J. N. Miller, J. C. Miller and R. D. Miller, Statistics and Chemometrics for Analytical Chemistry, Pearson Education Limited, Harlow, United Kingdom, 7th edn, 2018 Search PubMed.
- R. Wehrens, Chemometrics with R: Multivariate Data Analysis in the Natural and Life Sciences (Use R!), Springer, Berlin, Hiedelberg, 2nd edn, 2020 Search PubMed.
- A. S. Luna, Chemometrics: Methods, Applications and New Research, Nova, United Kingdom, 2017 Search PubMed.
- C. M. A. Iwegbue, G. E. Nwajei, O. Eguavoen and J. E. Ogala, Chemical fractionation of some heavy metals in soil profiles in vicinity of scrap dumps in Warri, Nigeria, Chem. Speciation Bioavailability, 2009, 21, 99–110 CrossRef CAS.
- J. Chen, J. Liu, H. Hong, S. Liang, W. Zhao, H. Jia, H. Lu, J. Li and C. Yan, Coastal reclamation mediates heavy metal fractions and ecological risk in saltmarsh sediments of northern Jiangsu Province, China, Sci. Total Environ., 2022, 825, 1–11 Search PubMed.
- W. Xu, L. Xiao, S. Hou, G. Rukh, M. Xu, Y. Pan, J. Xu, W. Lan, Z. Ruan, B. Zhong and D. Liu, Bioavailability and speciation of cadmium in contaminated paddy soil as alleviated by biochar from co-pyrolysis of peanut shells and maize straw, Environ. Sci. Eur., 2022, 34, 1–10 CrossRef.
- S. Cai, S. Zhou, J. Cheng, Q. Wang and Y. Dai, Distribution, Bioavailability and Ecological Risk of Heavy Metals in Surface Sediments from the Wujiang River Basin, Southwest of China, Pol. J. Environ. Stud., 2021, 30, 5479–5491 CAS.
- T. D. Nguyen and T. P. D. Dao, Analytical Chemistry, Questions and Problems in Ionic Equilibrium in Aqueous Solutions, Hanoi National University of Education Publishers, 2005 Search PubMed.
- M. B. McBride and C. E. Martínez, Environmental chemistry of soils, Royal Society of Chemistry, New York, 1st edn, 2023 Search PubMed.
- Inamuddin, M. I. Ahamed and E. Lichtfouse, Water Pollution and Remediation: Heavy Metals, Springer International Publishing, 2021 Search PubMed.
- H. Ji, H. Li, Y. Zhang, H. Ding, Y. Gao and Y. Xing, Distribution and risk assessment of heavy metals in overlying water, porewater, and sediments of Yongding River in a coal mine brownfield, J. Soils Sediments, 2018, 18, 624–639 CrossRef CAS.
- A. Naji, A. Ismail and A. R. Ismail, Chemical speciation and contamination assessment of Zn and Cd by sequential extraction in surface sediment of Klang River, Malaysia, Microchem. J., 2010, 95, 285–292 CrossRef CAS.
- M. Abidi, A. Yahyaoui, R. B. Amor, L. Chouba and M. Gueddari, Evaluation of heavy metal pollution risk in surface sediment of the South Lagoon of Tunis by a sequential extraction procedure, Sci. Mar., 2022, 86, 1–12 Search PubMed.
- B. A. Hakami, E.-S. S. A. Seif and A. A. El-Shater, Environmental pollution assessment of Al-Musk Lake, Jeddah, Saudi Arabia, Nat. Hazards, 2020, 101, 429–448 CrossRef.
- R. B. Amor, A. Yahyaoui, M. Abidi, L. Chouba and M. Gueddari, Bioavailability and Assessment of Metal Contamination in Surface Sediments of Rades-Hamam Lif Coast, around Meliane River (Gulf of Tunis, Tunisia, Mediterranean Sea), J. Chem., 2019, 2019, 1–11 CrossRef.
- M. M. Billah, E. Kokushi and S. Uno, Distribution, Geochemical Speciation, and Bioavailable Potencies of Cadmium, Copper, Lead, and Zinc in Sediments from Urban Coastal Environment in Osaka Bay, Japan, Water, Air, Soil Pollut., 2019, 230, 1–13 CrossRef CAS.
- R. G. Pearson, Hard and Soft Acids and Bases, J. Am. Chem. Soc., 1963, 85, 3533–3539 CrossRef CAS.
- C. E. Housecroft and A. G. Sharpe, Inorganic Chemistry, Pearson Prentice Hall, United Kingdom, 5th edn, 2018 Search PubMed.
- J. Kierczak, C. Neel, U. Aleksander-Kwaterczak, E. Helios-Rybicka, H. Bril and J. Puziewicz, Solid speciation and mobility of potentially toxic elements from natural and contaminated soils: a combined approach, Chemosphere, 2008, 73, 776–784 CrossRef CAS PubMed.
- H. Altundag, M. Imamoglu, S. Doganci, E. Baysal, S. Albayrak and M. Tuzen, Determination of Heavy Metals and Their Speciation in Street Dusts by Inductively Coupled Plasma-Optical Emission Spectrometry after a Community Bureau of Reference Sequential Extraction Procedure, J. AOAC Int., 2013, 96, 864–869 CrossRef CAS PubMed.
- O. I. Davutluoglu, G. Seckin, C. B. Ersu, T. Yilmaz and B. Sari, Heavy metal content and distribution in surface sediments of the Seyhan River, Turkey, J. Environ. Manage., 2011, 92, 2250–2259 CrossRef CAS PubMed.
- V. M. Goldschmidt, Geochemistry, Soil Sci., 1954, 78, 156 CrossRef.
- G. L. Miessler, P. J. Fischer and D. A. Tarr, Inorganic chemistry, Pearson, Boston, 5th edn, 2021 Search PubMed.
- A. Wali, G. Colinet, M. Khadhraoui and M. Ksibi, Trace metals in surface soil contamination by release of phosphate industry in the surroundings of Sfax-Tunisia, Environmental Research, Engineering and Management, 2013, 65, 20–25 CrossRef.
- K. Nemati, N. K. A. Bakar, M. R. Abas and E. Sobhanzadeh, Speciation of heavy metals by modified BCR sequential extraction procedure in different depths of sediments from Sungai Buloh, Selangor, Malaysia, J. Hazard. Mater., 2011, 192, 402–410 CAS.
- M. N. Islam, X. P. Nguyen, H.-Y. Jung and J.-H. Park, Chemical Speciation and Quantitative Evaluation of Heavy Metal Pollution Hazards in Two Army Shooting Range Backstop Soils, Bull. Environ. Contam. Toxicol., 2016, 96, 179–185 CrossRef CAS PubMed.
- F. Li, M. Xiao, J. Zhang, C. Liu, Z. Qiu and Y. Cai, Spatial Distribution, Chemical Fraction and Fuzzy Comprehensive Risk Assessment of Heavy Metals in Surface Sediments from the Honghu Lake, China, Int. J. Environ. Res. Public Health, 2018, 15, 1–17 CAS.
- X. Hao, L. Bai, X. Liu, P. Zhu, H. Liu, Y. Xiao, J. Geng, Q. Liu, L. Huang and H. Jiang, Cadmium Speciation Distribution Responses to Soil Properties and Soil Microbes of Plow Layer and Plow Pan Soils in Cadmium-Contaminated Paddy Fields, Front. Microbiol., 2021, 12, 1–12 Search PubMed.
- L. Gao, R. Li, Z. Liang, L. Hou and J. Chen, Seasonal variations of cadmium (Cd) speciation and mobility in sediments from the Xizhi River basin, South China, based on passive sampling techniques and a thermodynamic chemical equilibrium model, Water Res., 2021, 207, 1–11 CrossRef PubMed.
- J. D. Allison and T. L. Allison, Partition coefficients for metals in surface water, soil, and waste, U.S. Environmental Protection Agency Office of Research and Development, Washington, DC 20460, 2005 Search PubMed.
- J. E. Sedeño-Díaz, E. López-López, E. Mendoza-Martínez, A. J. Rodríguez-Romero and S. S. Morales-García, Distribution Coefficient and Metal Pollution Index in Water and Sediments: Proposal of a New Index for Ecological Risk Assessment of Metals, Water, 2020, 12, 1–20 Search PubMed.
- N. N. Anh, Attribution of sources to heavy metal accumulation in the anthropogenically impacted Bach Dang River Estuary, Vietnam, Mar. Pollut. Bull., 2023, 193, 115244 CrossRef CAS PubMed.
- H. H. Ho, R. Swennen, V. Cappuyns, E. Vassilieva, G. Neyens, M. Rajabali and T. Van Tran, Assessment on Pollution by Heavy Metals and Arsenic Based on Surficial and Core Sediments in the Cam River Mouth, Haiphong Province, Vietnam, Soil Sediment Contam., 2013, 22, 415–432 CrossRef CAS.
- N. Dang Hoai, H. Nguyen Manh, T. Tran Duc, T. Do Cong, L. Tran Dinh, R. Johnstone and D. Nguyen Thi Kim, An assessment of heavy metal contamination in the surface sediments of Ha Long Bay, Vietnam, Environ. Earth Sci., 2020, 79, 436 CrossRef.
- N. N. Anh, An insight into source apportionment of metals in superficial sediments from the Tien Hai nature reserve of the Red River delta, Vietnam, Mar. Pollut. Bull., 2022, 185, 114278 CrossRef CAS PubMed.
- L. T. Duong, B. Q. Nguyen, C. D. Dao, N. N. Dao, H. L. T. Nguyen, T. H. T. Nguyen, C. H. T. Nguyen, D. C. Duong and N. N. Pham, Heavy metals in surface sediments of the intertidal Thai Binh Coast, Gulf of Tonkin, East Sea, Vietnam: distribution, accumulation, and contamination assessment, Environ. Sci. Pollut. Res., 2022, 29, 41261–41271 CrossRef CAS PubMed.
- T. Nguyen Nhu, N. Bui Van, D.-A. Le, T. H. Tran Thi, T.-D. Nguyen, T. Dang Xuan, H.-N. Tran, P. N. Sang, M. Saiyad Musthafa and V.-H. Duong, Characteristics of heavy metals in surface sediments of the Van Don-Tra Co coast, northeast Vietnam, Regional Studies in Marine Science, 2024, 73, 103459 CrossRef.
- B. T. Nguyen, D. D. Do, T. X. Nguyen, V. N. Nguyen, D. T. Phuc Nguyen, M. H. Nguyen, H. T. Thi Truong, H. P. Dong, A. H. Le and Q.-V. Bach, Seasonal, spatial variation, and pollution sources of heavy metals in the sediment of the Saigon River, Vietnam, Environ. Pollut., 2020, 256, 113412 CrossRef CAS PubMed.
- Y. Ma, Y. Ma, W. Zhang, H. Zhang, T. Li, D. Kong, C. Xu, H. Shi, X. Xu and D. Wang, The Spatiotemporal Variation and Historical Evolution of Heavy Metal Pollution in Sediments from the Pearl River Estuary, China, Water, 2024, 16, 531 CrossRef CAS.
- W. Ali and S. Muhammad, Spatial distribution, eco-environmental risks, and source characterization of heavy metals using compositional data analysis in riverine sediments of a Himalayan River, Northern Pakistan, J. Soils Sediments, 2023, 23, 2244–2257 CrossRef CAS.
- S. Muhammad, S. Ullah, W. Ali, I. A. K. Jadoon and M. Arif, Spatial distribution of heavy metal and risk indices of water and sediments in the Kunhar River and its tributaries, Geocarto International, 2022, 37, 5985–6003 CrossRef.
- P. Mondal, M. Schintu, B. Marras, A. Bettoschi, A. Marrucci, S. K. Sarkar, R. Chowdhury, M. P. Jonathan and J. K. Biswas, Geochemical fractionation and risk assessment of trace elements in sediments from tide-dominated Hooghly (Ganges) River Estuary, India, Chem. Geol., 2020, 532, 119373 CrossRef CAS.
- F. Ustaoğlu and M. S. Islam, Potential toxic elements in sediment of some rivers at Giresun, Northeast Turkey: a preliminary assessment for ecotoxicological status and health risk, Ecol. Indic., 2020, 113, 106237 CrossRef.
- A. D. G. Al-Afify and A. M. Abdel-Satar, Risk assessment of heavy metal pollution in water, sediment and plants in the Nile River in the Cairo region, Egypt, Oceanol. Hydrobiol. Stud., 2020, 49, 1–12 CrossRef CAS.
- D. D. Macdonald, R. S. Carr, F. D. Calder, E. R. Long and C. G. Ingersoll, Development and evaluation of sediment quality guidelines for Florida coastal waters, Ecotoxicology, 1996, 5, 253–278 CrossRef CAS PubMed.
- D. D. MacDonald, C. G. Ingersoll and T. A. Berger, Development and Evaluation of Consensus-Based Sediment Quality Guidelines for Freshwater Ecosystems, Arch. Environ. Contam. Toxicol., 2000, 39, 20–31 CrossRef CAS PubMed.
- E. R. Long, D. D. Macdonald, S. L. Smith and F. D. Calder, Incidence of adverse biological effects within ranges of chemical concentrations in marine and estuarine sediments, Environ. Manage., 1995, 19, 81–97 CrossRef.
- J. Madondo, C. Canet, E. González-Partida, A. A. Rodríguez-Díaz, F. Núñez-Useche, P. Alfonso, A. Rajabi, T. Pi, L. Blignaut and N. Vafeas, Geochemical constraints on the genesis of the ‘Montaña de Manganeso’ vein-type Mn deposit, Mexican Plateau, Ore Geol. Rev., 2020, 125, 1–16 CrossRef.
- J. P. Vareda, A. J. M. Valente and L. Durães, Assessment of heavy metal pollution from anthropogenic activities and remediation strategies: a review, J. Environ. Manage., 2019, 246, 101–118 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4va00325j |
| ‡ Present address: Institut de Chimie Physique, Université Paris-Saclay, Centre National de la Recherche Scientifique (CNRS), UMR8000, 91405 Orsay, France. |
| This journal is © The Royal Society of Chemistry 2025 |