Open Access Article
Open Access ArticlePhytomanagement strategy leads to plant-derived catalysts for the sustainable synthesis of oxidized Hantzsch esters†
Anca-Elena Dascăluabc,
Christophe Waterlotd,
Jenna Caudle ae,
David Bulteeldf,
Guillaume Dhainautdf,
Adeline Janusg,
Tristan Debuigneg,
Jean-Yves Cornuh and
Alina Ghinet
ae,
David Bulteeldf,
Guillaume Dhainautdf,
Adeline Janusg,
Tristan Debuigneg,
Jean-Yves Cornuh and
Alina Ghinet *abc
*abc
aJUNIA, Health and Environment, Laboratory of Sustainable Chemistry and Health, F-59000 Lille, France. E-mail: alina.ghinet@junia.com
bUniversity of Lille, Inserm, CHU Lille, Institut Pasteur de Lille, U1167-RID-AGE-Facteurs de Risque et Déterminants Moléculaires des Maladies Liées Au Vieillissement, F-59000 Lille, France
cFaculty of Chemistry, Department of Organic Chemistry, ‘Al. I. Cuza’ University of Iasi, Bd. Carol I Nr. 11, 700506 Iasi, Romania
dUniversity of Lille, Institut Mines-Télécom, University Artois, JUNIA, ULR 4515 - LGCgE, Laboratoire de Génie Civil et Géo-Environnement, F-59000 Lille, France
eDepartment of Chemistry and Biochemistry, The University of Tulsa, 800 South Tucker Drive, Tulsa, OK 74104, USA
fIMT Nord Europe, Institut Mines-Télécom, Centre for Materials and Processes, F-59000, Lille, France
gIxsane, Parc des Moulins, F-59650 Villeneuve d’Ascq, France
hISPA, Bordeaux Sciences Agro, INRAE, F-33140 Villenave-d’Omon Cedex, France
First published on 12th May 2025
Abstract
This study proposes a phytomanagement strategy to produce plant-derived eco-catalysts from Lolium perenne (ryegrass) and Trifolium incarnatum (clover), cultivated in contaminated or nutrient-rich soils from two French regions (Pompey and Bordeaux, respectively) and two Belgium sites (Duferco and Vieille Montagne, respectively), for the sustainable oxidation of Hantzsch esters and other substrates. Accumulating plants, cultivated in nutrient-rich or contaminated soils, provide catalysts enriched with transition metals like copper and zinc, supporting green chemistry principles by minimizing hazardous waste and fostering resource circularity. These catalysts were evaluated for the oxidation of Hantzsch esters to pyridines, pyridine-4(1H)-thione to pyridine-4-thiol, cyclohex-2-enone to substituted phenol, and dihydroquinoxaline to hydroxyquinoxaline. Results demonstrated substrate-dependent performance influenced by electronic effects, metal composition, and reaction parameters, with conversions reaching up to 95% for BIO-P2 and 89% for BIO-V catalysts, surpassing traditional oxidants such as KMnO4. Solvent and catalyst loading optimizations further enhanced reaction efficiency, with acetonitrile identified as an appropriate solvent. This dual-purpose strategy combines environmental remediation with the development of efficient, plant-derived catalysts, offering a scalable, eco-friendly alternative for industrial and pharmaceutical oxidative processes while aligning with Sustainable Development Goals.
Environmental significanceThe increasing demand for sustainable chemical processes calls for greener catalytic alternatives. This study demonstrates how phytomanagement strategies can transform metal-accumulating plants (Lolium perenne L. and Trifolium incarnatum) into efficient, plant-derived catalysts for oxidation reactions. These biosourced catalysts achieve high yields while reducing reliance on hazardous oxidants, minimizing waste, and promoting circular resource utilization. By integrating environmental remediation with sustainable catalysis, this approach offers a dual benefit of detoxifying contaminated soils while generating eco-friendly catalysts. The strategy aligns with green chemistry principles and Sustainable Development Goal 12, fostering responsible production practices. Our findings highlight a nature-inspired solution for reducing the environmental footprint of oxidation reactions in industrial and pharmaceutical applications. |
1 Introduction
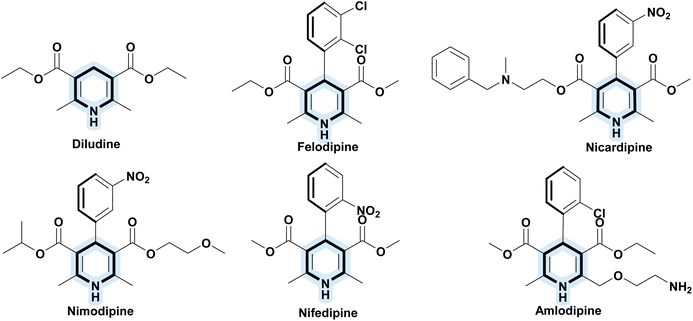
The field of oxidation catalysis is crucial in organic synthesis, as it is essential to the production of fine chemicals, pharmaceuticals, and agrochemicals. Traditional oxidation processes often rely on toxic reagents and produce substantial amounts of hazardous waste, posing significant environmental and health risks.1 As a result, this has led to the growing interest in developing greener alternatives, aligning with the principles of green chemistry which aim to reduce or eliminate the use and generation of hazardous substances in chemical processes (Anastas and Warner, 1998).2 An emerging solution involves the use of catalysts derived from natural and renewable sources, specifically from metal-(hyper)accumulating plants through phytomanagement strategies. Phytomanagement not only utilizes plants for environmental remediation but also transforms metal-rich biomass into valuable catalytic materials.3 In this study, the metal-accumulating plants Lolium perenne L. (ryegrass) and Trifolium incarnatum (clover), cultivated in contaminated or nutrient-rich soils, were processed to produce biosourced catalysts (or eco-catalysts). This approach offers dual functionality: it provides non-toxic alternatives in catalysis while simultaneously restoring polluted environments, supporting Sustainable Development Goal (SDG) 12:4 Responsible Consumption and Production. By employing Lolium perenne L. and Trifolium incarnatum-based eco-catalysts, this method aligns with green chemistry principles by reducing reliance on hazardous substances, minimizing waste, and fostering a circular approach that transforms biomass into functional catalytic materials.In this study, the oxidation of Hantzsch esters (1,4-dihydropyridines (1,4-DHPs)) was tested using catalysts derived from metal-accumulating plants, such as ryegrass and clover. Hantzsch esters are versatile intermediates in organic synthesis, particularly in pharmaceutical chemistry where they form the core structure of drugs such as nifedipine, amlodipine, and nicardipine, commonly used as calcium channel blockers for treating hypertension and angina (Fig. 1).5,6 The Hantzsch synthesis provides an efficient route for preparing 1,4-DHPs, which are subsequently oxidized to aromatic pyridines, a transformation with significant synthetic and medicinal relevance.7,8
The oxidation of Hantzsch esters to pyridines is a valuable transformation as it mirrors some metabolic oxidation processes in biological systems. This can be seen by the oxidative dehydrogenation that drugs like nifedipine undergo in vivo, catalyzed by cytochrome P450 enzymes in the liver. Replicating this bioinspired transformation in a sustainable manner could enhance our understanding of metabolic pathways while supporting industrial applications. Conventional oxidation methods for Hantzsch esters, however, still predominantly rely on reagents that are effective yet hazardous, requiring strict handling and disposal protocols.
Traditional methods for oxidizing 1,4-dihydropyridines (DHPs) often utilize potassium permanganate (KMnO4), chromium trioxide (CrO3), and various nitrate and nitrite salts, such as iron(III) nitrate (Fe(NO3)3). While effective, these reagents generate hazardous waste, necessitating careful management to mitigate associated risks.9 Iodine-based oxidants, including molecular iodine (I2), iodine(III) and hypervalent iodine(V) reagents, offer a more environmentally friendly alternative, providing efficiency under milder conditions.10,11 Yet, despite their reduced environmental impact, these reagents remain costly and require extensive preparation. Molecular oxygen (O2), when used with platinum or gold nanoclusters, is another sustainable option, although reliance on precious metals limits its scalability.12 Other eco-friendlier alternatives include calcium hypochlorite (Ca(OCl)2) in aqueous media, which offers stability and ease of use but presents challenges with disposal.13 Additionally, t-butylhydroperoxide (TBHP) combined with iron(III) phthalocyanine chloride has shown mild and efficient oxidation performance, although organic peroxides like TBHP pose safety concerns.14 Recently, approaches involving nitro urea and silica sulfuric acid (SiO2–OSO3H) have demonstrated benign and effective oxidation under mild conditions, though they may necessitate specific reaction setups.15 Enzymatic methods, such as laccase- or lipase-catalyzed oxidations of Hantzsch esters, have also been reported, but require controlled aerobic conditions with specific mediators.16,17 In contrast, this study employs sustainable, plant-based catalysts, to demonstrate an eco-friendly approach to Hantzsch ester oxidation. This approach minimizes hazardous waste and leverages plant-derived catalytic properties, offering a mediator-free alternative. This green oxidation strategy not only reduces the environmental footprint of pharmaceutical synthesis but also aligns with green chemistry goals by replacing toxic reagents with biologically sourced materials that embody both catalytic efficacy and environmental restoration potential.
2. Materials and methods
2.1 Reagents
All reagents and solvents were of analytical grade and used as received without further purification. Ethyl acetoacetate, benzaldehyde, and ammonium acetate were purchased from commercial suppliers and used as starting materials for the synthesis of the substrates used for oxidation. The commercial catalysts used for the oxidative reaction were purchased from Sigma-Aldrich, along with the pharmaceutical molecule nifedipine.After completion, as confirmed by TLC (or 1H NMR monitoring), the reaction mixture was allowed to cool to room temperature. The precipitate was collected by filtration, washed with cold ethanol, and dried under reduced pressure. If further purification was required, the crude product was recrystallized from ethanol. The conversion and purity of the final Hantzsch ester product were confirmed using 1H NMR and 13C NMR spectroscopy.
2.2 Conception of biosourced catalyst and analyses
Biosourced catalysts were obtained from ryegrass (Lolium perenne L.) grown on contaminated soils located in Belgium (Duferco and Vieille Montagne sites) and France (Pompey and STPI sites), and from clover (Trifolium incarnatum) grown on vineyard soil in Bordeaux, France. Air-dried plant biomass was incinerated in a muffle furnace (Nabertherm P330, Lilienthal, Germany).18 Metal extraction was carried out using hydrochloric acid (3 M for ryegrass, 2 M for clover), in a ratio solid/liquid: 1/10. The mixtures were then heated, stirred, filtered, concentrated, and thermally activated at 120 °C to obtain solid residues coined biosourced catalysts.18 The catalysts were named BIO-DUF, BIO-VM, BIO-P1 and BIO-P2, BIO-STPI, and BIO-V, depending on the location of the sites (Duferco, Vieille Montagne, Pompey, STPI, and Vineyard sites, respectively).The concentration of metals (Cd, Pb, Zn, Cu, Cr, Ni, Na, K, Ca, Mg, Fe, Mn, Al, and Si) in ryegrass and clover were determined following acid digestion by FAAS, according to the procedure described in a previous study.19 Catalyst characterization by X-ray diffraction was conducted using a BRUKER D8 diffractometer equipped with a cobalt anticathode (λKα1 = 1.74 Å) and a LynxEye detector. The X-ray patterns were acquired in the 2θ (10–80°) with a step size of 0.02° and 89.5 s per step.20
3 Results and discussion
3.1. Metal concentrations in biomass ashes and characterization of the crude mixture of Lewis acids
The metal composition of eco-biosourced catalysts derived from Lolium perenne L. (ryegrass) and Trifolium incarnatum (clover) is summarized in Table 1. This structured analysis, following the order of metals in the table, highlights the distinct roles these elements play in catalysis and their environmental relevance.| Ecocatalyst | BIO-P1 | BIO-P2 | BIO-DUF | BIO-V | BIO-STPI | BIO-VM |
|---|---|---|---|---|---|---|
| Metal | ||||||
| Cd | 2.12 × 10−4 | 1.02 × 10−4 | 5.06 × 10−4 | 6.25 × 10−5 | 1.64 × 10−3 | 3.10 × 10−3 |
| Pb | 7.52 × 10−3 | 6.31 × 10−3 | 4.48 × 10−2 | 2.99 × 10−3 | 2.93 × 10−2 | 1.38 × 10−2 |
| Zn | 2.52 × 10−2 | 9.88 × 10−3 | 2.42 × 10−2 | 4.42 × 10−3 | 2.85 × 10−2 | 3.61 × 10−2 |
| Cu | 7.16 × 10−3 | 6.45 × 10−3 | 1.91 × 10−2 | 3.70 × 10−2 | 4.69 × 10−3 | 6.00 × 10−3 |
| Cr | 1.76 × 10−3 | 1.23 × 10−3 | 1.33 × 10−2 | 9.70 × 10−4 | 1.39 × 10−3 | 1.16 × 10−3 |
| Ni | 4.30 × 10−3 | 9.54 × 10−3 | 1.32 × 10−2 | 4.98 × 10−3 | 3.58 × 10−3 | 5.25 × 10−3 |
| Na | 1.29 | 1.65 | 0.73 | 0.3 | 3.08 | 4.27 |
| K | 18.24 | 26.53 | 9.99 | 21.92 | 19.91 | 18.65 |
| Ca | 5.23 | 5.15 | 8.45 | 10.49 | 5.14 | 3.67 |
| Mg | 1.77 | 1.49 | 1.76 | 1.72 | 2.36 | 2.16 |
| Fe | 0.08 | 0.12 | 12.64 | 0.23 | 0.10 | 0.08 |
| Mn | 0.25 | 0.19 | 0.19 | 0.05 | 0.06 | 0.04 |
| Al | 0.08 | 0.04 | 0.63 | 0.21 | 0.07 | 0.13 |
| Si | 0.54 | 1.00 | 0.64 | 0.20 | 1.90 × 10−4 | 0.08 |
The composition of Cd, Pb, Ni and Cr, four toxic heavy metals, are low across all samples, with Cd below 0.005% in several, Pb consistently under 0.05% (BIO-DUF and BIO-P2 show the highest Pb at approximately 0.0448%), Ni up to 0.0133% in BIO-P2, and Cr up to 0.0133% in BIO-DUF and BIO-P2, suggesting a possible supporting role in redox activity. This minimal presence of toxic metals is crucial for green catalytic applications, as it reduces environmental risks. The high Zn and Cu to Cd and Pb ratios indicate selective uptake favouring catalytically beneficial metals, supporting the alignment of ecocatalysts with green chemistry principles.
Transition metals Zn and Cu, both recognized for their roles in oxidation catalysis, are well-represented. Zn, present up to 0.0361% in BIO-VM and 0.0285% in STPI, acts as a Lewis acid, facilitating electron transfer processes. Cu, at 0.0370% in BIO-V, is valuable for its redox flexibility, enabling redox cycling essential for oxidative catalysis. Recent studies have highlighted the efficiency of copper-based catalysts in 1,4-dihydropyridines oxidation reactions under mild conditions, supporting the relevance of copper in such transformations.21
Alkali and alkaline earth metals, including Na, K, Ca, and Mg, are found in substantial quantities. Potassium reaches up to 21.92% in BIO-V and 9.99% in BIO-P2, enhancing catalyst stability and potentially aiding in oxygen activation. Ca, with concentrations up to 10.49% in BIO-V, contributes to the structural integrity and provides Lewis acidic sites for stabilizing oxidation intermediates. Na (up to 4.27% in BIO-VM) and Mg (up to 2.36% in BIO-STPI) serve as stabilizers, with Mg enhancing Lewis acidity and Na contributing to matrix stability, improving the eco-catalysts' durability.
The final group, Fe, Mn, Al, and Si, plays a role in structural support. Fe, concentrated in BIO-DUF at 12.64%, is active in electron transfer, enhancing catalytic potential in oxidation. Manganese, at 0.25% in BIO-P1 and at 0.19% in BIO-P2 and BIO-DUF, is another transition metal useful in redox reactions. Aluminium, found in trace amounts, and Si (up to 1% in BIO-P2), contribute to the structural robustness of the biosourced catalysts, supporting sustained catalytic performance.
This analysis highlights the composition of metal-accumulating plants as a crude mixture of metals with potential applications in sustainable catalysis. The concentration profiles across different biosourced catalysts demonstrate that both Lolium perenne L. and Trifolium incarnatum effectively accumulate beneficial metals while limiting toxic metals, supporting their use in green catalytic processes without the need for additional purification steps or supports. This intrinsic selectivity toward beneficial metals like Zn, K, and Ca positions these plant-derived ashes as viable candidates for environmentally benign catalysts.
3.2 Oxidative applications
Achieving efficient oxidation across diverse substrates with minimal environmental impact remains a significant challenge. By employing plant-derived catalysts, this study advances green chemistry principles and supports a circular economy approach, transforming waste biomass into valuable catalytic materials. This methodology shows broad potential for applications in pharmaceutical synthesis, fine chemical production, and other industries where selective oxidation is essential.The study aligns with Sustainable Development Goal 12 (Responsible Consumption and Production) by fostering sustainable practices, minimizing reliance on hazardous substances, reducing waste, and promoting environmentally responsible chemical processes.22
The oxidation reactions were conducted under the general procedure described in Fig. S1.† The substrate was dissolved in acetonitrile (10 mL) in a round-bottom flask. Catalyst (20 wt% relative to substrate) was added to the solution, and the reaction mixture was stirred at 80 °C under reflux for 24 hours. The reported conversion values across all oxidation studies represent the average of two independent experiments. Standard deviation was within ±3%, supporting the reproducibility of the observed trends despite the exploratory scale of the study.
Upon completion, the reactions were monitored using 1H-NMR after calibration, with TMS as the internal standard. The conversion for each derivative was determined based on the NMR results. The catalysts evaluated include BIO-P1, BIO-P2, BIO-V, BIO-STPI, BIO-VM, BIO-DUF, MnO2, KMnO4, and Ca(ClO)2. Each catalyst displayed a different efficiency in facilitating the oxidation reaction, as summarized in Table 2.
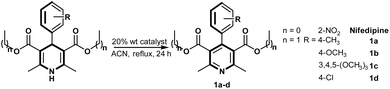
The oxidation of Hantzsch ester derivatives 1a–d and nifedipine using various biosourced catalysts derived from ryegrass and clover highlights significant differences in conversion, which can be correlated with both the structure of the substrate and the specific catalyst composition. The performance of these plant-based catalysts is compared with traditional oxidants like KMnO4, MnO2, and Ca(OCl)2, with an emphasis on catalytic efficiency and environmental impact. The results show clear substrate-specific performance across catalysts (Table 2). The methoxy-substituted derivative 1b consistently yields the highest conversions, reaching up to 92% with BIO-V and 82% with BIO-P1. This high reactivity can be attributed to the mesomere electron-donating (+M) nature of the methoxy group, which enhances the nucleophilicity of the 1,4-dihydropyridine core, making it more susceptible to oxidation. In contrast, 1a, the 4-methyl-substituted derivative, shows lower conversions, with yields of only 60% using BIO-V and 40% using BIO-P1, suggesting that the inductive electron-donating group (+I) reduces the ease of oxidation under these conditions.
The presence of an electron-withdrawing chloro substituent in compound 1d appears to stabilize the 1,4-dihydropyridine structure, resulting in lower conversions across all tested catalysts. For example, BIO-V achieves only a 55% conversion for 1d, and BIO-P1 provides a 16% conversion. This trend highlights the influence of electronic effects in determining the reactivity of Hantzsch esters in oxidation reactions, with electron-donating groups facilitating the process and electron-withdrawing groups inhibiting it.
The oxidation of nifedipine, which bears a strong electron-withdrawing nitro (NO2) group, shows intermediate conversions across the catalysts, with BIO-P1 achieving a conversion of 56% and BIO-P2 reaching 73%. BIO-V also provides a moderate conversion of 57%. These relatively reduced conversions align with the nitro group's stabilizing effect on the 1,4-dihydropyridine ring, which reduces its susceptibility to oxidation. However, the reasonable performance of these biosourced catalysts on a more electron-deficient substrate like nifedipine indicates their versatility and effectiveness even for more stabilized derivatives, which are generally more resistant to oxidation.
The biosourced catalysts present varying efficiencies across substrates, with BIO-V demonstrating the highest activity overall, especially for substrates 1a, 1b, and 1c. This catalyst's superior performance can likely be attributed to its metal composition, which includes high levels of copper (Cu) and zinc (Zn). These metals are known to promote oxidative transformations through redox cycling and electron transfer processes. BIO-P1 and BIO-P2 also exhibit high reactivity, with conversions of up to 82% for 1b and 73% for nifedipine, respectively. These results suggest that the combination of metals in these biosourced catalysts creates a favourable environment for oxidation, potentially due to synergistic effects that facilitate intermediate stabilization and transition state formation.
In contrast, the BIO-DUF catalyst shows no detectable activity under the tested conditions. This lack of reactivity could be attributed to the absence or insufficient concentration of essential redox-active metals in its composition. Similarly, traditional oxidants like KMnO4 and MnO2 produce moderate conversions, with KMnO4 achieving up to 20% for the oxidation of 1a and 51% for the oxidation of nifedipine.
To evaluate the impact of catalyst loading on the oxidation efficiency of Hantzsch ester derivative 1b, varying amounts of BIO-P1 catalyst (5%, 10%, 20%, and 30% wt relative to substrate) were tested under standard reaction conditions. As shown in Table 3 and the NMR spectra, increasing the catalyst loading significantly improved the conversion. At 5% loading, a modest conversion of 15% was observed, while increasing the catalyst loading to 10% and 20% resulted in conversions of 30% and 85%, respectively. The highest loading of 30% yielded a near-quantitative conversion of 91%. These results indicate a clear correlation between catalyst loading and reaction efficiency, with higher catalyst concentrations facilitating greater substrate conversion. Kinetic analysis, detailed in the ESI (Fig. S4),† revealed that the reaction follows second-order kinetics, where the rate depends on both the reactant and catalyst concentrations. This was confirmed by the linearity of the 1/([A]0 − [A]) vs. time plot, consistent with a bimolecular rate-determining step. The first-order plot, ln ([A]0 − [A]) vs. time, deviates from linearity in the early stages, suggesting additional mechanistic complexity, possibly catalyst activation or intermediate formation, during the initial reaction phase (Table 4).
The 20% catalyst loading optimizes catalytic efficiency and material economy, providing sufficient active sites for effective oxidation without the diminishing returns observed at higher loadings. This choice maximizes resource use and conversion, demonstrating the practical scalability of BIO-P1 for Hantzsch ester oxidation.
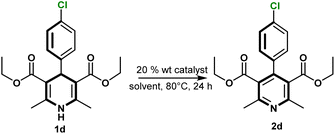
The solvent evaluation for the oxidation of Hantzsch ester derivative 1d revealed that dioxane provided the highest conversion at 85%, followed by acetonitrile at 55%, while other solvents (2-MeTHF, 4-MTHP, cyrene) yielded less than 10%. Despite dioxane's high conversion, its environmental persistence and classification as a probable human carcinogen limit its suitability.23 Water and buffer conditions were also tested, but no oxidation was observed. Additionally, the temperature effect in acetonitrile was verified. When the reaction was conducted at room temperature, no significant conversion was observed for compound 1d after 24 h. Acetonitrile offers good solubility across all substrates, allowing a reduction in solvent volume and operational efficiency. This makes acetonitrile a practical choice for the current catalytic system.
Under standard conditions (20 wt% catalyst, acetonitrile, reflux, 24 h), the conversion of pyridine-4(1H)-thione 3 to pyridine-4-thiol 4 yielded different efficiencies across the catalysts. BIO-V demonstrated the highest conversion at 89%, followed by BIO-P1 with a 66% conversion. BIO-P2 and Ca(OCl)2 showed lower conversions of 50% and 42%, respectively. BIO-V stands out as a promising eco-friendly catalyst for the conversion of thiones to thiols in this pyridine derivative (Table 5).
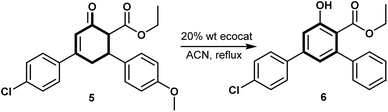
Cyclohex-2-enone, a classic Robinson annulation product, serves as a key precursor in constructing complex aromatic systems like terphenyls through oxidative transformation of the enone core to phenolic structures. This section evaluates the efficiency of biosourced catalysts under sustainable conditions for accessing these bioactive terphenyl derivatives.
Some previous methodologies for the oxidation of cyclohex-2-enone derivatives to phenols involve the use of iodine-catalyzed oxidative aromatization, as demonstrated by Wang et al., which provides a metal-free alternative that avoids harsh reagents and efficiently yields meta-substituted phenols under mild conditions.25 Other approaches employ transition metal catalysts, such as magnetite or cerium oxide composites,26 leveraging redox-active metal centres to facilitate oxygen transfer and enhance catalytic efficiency. Chloramine-T, another oxidant used for this transformation, introduces reactivity that can lead to over-oxidation or side reactions, making it suitable mainly for robust substrates or carefully controlled reactions.27 These methodologies illustrate the range of options available, from metal-free conditions to transition metal-based systems, each with unique advantages and considerations in terms of sustainability and efficiency.
Building on established oxidative methods, we evaluated biosourced catalysts derived from metal-accumulating plants for the oxidation of cyclohex-2-enone to its phenolic derivative (Table 6). Among the tested catalysts, BIO-V achieved the highest conversion at 67%, followed by BIO-P2 and BIO-P1 with conversions of 65% and 56%, respectively. These results suggest that the copper and zinc content in these catalysts may facilitate efficient electron transfer and redox processes, comparable to established metal-catalyzed methods.
Traditional oxidants, including MnO2, KMnO4, and CaO, resulted in lower conversions of 32%, 20%, and 18%, highlighting the limitations of conventional oxidants in stabilizing intermediates necessary for aromatization. The superior performance of BIO-V, BIO-P1, and BIO-P2 catalysts underscores their potential as green alternatives, combining sustainability with effective catalytic activity.
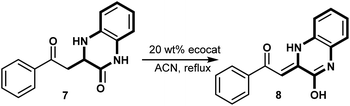
Under standardized conditions (20 wt% catalyst, acetonitrile, reflux, 24 h), we observed a range of catalytic activities across our biosourced catalysts. BIO-P2 achieved the highest conversion, reaching 95%, suggesting it provided the optimal environment for this transformation. BIO-V also demonstrated a strong catalytic effect, with a 68% conversion, while BIO-P1 yielded a moderate 40% conversion. Comparatively, traditional oxidants, such as MnO2 and KMnO4, delivered lower conversions, with MnO2 reaching only 32% and KMnO4 stopping at 25%.
The high efficiency of BIO-P2 in facilitating this oxidation may be attributed to its unique metal composition, potentially providing sufficient catalytic sites and redox potential necessary for activating the quinoxaline substrate. BIO-V, while not as efficient as BIO-P2, still displayed notable activity, likely due to its metal composition that aligns with oxidation requirements for complex structures like quinoxalines.
4 Conclusion
This study demonstrates the potential of biosourced catalysts derived from Lolium perenne L. and Trifolium incarnatum for selective oxidation reactions, showcasing their applicability across a range of substrates, including Hantzsch esters, pyridine-4(1H)-thione, cyclohex-2-enone, and dihydroquinoxaline derivatives. The copper- and zinc-enriched catalysts, especially BIO-V and BIO-P2, showed high conversion yields and substrate versatility, rivalling or surpassing conventional oxidants while offering improved environmental compatibility. In contrast to enzymatic oxidation methods, which require isolated enzymes, specific mediators, controlled pH, and aerobic conditions, our approach avoids such constraints by using phytosourced solid catalysts under simple reflux conditions, contributing to operational simplicity. Notably, these plant-based catalysts showed comparable or superior activity to traditional reagents, with the added advantage of reduced environmental toxicity and resource sustainability. The demonstrated applicability of plant-based catalysts in these oxidation reactions highlights their potential for eco-friendly applications in organic synthesis. Future studies should explore the mechanistic pathways of these reactions, including the elucidation of the contribution of metallic species to catalytic activity of these eco-materials, solidifying the broad application of plant-derived catalysts as practical tools in sustainable organic and medicinal chemistry.Data availability
The data supporting this article have been included as part of the ESI.† Raw data that support the findings of this study are available from the corresponding author, upon reasonable request.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors gratefully acknowledge Interreg North-West Europe (NWE) and AMI CIVB “Recherche, Innovation & Transfert” for financial support of this work which was part of the projects “REGENERATIon of Past Metallurgical Sites and Deposits through innovative circularity for raw materials” (REGENERATIS) and “Phytoextraction of copper in vineyard soils” (Extracuivre), respectively.Notes and references
- R. A. Sheldon, Green chemistry and resource efficiency: towards a green economy, Green Chem., 2016, 18, 3180–3183 RSC.
- P. T. Anastas and J. C. Warner, Green Chemistry: Theory and Practice, Oxford University Press, 1998 Search PubMed.
- C. Grison, Combining phytoextraction and ecocatalysis: a novel concept for greener chemistry, an opportunity for remediation, Environ. Sci. Pollut. Res., 2015, 22, 5589–5591 CrossRef.
- https://www.un.org/sustainabledevelopment/sustainable-consumption-production/accessed 09.11.2024.
- N. Robles, F. Fici and G. Grassi, Dihydropyridine calcium channel blockers and renal disease, Hypertens. Res., 2017, 40, 21–28 CrossRef CAS PubMed.
- D. M. Stout and A. I. Meyers, Recent advances in the chemistry of dihydropyridines, Chem. Rev., 1982, 82, 223–243 CrossRef CAS.
- A. Hantzsch, Synthesis of 1,4-dihydropyridines, Ber. Dtsch. Chem. Ges., 1882, 15, 1455–1458 Search PubMed.
- C. O. Kappe, 100 years of the Hantzsch dihydropyridine synthesis, Angew. Chem., Int. Ed., 2000, 39, 3418–3437 Search PubMed.
- A. Ghorbani-Choghamarani, M. A. Zolfigol, M. Hajjami, S. Rastgoo and S. Mallakpour, Metal-free oxidation of urazole and 1,4-dihydropyridine derivatives under mild and heterogeneous conditions by nitro urea, derived from urea nitrate, and silica sulfuric acid, Lett. Org. Chem., 2010, 37, 2317–2324 Search PubMed.
- M. Filipan-Litvic, M. Litvic and V. Vinkovic, An efficient, metal-free, room temperature aromatization of Hantzsch-1,4-dihydropyridines with urea-hydrogen peroxide adduct, catalyzed by molecular iodine, Tetrahedron, 2008, 64, 5649–5656 CrossRef CAS.
- S. Ko, M. N. V. Sastry, C. Lin and C. F. Yao, Molecular iodine-catalyzed one-pot synthesis of 4-substituted-1,4-dihydropyridine derivatives via Hantzsch reaction, Tetrahedron Lett., 2005, 46, 5771–5774 CrossRef CAS.
- H. Miyamura, K. Maehata and S. Kobayashi, In situ coupled oxidation cycle catalyzed by highly active and reusable Pt-catalysts: dehydrogenative oxidation reactions in the presence of a catalytic amount of o-chloranil using molecular oxygen as the terminal oxidant, Chem. Commun., 2010, 46, 8052–8054 RSC.
- F. Tamaddon and Z. Razmi, Oxidation of 1,4-dihydropyridines and 3,4-dihydropyrimidin-2(1H)-ones to substituted pyridines and pyrimidinones using Ca(OCl)2 in aqueous media, Synth. Commun., 2011, 41, 485–492 CrossRef CAS.
- M. Filipan-Litvic, M. Litvic and V. Vinkovic, A highly efficient biomimetic aromatization of Hantzsch-1,4-dihydropyridines with t-butylhydroperoxide, catalyzed by iron(III) phthalocyanine chloride, Bioorg. Med. Chem., 2008, 16, 9276–9282 CrossRef CAS PubMed.
- A. Ghorbani-Choghamarani, M. A. Zolfigol, M. Hajjami, S. Rastgoo and S. Mallakpour, Metal-free oxidation of urazole and 1,4-dihydropyridine derivatives under mild and heterogeneous conditions by nitro urea, derived from urea nitrate, and silica sulfuric acid, Lett. Org. Chem., 2010, 7, 249–254 CrossRef CAS.
- S. Simić, S. Jeremic, L. Djokic, N. Božić, Z. Vujčić, N. Lončar, R. Senthamaraikannan, R. Babu, I. M. Opsenica and J. Nikodinovic-Runic, Development of an efficient biocatalytic system based on bacterial laccase for the oxidation of selected 1,4-dihydropyridines, Enzyme Microb. Technol., 2020, 132, 109411 CrossRef.
- J. Jayakumar, D. Priyadarshini, A. Parthasarathy and S. R. Reddy, Recent advances in molecular oxygen assisted laccase catalyzed sustainable organic transformations, Asian J. Org. Chem., 2023, 12, e202200564 CrossRef CAS.
- C. Waterlot, P. Dufrénoy, M. Hechelski, B. Louvel, A. Daïch and A. Ghinet, Benefits of ryegrass on multicontaminated soils. Part 2: Green process to provide Idrocilamide, Sustainability, 2019, 11, 6685–6694 CrossRef CAS.
- C. Waterlot and M. Hechelski, Benefits of ryegrass on multicontaminated soils. Part 1: Effects of fertilizers on bioavailability and accumulation of metals, Sustainability, 2019, 11, 5093–6013 CrossRef CAS.
- J. Kleib, G. Aouad, G. Louis, M. Zakhour, M. Boulos, A. Rousselet and D. Bulteel, The use of calcium sulfoaluminate cement to mitigate the alkali silica reaction in mortars. Construct, Build. Mater., 2018, 184, 295–303 CrossRef CAS.
- H. Veisi, A. Rostami, K. Amani and P. Hoorijani, Copper nitrate as a powerful and eco-friendly natural catalyst for mediator-free aerobic oxidation of 2,3-dihydroquinazolinones and 1,4-dihydropyridines under mild conditions, J. Mol. Struct., 2025, 1323, 140621 CrossRef CAS.
- United Nations Environment Programme (UNEP), 10-Year Framework of Programmes on Sustainable Consumption and Production Patterns, United Nations Conference on Sustainable Development (Rio+20), 2012 Search PubMed.
- U.S. Environmental Protection Agency (EPA). “Toxicological Review of 1,4-Dioxane (with Inhalation Update).” https://iris.epa.gov/ChemicalLanding/%26substance_nmbr=326.
- J. K. Liu, Natural terphenyls: developments since 1877, Chem. Rev., 2006, 106, 2209–2223 CrossRef CAS PubMed.
- S.-K. Wang, M.-T. Chen, D.-Y. Zhao, X. You and Q.-L. Luo, Iodine-catalyzed oxidative aromatization: a metal-free concise approach to meta-substituted phenols from cyclohex-2-enones, Adv. Synth. Catal., 2016, 358, 1123–1130 Search PubMed.
- A. V. Petkevich, J. V. Siniutsich and D. S. Martsinkevich, et al., Magnetite and cerium dioxide nanocomposites as catalysts for cyclocondensation reactions of β-dicarbonyl compounds in the synthesis of pyrimidines and m-terphenyls, Russ. J. Gen. Chem., 2023, 93(Suppl 1), S42–S55 CrossRef CAS.
- S. Samshuddin, B. Narayana and B. K. Sarojini, Ethyl 4,4-difluoro-5-hydroxy-1,1:3,1-terphenyl-4-carboxylate, Molbank, 2011, 2011, M745 CrossRef.
- C. Waterlot, A. Ghinet, P. Dufrénoy, M. Hechelski, A. Daïch, D. Betrancourt and D. Bulteel, Sustainable pathways to oxicams via heterogeneous biosourced catalysts - Recyclable and reusable materials, J. Cleaner Prod., 2024, 437, 140684 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5va00028a |
| This journal is © The Royal Society of Chemistry 2025 |