Open Access Article
Open Access ArticleOptimized colorimetric detection of chromium ions (Cr3+) using black garlic-Ag nanoparticles†
Rintumoni
Paw
 abc,
Kangkan
Sarmah
d,
Ankur K.
Guha
abc,
Kangkan
Sarmah
d,
Ankur K.
Guha
 d and
Chandan
Tamuly
d and
Chandan
Tamuly
 *ab
*ab
aNatural Product Chemistry Section, CSIR-North East Institute of Science and Technology, Branch Itanagar, Arunachal Pradesh-791110, India. E-mail: c.tamuly@gmail.com
bAcademy of Scientific and Innovative Research (AcSIR), Ghaziabad-201002, India
cDept of Chemistry, Silapathar Science College, Silapathar, Assam-787059, India
dDept of Chemistry, Cotton University, Guwahati, Assam-781001, India
First published on 2nd June 2025
Abstract
In this study, AgNPs were synthesized using black garlic (BG) extract and applied for precise and sensitive colorimetric detection of chromium (Cr3+) ions in solution. The nanoparticles (BG-AgNPs) were characterised using UV-visible spectroscopy, FTIR, SEM, TEM and XPS analysis. The UV-visible absorbance peak appeared at 411 nm. The pattern of X-ray diffraction (XRD) confirmed a face-centered cubic (FCC) structure of the AgNPs. The SEM images displayed a fusiform (spindle-shaped) morphology of the AgNPs. Transmission electron microscopy (TEM) images presented the sphere-shaped nature of the nanoparticles having a mean diameter of 16.02 ± 1.75 nm. The addition of Cr3+ ions turned the brownish BG-AgNP solution colourless. The sensor displayed a linear response to Cr3+ concentrations between 2 nM and 20 nM, with a limit of detection (LoD) of 0.07 nM. Optimal sensor performance was achieved at pH 7 and a temperature of 45 °C. Selectivity tests confirmed minimal interference from other metal ions. These results indicate that the developed nanosensor is exceptionally efficient and ultrasensitive at precisely detecting low concentrations of Cr3+ ions in environmental applications. A computational study with one of the most abundant compounds during the early stages of the aging process of black garlic formation, γ-glutamyl-S-allylmercaptocysteine (GSAMC), showed a binding energy of −6.67 kcal mol−1 for the formation of GSAMC-AgNPs and −8.82 kcal mol−1 for the formation of the GSAMC-AgNP-Cr complex.
Environmental significanceThis study is highly significant from an environmental point of view. Chromium is a major pollutant in different industries affecting soil and water. Therefore, method development for the detection of Cr3+ ions at the nM level has significant importance for health, hygiene and human life. |
Introduction
Chromium is highly valued for its shiny appearance and resistance to corrosion, making it a key material in industries such as steel production, electroplating, tanning, and chemical processing.1 However, its widespread industrial use has contributed to environmental contamination. Both trivalent and hexavalent forms of chromium are used in various sectors, such as fossil fuel processing, metallurgy, leather treatment, dye production, metal fabrication, cement manufacturing, munitions, and timber preservation.2–5Cr3+ is an essential trace nutrient necessary for human health.6 Hexavalent chromium (Cr6+) is reported to be highly carcinogenic, while the toxicological mechanisms of trivalent chromium (Cr3+) are not well understood, leading to its classification as a category-III carcinogen.7–9 Cr3+ can induce a wide range of responses in aquatic and terrestrial plants, including inhibited growth and seed germination, chloroplast damage, reduced photosynthesis, oxidative stress, and alterations in nutrient balance, organelle function, and cellular processes.10
Moreover, growing evidence suggests that Cr3+ is embryotoxic, with exposure during the preimplantation stage causing significant developmental issues in the in vitro fertile (IVF) mouse embryos, including growth arrest, reduced blastocyst formation, and increased cellular damage, even at low concentrations.11
Non-occupational exposure to chromium mainly comes from consuming contaminated food and drinking water, as well as from exposure to tobacco products that release chromium during pyrolysis.12–15 Therefore, the detection of Cr3+ ions in drinking water is essential for safeguarding human health. Various qualitative and quantitative analytical methods exist for determining Cr3+. Conventional methods encompass spectrometric techniques based on atomic absorption, atomic emission, and mass detection, along with other approaches such as X-ray fluorescence and voltammetry.16–20 While these methods are highly accurate and sensitive, they have limitations, such as time-consuming sample preparation, high costs, the need for trained personnel to operate the instruments, and unsuitability for on-site applications.21
Colorimetric sensors are a simple, low-cost method for detecting Cr3+ as they do not require sophisticated instruments and can be easily visualized by the naked eye.22–26 Silver nanoparticles (AgNPs) have been extensively used as colorimetric sensors for detecting various analytes, including metal ions, pesticides, and DNA, due to their surface plasmon resonance (SPR) absorption properties, which are highly sensitive to the shape, size, and surrounding chemical environment.27–33 The mechanism behind AgNP-based colorimetric sensors involves a visible colour change from yellow to other colours, triggered by analyte-induced aggregation of AgNPs, leading to inter-particle plasmon coupling.34
Various nanosensors have been developed to detect Cr3+. These include AuNPs functionalized with polyethyleneimine, 4-mercaptobenzoic acid, 4-amino hippuric acid, 4-nitrobenzene mercaptan, citrate, and gallic acid, as well as Ag@mannose and AuNPs modified with 5,5′-dithiobis(2-nitrobenzoic acid). Ag@mannose nanoparticles detect Cr3+ ions via colour changes induced by nanoparticle aggregation involving surface-bound mannose. Cr3+ detection has also been demonstrated using AuNPs modified with 5,5′-dithiobis(2-nitrobenzoic acid), achieving a detection limit of 25 ppb at pH 6. Additionally, colorimetric detection of Cr3+ in aqueous solutions has been reported using citrate-capped AgNPs. Another approach involves using AuNPs functionalized with 4-mercaptobenzoic acid for Cr3+ detection.35
AuNPs have also been designed using 4-mercaptobenzoic acid, polyethyleneimine, and 4-nitrobenzene mercaptan.36,37 Among these, 4-MBA and PEI co-functionalized AuNPs demonstrated superior sensitivity, allowing for visual Cr3+ detection and analysis via UV-vis absorption spectroscopy, with a detection limit of 1 μM.36
In addition, colorimetric detection of Cr3+ has been demonstrated using AuNPs functionalized with 4-amino hippuric acid.38 Elavarasi et al. designed a colorimetric assay and visual colour change method on paper to detect Cr3+ in aqueous solutions using citrate-capped AuNPs.39 This approach relied on the clustering of citrate-functionalized AuNPs triggered by Cr3+ ions. This clustering resulted in a redshift of the colorimetric absorption peak from 526 nm to 714 nm. Furthermore, a selective colorimetric assay for detecting both Cr3+ and Cr6+ using gallic acid-capped AuNPs has also been reported, where colour changes are induced by nanoparticle aggregation.40
The objective of this study is to develop a colorimetric technique for detecting Cr3+ ions, utilizing AgNPs synthesized using black garlic (BG) extract. Black garlic is a health-promoting ingredient that is widely available in international markets. It is produced by roasting fresh garlic (Allium sativum L.) under controlled conditions involving high temperature and humidity over an extended period. This process darkens the garlic cloves, enhances their sweetness, and transforms their texture into a chewy, jelly-like consistency. The fermentation duration varies based on cultural practices, manufacturers, and intended purposes.41 It is well known that the aging period is shorter at higher temperatures.42 For example, at 70 °C, the aging process is twice as fast compared to 60 °C.43 During aging, the cloves turn dark brown and develop a sweet, tangy taste as the pungent odour diminishes.44 Significant chemical changes occur during heating, primarily through the Maillard reaction, which involves the condensation of amino groups with reducing sugars.45,46 Organosulfur compounds are key components in garlic. Eight major compounds identified in black garlic are diallyl disulfide, allicin, ajoene, S-allyl-mercapto-L-cysteine, S-allyl-L-cysteine, S-(propenyl)-L-cysteine, γ-glutamyl-S-allylcysteine, and γ-glutamyl-S-(1-propenyl)-L-cysteine.47–50 Additionally, γ-glutamyl-S-allylmercaptocysteine (GSAMC) is predominantly formed during the early stages of aging. It is derived from γ-glutamyl-S-allylcysteine or γ-glutamyl-S-1-propenyl-L-cysteine, with its concentration peaking at around one month before declining as aging continues.51–55
Materials and methods
Materials
Forty-day fermented peeled black garlic was procured from Aasward Implex LLP, Vadodara, India. Analytical grade ZnSO4·7H2O (99.99%), CrCl3 (99.9%), MgCl2·6H2O (99.99%), PdCl2 (99%), NiCl2 (98%), CoCl2, MnCl2·2H2O, Pb(NO3)2, AlCl3, BaCl2·2H2O (99%), and CaCO3 (99%) were purchased from Sigma-Aldrich.Preparation of BG-AgNPs
Four cloves of black garlic were ground using a mortar and pestle, and 200 mL of H2O was added to it. The mixer was then boiled for 15 min. Once cooled, the solution was passed through a microporous cellulose filter with a 20–25 μm pore size to collect the garlic extract (filtrate). The filtrate was kept at 4 °C for later use. A mixture was prepared by adding 10 mL of 1 mM AgNO3 solution to 1 mL of the black garlic extract, followed by exposing it to sunlight for 15 min. The colour changed to brown and the UV peak was observed at 411 nm (Fig. 1).56 | ||
| Fig. 1 Diagrammatic overview of BG-AgNP synthesis and the evaluation of their metal ion sensitivity. | ||
Characterization of the synthesized AgNPs
The synthesized nanoparticles were characterized using a range of advanced techniques. UV-visible absorbance spectra were studied to confirm the synthesis of AgNPs and to analyze their plasmonic behaviour. Light beams from 300 to 800 nm wavelength were used for this purpose (UV-2600 spectrometer, Shimadzu Corp., Japan). Powder X-ray diffraction analysis using a source of Cu Kα radiation (λ = 1.5406 Å) was performed at a scanning speed of 3° per min (Bruker D8 Advance powder XRD, Germany) to characterize the crystalline nature. Transmission electron microscopy (TEM) images were acquired using an accelerating voltage of 200 kV (JEM-2100 microscope, JEOL Ltd, Japan). Nanoparticle size, interplanar spacing, and d-spacing values were determined through a selected area electron diffraction (SAED) study and analysis using the software ImageJ. A field emission scanning electron microscope (Sigma 300 VP FE-SEM, ZEISS, Germany) operated at 15 kV accelerating voltage was used to obtain the surface morphology image of the nanoparticles. An X-ray photoelectron spectrometer (ESCALAB Xi + XPS, Thermo Fisher Scientific Inc., USA) was used to characterize the elemental composition, empirical formula, and electronic states of the constituent elements. Energy dispersive X-ray spectroscopy (EDX) based elemental composition of the nanoparticles was analyzed using an Aztec (Oxford Instruments, UK). The FTIR spectra were recorded using a Spectrum Two, PerkinElmer Inc., USA.Selectivity as a metal ion sensor
To evaluate the metal ion selectivity of the synthesized BG-AgNPs, 1 mM solutions of the metal ions Zn2+, Cr3+, Mg2+, Pd2+, Ni2+, Co2+, Mn2+, Pb2+, Al3+, Ba2+, and Ca2+ were prepared in deionized water. A 0.2 mL aliquot of each 1 mM metal ion solution was added to 5 mL of a diluted BG-AgNP solution. The resulting mixture was vortexed for 2 min at room temperature to facilitate possible interactions. The chromogenic changes in the solutions were visually observed, and the spectrophotometric absorbance corresponding to the mixtures was measured in the wavelength range from 300 nm to 800 nm. Variations in peak positions were analyzed to determine the interactions with the metal ions and to evaluate the specificity of the nanoparticles.Optimization of detection parameters
The peak absorbance of 5 mL BG-AgNPs was measured at room temperature after adding 10 μL of 0.01 mM Cr3+ across different pH levels: 1, 4, 7, 9, and 12 to optimize the pH. For optimization of temperature, the peak absorbance of 5 mL BG-AgNP solution was measured after adding 10 μL of 0.01 mM Cr3+ at pH 7, across a temperature range of 35–95 °C, at an interval of 10 °C. The study examined the impact of temperature and pH on the sensitivity of BG-AgNPs for Cr3+ by calculating the relative activity. The relative activities were calculated from the absorbance peak using the following formula (eqn (1)):57| Relative activity (%) = [(M2 − M1)/M2] × 100 | (1) |
Limit estimation for sensing Cr3+ ions
Initially, 10–100 μL from 0.01 mM solution of Cr3+ (2–20 nM) was added to 5 mL of BG-AgNPs, and their absorbance was measured at a wavelength of 411 nm. To determine the lowest detectable quantity of Cr3+, a linear calibration curve was plotted using the formula (eqn (2)):| Y = a + bX, | (2) |
The limit of detection and quantification (LoD and LoQ) were determined using eqn (3) and (4):58
 | (3) |
 | (4) |
Reaction kinetics between BG-AgNPs and Cr3+
To explore the interaction kinetics between BG-AgNPs and Cr3+, varying volumes (25, 50, and 75 μL) of a 0.01 mM Cr3+ solution were added to 5 mL of BG-AgNPs. The spectroscopic absorbance of the resulting mixtures was recorded at an interval of 5 min. The following linear kinetic models were evaluated in the current study (eqn (5)–(7)):59| Zero order: [At] = [A0] − k0t | (5) |
| First order: ln([At]/[A0]) = − k1t | (6) |
| Second order: 1/[At] = k2t + 1/[A0] | (7) |
Interference study
To evaluate the potential interference of common ions, 0.2 mL of 1 mM solution of CrCl3 was added to 3 mL of BG-AgNPs. Next, 0.2 mL of 1 mM salt solution (ZnSO4, MgCl2, PdCl2, NiCl2, CoCl2, MnCl2, Pb(NO3)2, AlCl3, BaCl2, and CaCO3) was added individually to this mixture. The solutions were vortexed for 2 min to facilitate possible reactive interactions, and then the colorimetric absorbance spectra were recorded.60Five replicate measurements were conducted for the control (containing only Cr3+) and for each salt-added mixture. F-test (ANOVA) was performed using the software Origin 9 to determine whether the addition of other ions caused significant changes in absorbance.
Computational details
Density Functional Theory (DFT) was utilized to analyze the bonding interactions between Ag and the COOH group of the GSAMC molecules. The computations were performed using the M06-2X functional in conjunction with the def2-TZVP basis set,61 within the Gaussian 16 software suite.62 To understand the molecular orbital interactions, natural bond orbital (NBO) analysis was performed.63To calculate the Gibbs free energy of the proposed reaction of AgNO3 with GSAMC and the Cr complex of the GSAMC-Ag nanoparticles, we used the following equations (eqn (8) and (9)).
| ΔG1 = [{GGSAMC-Ag-NPs} − {GAgNO3 + GGSAMC}] | (8) |
| ΔG2 = [{GGSAMC-Ag-NP-Cr } – {GGSAMC-Ag-NPs + GCr}] | (9) |
Results and discussion
Characterization of the BG-AgNPs
Upon exposure to sunlight, the addition of black garlic extract into a colourless AgNO3 solution induced the development of a yellowish-brown colouration within 15 minutes, signifying the synthesis of AgNPs. The reduction of silver ions (Ag+) to metallic silver (Ag0), leading to the synthesis of silver nanoparticles (AgNPs), was additionally confirmed through UV-visible spectroscopy, which displayed a characteristic SPR peak at 411 nm. This SPR peak confirms the successful formation of AgNPs (Fig. 2A).56 | ||
| Fig. 2 (A) UV-vis spectra of black garlic extract, BG-AgNPs and BG-AgNPs + Cr3+ complex solution. (B) XRD crystallography of black garlic extract, BG-AgNPs and BG-AgNPs + Cr3+ complex. | ||
X-ray diffraction (XRD) analysis
The XRD data of the synthesized BG-AgNPs showed strong diffraction peaks at angles corresponding to 2θ values of 38.22°, 44.56°, 64.34°, and 78.20°. These peaks are associated with the (111), (200), (220), and (311) crystallographic planes, and are characteristic indicators of the typical face-centered cubic (FCC) lattice structure of silver (Fig. 2B). The XRD analysis is consistent with the reference data from the International Centre for Diffraction Data (ICDD), specifically PDF Card No. 00-004-0783.64 The similarity in the peaks with the reference pattern confirms the crystalline nature of the BG-AgNPs.Electron microscopy analysis (SEM and TEM)
The scanning electron micrograph (Fig. 3A) of BG-AgNPs revealed a fusiform (spindle-shaped) morphology of the AgNPs, with a length of 0.85 ± 0.059 μm and a width of 0.22 ± 0.01 μm (N = 29) at the broadest point. Similar fusiform structures have been reported for CuO nanoparticles, Au nanoparticles, β-FeOOH, and α-Fe2O3 nanorods in other studies.65,66Fig. 3B presents a transmission electron micrograph of the BG-AgNPs, confirming the spherical morphology of the nanoparticles. The nanoparticle diameter was 16.02 ± 1.75 nm (range 5.298 to 50.344 nm) (N = 31), as shown in Fig. 3E. Notably, nanoparticles in the 5–10 nm range were found to be the most prevalent. The SAED of the BG-AgNPs was indicative of the crystalline nature of the sample. The SAED analysis exhibited a face-centred cubic (FCC) crystal lattice with measured d-spacings of 3.21 ± 0.61 1/nm, 5.86 ± 0.53 1/nm, 9.22 ± 0.25 1/nm, and 12.45 ± 0.37 1/nm, associated with diffraction angles of 38.22°, 44.56°, 64.34°, and 78.20°, respectively (Fig. 3D). The interplanar distance calculated from the TEM measured 0.09 nm for the (311) plane at 2θ = 78.20° (Fig. 3C).
X-ray photoelectron spectroscopy (XPS)
The XPS data revealed binding energy signals for S2p, C1s, Ag3d, N1s and O1s (Fig. 4). The S 2p peak is a doublet split into S 2p3/2 and S 2p1/2, with a typical spin–orbit splitting of ∼1.2 eV. The S 2p binding energy at 167.7 eV and 168 eV suggests the presence of oxidized or covalently bonded sulfur species, and –S–S– (disulfide) is a likely assignment. It also indicates sulfur in one chemical state. This data envelope is fit to a spin–orbit doublet (the S 2p3/2 and 2p1/2 peaks) with the correct separation (1.2 eV).67 The peaks for C1 are observed at 284.8 eV and 285.95 eV. A binding energy of 284.8 eV in the C1s XPS spectrum corresponds to C–C (sp3 hybridized carbon) and C–H bonds, which are typically found around ∼285 eV.68 The detection of double peaks in the Ag 3d region suggests metallic silver (Ag0) as the predominant constituent, with binding energies of 368.2 eV for Ag0 3d5/2 and 373.95 eV for Ag0 3d3/2, related to the AgNPs (Fig. 4B). Primary amine (–NH2) has an N1s binding energy around ∼399.5–400.5 eV. So, the binding energy peak observed at 399.9 eV indicated the presence of the –NH2 group.69 Binding energy peaks for O1s were observed at 532.45 eV and 532.55 eV in the O1s XPS spectrum, typically corresponding to –C–OH and –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O.70
O.70
 | ||
| Fig. 4 (A) XPS survey scan of BG-AgNPs, (B) XPS Ag 3d scan, (C) XPS C1s scan (D) XPS N1s scan, (E) XPS O1s scan, and (F) XPS S2p scan. | ||
BG-AgNPs as selective Cr3+ nanosensors
The selectivity of BG-AgNPs was analyzed for the metal ions Zn2+, Cr3+, Mg2+, Pd2+, Ni2+, Co2+, Mn2+, Pb2+, Al3+, Ba2+, and Ca2+. Among these, Cr3+ was the only metal ion that induced a significant change in the colour of the BG-AgNP solution, shifting from reddish-brownish to transparent. Cr3+ also significantly reduced the peak absorbance compared to other studied ions (Fig. 5A and S2†). The findings indicated that solutions of other ions were unable to significantly alter the plasmonic characteristics of the BG-AgNP solution (Fig. 5A and B). The LoD and LoQ were calculated to be 0.07 nM and 0.21 nM, respectively, using concentrations in the range of 2–20 nM. The slope of the regression line (Y = 1.339–0.02481X), which described the relationship between Cr3+ ion concentration and absorbance at 411 nm, was determined to be 0.02481. The Pearson correlation coefficient was r = 0.988, with an R2 value of 0.97, indicating a strong linear correlation. The standard deviation of the absorbance at 411 nm, based on measurements from five samples, was 0.00053 (Fig. 5C and D). The change in colour and the absorbance may be due to the aggregation of the AgNPs resulting from AgNPs-Cr complex formation. Cr3+ induced aggregation in AgNPs and other metal nanoparticles has also been reported in previous studies (Table 1).21,37–39,71| Sl. No. | Probe | Absorbance wavelength (nm) | Calibration range | LoD |
|---|---|---|---|---|
| 1 | Atomic absorption spectrometry78 | — | — | 0.577 μM |
| 2 | Fluorescence79 | — | 17–667 μM | 1.7 × 104 μM |
| 3 | Hyper Rayleigh Scattering (HRS) using AuNPs functionalized with DNTB80 | 630 | 0–250 ppb | 3.035 μM |
| 4 | Pyridoxal conjugated AuNPs assay81 | 625 | 7.5 × 10−5–1.2 × 10−5 M | 11.5 μM |
| 5 | Rhodamine capped AuNPs assay82 | — | — | 9.28 μM |
| 6 | EDTA- tannic acid-AgNPs21 | 429 | 2–5 mg L−1 | 29.2 μM |
| 7 | Citrate-capped AuNPs83 | 520 | 0.2–1.0 ppm | 0.98 μM |
| 8 | Citrate capped AuNPs39 | 526 | 10−3–10−6 M | 0.106 μM |
| 9 | 5,5′-Dithiobis (2-nitrobenzoic acid) (DTNBA) AuNPs84 | 650/524 | 0–6 μM | 1.8 μM |
| 10 | 4-Amino hippuric acid (PAH)-AuNPs38 | 520 | 5 to 120 μM | 1.17 μM |
| 11 | 4- Mercaptobenzoic acid AuNPs71 | 635/520 | 20 to 25 μM | 5 μM |
| 12 | 4-Mercaptobenzoic acids (4-MBA)-Au@Ag NPs37 | 675/410 | 10 to 500 μM | 9 μM |
| 13 | Meso-2,3-dimercaptosuccinic acid (DMSA)-Au NPs85 | 650/525 | 10–500 nM | 10 nM |
| 14 | 6- Mercaptonicotinic acid (MNA) and melamine (MA) functionalized Ag NPs86 | 535 | 10–370 μM | 64.51 μM |
| 15 | BG-AgNPs (present study) | 411 | 2-20 nM | 0.07 nM |
Energy-dispersive X-ray spectrometry (EDX) was used to determine the chemical composition of the nanoparticle surface. As shown in Fig. S1† the nanoparticle surface contained C K (weight % 65.37, atomic % 71.84), O K (weight % 34.05, atomic % 28.09), and Ag L (weight% 0.59, atomic % 0.07), and after addition of Cr3+ ions, the chemical composition of the surface was C K (weight % 73.84%, atomic % 79.06), O K (weight % 26.01, atomic % 20.91), Cr K (weight % 0.08, atomic % 0.02), and Ag L (weight% 0.08, atomic % 0.01). The high percentage of C indicated the presence of organic molecule.72 The oxygen at the material surface indicated the presence of functional groups.73 A decrease in oxygen content after the addition of Cr3+ suggested possible interaction or reduction reactions occurring due to Cr3+ ions. The presence of chromium confirms that Cr3+ ions were successfully introduced to the nanoparticle surface. The minimal change in Ag content suggests that Ag is not significantly affected by Cr3+ addition. Though sulfur (S) was found in the XPS analysis, there was no visible S peak in the spectrum. The possibility may be that S was below the detection limit or sulfur may be embedded inside the nanoparticle, and it might not appear in the spectrum.
The FTIR spectra reveal key functional groups present in the samples before and after the addition of Cr3+ ions (Fig. S3†). A broad peak observed around 3276–3262 cm−1 corresponds to O–H stretching, likely from hydroxyl (–OH). A distinct peak at 1636 cm−1 revealed the presence of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching. The low frequency of carbonyl indicated the presence of amide carbonyl. A weak peak at 2156–2158 cm−1 may indicate the presence of unbound gaseous CO. The spectrum of AgNPs synthesized using black garlic extract showed the same functional groups as BG extract but confirmed the formation of nanoparticles through the retention of stabilizing biomolecules.74
O stretching. The low frequency of carbonyl indicated the presence of amide carbonyl. A weak peak at 2156–2158 cm−1 may indicate the presence of unbound gaseous CO. The spectrum of AgNPs synthesized using black garlic extract showed the same functional groups as BG extract but confirmed the formation of nanoparticles through the retention of stabilizing biomolecules.74
The X-ray diffraction (XRD) analysis was performed on three different samples: black garlic extract, BG-AgNPs, and BG-AgNPs + Cr3+, to examine their crystallinity and phase composition. The XRD patterns revealed significant differences between the samples, particularly regarding the formation of face-centred cubic (FCC) AgNPs. The XRD pattern of black garlic extract exhibited broad peaks with no distinct sharp diffraction signals, suggesting its amorphous nature. No FCC peaks corresponding to Ag were detected, confirming the absence of crystalline Ag phases. Like BG-AgNPs, the Cr3+-BG-AgNP complex also showed strong diffraction peaks at angles corresponding to 2θ values of 38.22°, 44.56°, 64.34°, and 78.20°. These peaks are associated with the (111), (200), (220), and (311) crystallographic planes, and are characteristic indicators of the typical FCC lattice structure of Ag (Fig. 2B).64
Optimization of pH and temperature
The UV-visible absorbance spectra indicated that peak absorbance decreases in the presence of Cr3+ ions. Fig. 5E and G show that, when comparing UV absorbance at different pH levels, pH 7 exhibited the highest reduction in peak absorbance and the highest relative activity (94.3%). In case of temperature, the highest reduction in peak absorbance was observed at a temperature of 45 °C with the highest relative activity (Fig. 5F and H). These results suggested that pH 7 and 45 °C are the optimal conditions for detecting Cr3+ ions. Hamada et al., in a study of metal complex formation resulting from the reactions between Cr3+ and aspartic acid at different pH ranges, reported that the absorbance of the Cr3+-aspartic acid system in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 ratio decreased as the pH increased from 1.55 to 4.10.75 Present observation also aligns with the findings by Imai and Gloyna, who demonstrated that Cr3+ adsorption onto biological solids in activated sludge systems is maximized around neutral pH due to favourable ionic interactions and reduced competition from H+ or OH− ions.76 However, the optimal conditions may vary with the nanoparticles as observed from the UV-vis spectra of 4-MBA-AuNP solutions in the presence of Cr3+, which indicated pH 6 as its optimal condition over the range from 6 to 10.71 The observation regarding temperature is strongly supported by Liu et al., who reported that the adsorption of Cr3+ onto a spheroidal cellulose adsorbent was endothermic.77 Their study showed that the adsorption capacity increased when the temperature was increased from 25 °C to 45 °C, highlighting a clear enhancement in Cr3+ binding.
2 ratio decreased as the pH increased from 1.55 to 4.10.75 Present observation also aligns with the findings by Imai and Gloyna, who demonstrated that Cr3+ adsorption onto biological solids in activated sludge systems is maximized around neutral pH due to favourable ionic interactions and reduced competition from H+ or OH− ions.76 However, the optimal conditions may vary with the nanoparticles as observed from the UV-vis spectra of 4-MBA-AuNP solutions in the presence of Cr3+, which indicated pH 6 as its optimal condition over the range from 6 to 10.71 The observation regarding temperature is strongly supported by Liu et al., who reported that the adsorption of Cr3+ onto a spheroidal cellulose adsorbent was endothermic.77 Their study showed that the adsorption capacity increased when the temperature was increased from 25 °C to 45 °C, highlighting a clear enhancement in Cr3+ binding.
Kinetics of the reactive interaction between BG-AgNPs and Cr3+
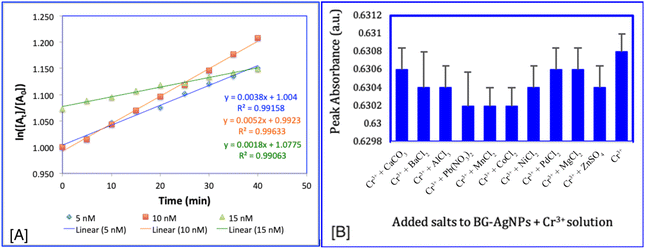
To assess the kinetics of the binding between Cr3+ ions and BG-AgNPs, the change in absorbance over time was measured using UV-visible spectroscopy at different Cr3+ concentrations. UV-visible spectra were recorded every 5 min, showing a gradual decrease in absorbance of the Ag NPs in the presence of Cr3+ over time under uniform reaction conditions. This decline in absorbance was also associated with a decrease in the rate constant as the Cr3+ concentration decreased (Table S1†).The investigation of the reaction dynamics between BG-AgNPs and Cr3+ demonstrated that a first-order kinetic framework provided the best fit for the data. It showed the highest R2 coefficient when compared to zero and second-order models (Fig. 6A). Although two reactants, the nanoparticles and Cr3+ ions, are involved, the reaction followed pseudo-first-order kinetics. This behaviour indicated that the reaction rate primarily depends on the concentration of one reactant in the solution. The rate constants were determined to be 0.0038, 0.0052, and 0.0018 for Cr3+ concentrations of 25 μM, 50 μM, and 75 μM, respectively. These values were higher compared to those obtained for a second-order reaction (Table S1†).
Interference study
An F-test (ANOVA) indicated no statistical variation in the absorbance between the BG-AgNP + Cr3+ and the BG-AgNP + Cr3+ solutions with the addition of other ions (F = 0.54; P = 0.85; df = 10, 44). This suggests that the occurrence of these ions did not interfere with the detection of Cr3+ ions by the BG-AgNPs (Fig. 6B). The lack of interference can be attributed to the sensor's higher binding affinity for Cr3+ relative to other ions. The results of the selectivity assessment confirm that the BG-AgNP probe exhibited strong specificity to Cr3+ ions, with no significant impact from other commonly encountered ions. This high level of specificity is crucial for the accurate detection of Cr3+ in aquatic samples, enabling reliable and precise assessments.Computational analysis
γ-Glutamyl-S-allylmercaptocysteine (GSAMC) is a compound derived from aged garlic extract. It primarily develops from GSAC/GS1PC during the initial stages of aging, attaining its maximum concentration around one month before diminishing in the later phases of the aging process.55 Based on previous literature, it is hypothesized that GSAMC might be the bioactive compound responsible for the formation of BG-AgNPs and the detection of Cr3+. To investigate this hypothesis, a computational study was conducted with GSAMC as the active compound.The geometries of γ-glutamyl-S-allylmercaptocysteine (GSAMC) and Ag containing nanoparticles are optimized with the help of MO6-2X functional in combination with the def2-TZVP basis set.61 The synthesis pathways of γ-glutamyl-S-allylmercaptocysteine (GSAMC)-silver nanoparticles (AgNPs) are illustrated in Fig. 7A, accompanied by the calculated Gibbs free energy values. The negative Gibbs free energy indicated that the reaction is thermodynamically favourable. After the study of the AgNP synthesis reaction, Fig. 7B depicts the synthesis pathways of the Cr containing γ-glutamyl-S-allylmercaptocysteine (GSAMC)-Ag complexes. Here also the Gibbs free energy is negative, indicating the thermodynamically favourable chemical reaction. All the structures shown in Fig. 7A and B are optimized.
In this synthesized GSAMC-AgNPs, the Ag atom interacts with the carboxylate group of the GSAMC molecule and the distance between the oxygen atom and Ag atom is 1.98 Å. The various types of bonding are described with the help of the molecular orbital analysis. In Fig. 7C(a), the Ag–Ag bond (HOMO-5) is shown, with a bond distance of 2.67 Å. The Ag atom linked with the carboxylate group is described by the help of molecular orbitals in Fig. 7C(b and c). After the addition of Cr, it linked with carboxylate and CH2 groups of the S–S branch of GSAMC-AgNPs, and it is shown with help of the molecular orbital (HOMO-15) in Fig. 7C(d), The orbital in Fig. 7C(e) also represented the linkage of the oxygen atom and Ag in Cr complexes of GSAMC-AgNPs.
The molecular orbital analysis and Gibbs free energy analysis showed that the Ag-containing GSAMC formation is a feasible reaction, and after the addition of Cr, it forms a stable GSAMC-AgNP-Cr complex.
Conclusion
The advantages of the proposed colorimetric sensing approach are manifold. It offers a simple and cost-effective method that does not require sophisticated instrumentation, making it suitable for on-site environmental monitoring and resource-limited settings. Additionally, the BG-AgNPs demonstrate remarkable sensitivity in detecting Cr3+ ions at extremely low concentrations. Moreover, BG-AgNPs offer high selectivity, as they can discriminate Cr3+ ions from other metal ions commonly present in environmental samples.Data availability
The data supporting this article have been included as part of the ESI.†Author contributions
RP: conceptualization, investigation, analysis, manuscript preparation. KS & AKG: validation, software. CT: conceptualization, methodology, supervision, visualization, writing – review & editing. All read and approved the final version of the manuscript.Conflicts of interest
The authors have no conflicts to declare.Acknowledgements
The authors express their sincere appreciation to the Director, CSIR-North East Institute of Science and Technology, Jorhat, Assam, for the continuous support and valuable suggestions provided throughout the research (Manuscript ref: CSIR-NEIST/PUB/2024/099). The author thanks the Analytical Chemistry Group & SAIF, CSIR-NEIST, Jorhat, for their instrumental support. The authors thank the SEED Division, DST New Delhi (DST/SEED/TSP/STI/2022/915) for financial support.References
- P. J. Cunat, Alloying Elements in Stainless Steel and Other Chromium-Containing Alloys, Euro Inox, 2004 Search PubMed.
- P. B. Tchounwou, C. G. Yedjou, A. K. Patlolla and D. J. Sutton, Heavy metal toxicity and the environment, in Molecular, Clinical and Environmental Toxicology, Experientia Supplementum, ed. A. Luch, Springer, Basel, 2012, vol 101, pp. 133–164 Search PubMed.
- R. Chakraborty, K. Renu, M. A. Eladl, M. El-Sherbiny, D. M. A. Elsherbini, A. K. Mirza, B. Vellingiri, M. Iyer, A. Dey and A. V. Gopalakrishnan, Biomed. Pharmacother., 2022, 151, 113–119 CrossRef PubMed.
- J. P. Wise, J. L. Young, J. Cai and L. Cai, Environ. Int., 2022, 158, 106877 CrossRef CAS.
- D. Y. Shin, S. M. Lee, Y. Jang, J. Lee, C. M. Lee, E. M. Cho and Y. R. Seo, Int. J. Mol. Sci., 2023, 24, 3410 CrossRef CAS PubMed.
- V. Gómez and M. P. Callao, Trends Anal. Chem., 2006, 25, 1006–1015 CrossRef.
- G. M. Keegan, I. D. Learmonth and C. Case, Crit. Rev. Toxicol., 2008, 38, 645–674 CrossRef CAS PubMed.
- D. Beyersmann and A. Hartwig, Arch. Toxicol., 2008, 82, 493–512 CrossRef CAS PubMed.
- ATSDR, Toxicological Profile for Chromium, Public Health Service U.S. Department of Health and Human Services, Agency for Toxic Substances and Disease Registry, Atlanta, Georgia, 2012 Search PubMed.
- E. Salles, V. Normant and D. A. L. Vignati, A critical evaluation of chromium(III) ecotoxicity to aquatic and terrestrial plants, in Chromium in Plants and Environment, ed. N. Kumar, C. Walther and D. K. Gupta, Springer, Cham, 2023, pp. 63–90 Search PubMed.
- Y. Tian, Q. Zhu, J. Yuan, R. Kneepkens, Y. Yue and C. Zhang, J. Reprod. Dev., 2021, 67, 283–290 CrossRef CAS PubMed.
- K. P. Nickens, S. R. Patierno and S. Ceryak, Chem.-Biol. Interact., 2010, 88, 276–288 CrossRef.
- H. Sun, J. Brocato and M. Costa, Oral chromium exposure and toxicity, Curr. Environ. Health Rep., 2015, 2, 295–303 CrossRef CAS.
- T. L. DesMarais and M. Costa, Curr. Opin. Toxicol., 2019, 14, 1–7 CrossRef.
- V. Singh, N. Singh, M. Verma, R. Kamal, R. Tiwari, M. Sanjay Chivate, S. N. Rai, A. Kumar, A. Singh, M. P. Singh, E. Vamanu and V. Mishra, Antioxidants, 2022, 11, 2375 CrossRef CAS.
- N. J. Miller Ihli, J. Food Compos. Anal., 1996, 9, 290–300 CrossRef CAS.
- K. Oktor, S. Yılmaz, G. Türker and E. Erkuş, Environ. Monit. Assess., 2008, 141, 97–103 CrossRef CAS PubMed.
- J. J. Thompson and R. S. Houk, Anal. Chem., 1986, 58, 2541–2548 CrossRef CAS.
- A. Rajib, I. Saiful, A. Razu, R. Tariqur, R. Atowar and B. I. Abu, Am. J. Eng. Res., 2016, 5, 243–247 Search PubMed.
- D. C. Prabhakaran, J. Riotte, Y. Sivry and S. Subramanian, Electroanalysis, 2017, 29, 1222–1231 CrossRef CAS.
- S. Sangsin, P. Dornbundit and P. Tongraung, Spectrochim. Acta, Part A, 2021, 246, 119050 CrossRef CAS PubMed.
- A. J. Weerasinghe, C. Schmiesing and E. Sinn, Tetrahedron Lett., 2009, 50, 6407–6410 CrossRef CAS.
- S. Wu, K. Zhang, Y. Wang, D. Mao, X. Liu, J. Yu and L. Wang, Tetrahedron Lett., 2014, 55, 351–353 CrossRef CAS.
- D. Xue, C. Zheng, C. Fan, G. Liu and S. Pu, J. Photochem. Photobiol., A, 2015, 303–304, 59–66 CrossRef CAS.
- H. Zhang, G. Zhang, J. Xu, Y. Wen, S. Ming, J. Zhang and W. Ding, Spectrochim. Acta, Part A, 2018, 191, 79–87 CrossRef CAS PubMed.
- M. Zhang, L. Gong, C. Sun, W. Li, Z. Chang and D. Qi, Spectrochim. Acta, Part A, 2019, 214, 7–13 CrossRef CAS PubMed.
- Y. Zhou, H. Zhao, C. Li, P. He, W. Peng, L. Yuan, L. Zeng and Y. He, Talanta, 2012, 97, 331–335 CrossRef CAS PubMed.
- M. L. Firdaus, I. Fitriani, S. Wyantuti, Y. W. Hartati, R. Khaydarov, J. A. McAlister, H. Obata and T. Gamo, Anal. Sci., 2017, 33, 831–837 CrossRef CAS PubMed.
- G. S. Ghodake, S. K. Shinde, R. G. Saratale, A. A. Kadam, G. D. Saratale, A. Syed, F. Ameen and D. Y. Kim, Beilstein J. Nanotechnol., 2018, 9, 1414–1422 CrossRef CAS PubMed.
- W. Wei, Y. Du, L. Zhang, Y. Yang and Y. Gao, J. Mater. Chem. C, 2018, 6, 8793–8803 RSC.
- D. G. Thompson, A. Enright, K. Faulds, W. E. Smith and D. Graham, Anal. Chem., 2008, 80, 2805–2810 CrossRef CAS PubMed.
- N. E. Motl, A. F. Smith, C. J. DeSantis and S. E. Skrabalak, Chem. Soc. Rev., 2014, 43, 3823–3834 RSC.
- H. H. Nguyen, J. Park, S. Kang and M. Kim, Sensors, 2015, 15, 10481–10510 CrossRef CAS.
- E. Petryayeva and U. Krull, Anal. Chim. Acta, 2011, 706, 8–24 CrossRef CAS PubMed.
- S. Jayalakshmi, P. K. Suresh, D. Govindan, M. Mariappan, F. Ameen and A. Veerappan, Opt. Mater., 2024, 154, 115679 CrossRef CAS.
- Z. Zhang, Q. Dong, Y. Cao, Y. Liu and Y. Zhao, Nano, 2022, 17, 14 Search PubMed.
- Z. Zhang, H. Li, D. Guo, C. Hu and Y. Liu, J. Anal. Sci. Technol., 2023, 14, 40 CrossRef CAS.
- W. Jin, P. Huang and Y. Chen, et al. , J. Nanopart. Res., 2015, 17, 358 CrossRef.
- M. Elavarasi, A. Rajeshwari, N. Chandrasekaran and A. Mukherjee, Anal. Methods, 2013, 5, 6211–6218 RSC.
- C. Dong, G. Wu, Z. Wang, W. Ren, Y. Zhang, Z. Shen, T. Li and A. Wu, Dalton Trans., 2016, 45, 8347–8354 RSC.
- H. Yuan, L. Sun, M. Chen and J. Wang, J. Food Sci., 2016, 81, C1662–C1668 CAS.
- M. A. Toledano-Medina, J. Perez-Aparicio, R. Moreno-Rojas and T. Merinas-Amo, Food Chem., 2016, 199, 135–139 CrossRef CAS PubMed.
- X. Zhang, N. Li, X. Lu, P. Liu and X. Qiao, J. Sci. Food Agric., 2015, 96, 2366–2372 CrossRef PubMed.
- S. Kimura, Y. C. Tung, M. H. Pan, N. W. Su, Y. J. Lai and K. C. Cheng, J. Food Drug Anal., 2017, 25, 62–70 CrossRef CAS PubMed.
- W. C. W. Chang, Y. T. Chen, H. J. Chen, C. W. Hsieh and P. C. Liao, J. Agric. Food Chem., 2020, 68, 14049–14058 CrossRef CAS.
- F. Li, J. Cao, Q. Liu, X. Hu, X. Liao and Y. Zhang, Food Chem., 2020, 318, 126517 CrossRef CAS PubMed.
- M. Iciek, I. Kwiecien and L. Wlodek, Environ. Mol. Mutagen., 2009, 50, 247–265 CrossRef CAS.
- P. Z. Trio, S. You, X. He, J. He, K. Sakao and D. X. Hou, Food Funct., 2014, 5, 833–844 RSC.
- T. Kamoi, Phosphorus, Sulfur Silicon Relat. Elem., 2019, 194, 698–700 CrossRef CAS.
- Y. Yamaguchi and H. Kumagai, Exp. Ther. Med., 2019, 19, 1528–1535 Search PubMed.
- The Science and Therapeutic Application of Allium Sativum L and Related Species, H. P. Koch and L. D. Lawson, Williams & Wilkins, Baltimore, Md, USA, 1996 Search PubMed.
- H. Amagase, B. L. Petesch, H. Matsuura, S. Kasuga and Y. Itakura, J. Nutr., 2001, 131, 955S–962S CrossRef CAS.
- H. Amagase, J. Nutr., 2006, 136, 716S–725S CrossRef CAS PubMed.
- F. Albrecht, R. Leontiev, C. Jacob and A. J. Slusarenko, Molecules, 2017, 22, 770 CrossRef PubMed.
- T. Fujii, T. Matsutomo and Y. Kodera, J. Agric. Food Chem., 2018, 66, 10506–10512 CrossRef CAS PubMed.
- R. Paw, M. Hazarika, P. K. Boruah, A. J. Kalita, A. K. Guha, M. R. Das and C. Tamuly, RSC Adv., 2021, 11, 14700–14709 RSC.
- N. Borah, D. Gogoi, N. C. Ghosh and C. Tamuly, Food Chem., 2023, 15, 133975 CrossRef PubMed.
- ICH Q2, Validation of Analytical Procedures: Test and Methodology, 2005 Search PubMed.
- R. Painuli, S. Raghav and D. Kumar, ACS Omega, 2019, 4, 3635–3645 CrossRef CAS PubMed.
- R. Paw, A. K. Guha and R. Tamuly, RSC Adv., 2024, 14, 22701–22713 RSC.
- Y. Zhao and D. G. Truhlar, Theor. Chem. Acc., 2008, 120, 215–241 Search PubMed.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel and G. E. Scuseria, et al., Gaussian 16, Revision A.03, Gaussian, Inc., Wallingford CT, 2016 Search PubMed.
- A. E. Reed, L. A. Curtiss and F. Weinhold, Chem. Rev., 1988, 88, 899–926 CrossRef CAS.
- D. H. Wi, H. Yang, Y. Kim, H. Ahn, J. W. Hong and S. W. Han, J. Mater. Chem. A, 2023, 11, 1343–1350 RSC.
- L. Q. Lu and Y. Wang, J. Mater. Chem., 2011, 21, 17916 RSC.
- M. Li, H. Liu, S. Pang, P. Yan, M. Liu, M. Ding and B. Zhang, Nanomaterials, 2021, 11, 2650 CrossRef CAS PubMed.
- G. H. Major, J. W. Pinder, D. E. Austin and D. R. Baer, et al. , J. Vac. Sci. Technol., A, 2023, 41, 038501 CrossRef CAS.
- J. W. Pinder, G. H. Major, D. R. Baer and J. Terry, et al. , Appl. Surf. Sci. Adv., 2024, 19, 100534 CrossRef.
- S. Ravi, S. Zhang, Y. R. Lee, K. K. Kang, J. M. Kim, J. W. Ahn and W. S. Ahn, J. Ind. Eng. Chem., 2018, 67, 210–218 CrossRef CAS.
- J. Zhang, M. Zhang, H. Wan, J. Zhou and A. Lu, Nat. Commun., 2025, 16, 320 CrossRef CAS PubMed.
- Z. Zhang, X. Ye, Q. Liu, Y. Liu and R. Liu, J. Anal. Sci. Technol., 2020, 11, 10 CrossRef CAS.
- F. K. Alqarawi, M. F. Alkahtany, K. H. Almadi, G. A. A. Ben, F. A. Alshahrani, M. H. AlRefeai, I. Farooq, F. Vohra and T. Abduljabbar, Polymers, 2021, 13, 1555 CrossRef CAS PubMed.
- R. Moreno-Tovar, E. Terres and J. R. Rangel-Mendez, Appl. Surf. Sci., 2014, 303, 373–380 CrossRef CAS.
- L. Wade and J. Simek, Organic Chemistry, 9th edn, Pearson, 2016, p.189 Search PubMed.
- Y. Z. Hamada, N. Bayakly, M. Shafi, S. Painter, V. Taylor, J. Greene and K. Rosli, Complex Met., 2014, 1, 46–51 CrossRef.
- A. Imai and E. F. Gloyna, Water Res., 1990, 24, 1143–1150 CrossRef CAS.
- M. Liu, H. Zhang, X. Zhang, Y. Deng, W. Liu and H. Zhan, Water Environ. Res., 2001, 73, 322–328 CrossRef CAS PubMed.
- J. Chwastowska, W. Skwara, E. Sterlinska and L. Pszonicki, Talanta, 2005, 66, 1345–1349 CrossRef CAS PubMed.
- S. Panda, P. B. Pati and S. S. Zade, Chem. Commun., 2011, 47, 4174–4176 RSC.
- S. I. Hughes, S. S. R. Dasary, A. K. Singh, Z. Glenn, H. Jamison, P. C. Ray and H. Yu, Sens. Actuators, B, 2013, 178, 514–519 CrossRef CAS PubMed.
- S. Bothra, R. Kumar and S. K. Sahoo, New J. Chem., 2017, 41, 7339–7346 RSC.
- N. Manjubaashini, D. T. Thangadurai, G. Bharathi and D. Nataraj, J. Lumin., 2018, 202, 282–288 CrossRef CAS.
- M. L. Firdaus, H. Apriyoanda, I. Isnan, S. Wyantuti and D. R. Eddy, Iran. J. Chem. Chem. Eng., 2023, 42, 722–730 CAS.
- Y. Q. Dang, H. W. Li, B. Wang, L. Li and Y. Wu, ACS Appl. Mater. Interfaces, 2009, 1, 1533–1538 CrossRef CAS PubMed.
- W. Chen, F. Cao, W. Zheng, Y. Tian, Y. Xianyu, P. Xu, W. Zhang, Z. Wang, K. Deng and X. Jiang, Nanoscale, 2015, 7, 2042–2049 RSC.
- R. P. Modi, V. N. Mehta and S. K. Kailasa, Sens. Actuators, B, 2014, 195, 562–57 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5va00100e |
| This journal is © The Royal Society of Chemistry 2025 |