Open Access Article
Open Access ArticlePlastic waste gasification for low-carbon hydrogen production: a comprehensive review
Muhammad Aamir
Bashir†
 ab,
Tuo
Ji†
ab,
Tuo
Ji†
 ab,
Jennifer
Weidman
ab,
Jennifer
Weidman
 ab,
Yee
Soong
ab,
Yee
Soong
 a,
McMahan
Gray
a,
McMahan
Gray
 a,
Fan
Shi
a,
Fan
Shi
 *a and
Ping
Wang
*a and
Ping
Wang
 *a
*a
aNational Energy Technology Laboratory, 626 Cochrans Mill Road, P.O. Box 10940, Pittsburgh, PA 15236-0940, USA. E-mail: fan.shi@netl.doe.gov; Ping.Wang@NETL.DOE.GOV
bNETL Support Contractor, 626 Cochrans Mill Road, P.O. Box 10940, Pittsburgh, PA 15236-0940, USA
First published on 15th January 2025
Abstract
Hydrogen is one of the most important feedstocks for the chemical industry, power production, and the decarbonization of other sectors that rely on natural gas. The production of hydrogen from plastics enables sustainable use of plastic waste and offers significant environmental benefits. Gasification emerges as a promising route for chemical recycling, converting plastic into hydrogen and other valuable chemicals. Although the gasification of plastic waste has recently gained attention, the number of studies regarding low-carbon hydrogen production is still limited. The effective integration of carbon capture, utilization, and storage (CCUS) is essential for achieving low-carbon hydrogen production via gasification, which enables the efficient capture and storage of CO2 emissions. Incorporating coal waste and biomass into plastic gasification can synergistically enhance reforming reactions for hydrogen production, reduce tar content, and resolve feeding issues caused by plastic stickiness. Based on the previous studies, this paper briefly reviews the mechanisms of plastic gasification including plastic depolymerization, reforming, tar and char formation, and gasification; the discussions on feedstocks and effects of operating conditions on H2 production including plastic-type, temperature, steam/carbon ratio, equivalence ratio, and catalysts; and the integration of CCUS and alternative recovery processes in plastic gasification for low-carbon hydrogen.
1. Introduction
Plastics revolutionized various industries from the early 1900s, with mass production and consumption by 1950. Although plastics have transformed various sectors, their widespread adoption soon exceeded the capacity of waste management systems, leading to accumulations in various environments. The current use of plastics is increasingly becoming a global concern due to the tremendous amount of discarded plastic waste accumulating as debris in landfills, oceans, and other natural habits across the world.1–3 According to an estimate, 6.3 billion metric tons of plastic waste was generated in the last 6 decades, out of which, 60% accumulated in the landfills/natural environment.4 As shown in Fig. 1a, the plastic waste problem escalated so quickly that by 2015, 8300 million metric tons of plastic had been generated. Of this, only approximately 9% was recycled; the remainder was either incinerated or littered in landfills and the environment.5Fig. 1b shows common plastics, their recycling codes, the amount produced, and the percentage recycled.6 The inadequate management of plastic waste (e.g. landfills) causes serious environmental problems such as ground water contamination, and sanitary related issues, among others. In addition, it leads to sustainability issues due to the loss of valuable and natural resources because most of the plastics are made from fossil fuels.7,8 The current handling of plastic waste cannot continue unchecked, as it is causing serious environmental problems such as ground water contamination, harm to wildlife, and burdens to ecosystems.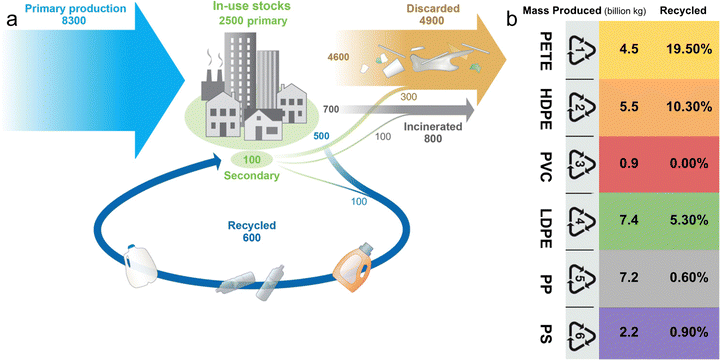 | ||
| Fig. 1 (a) Global production, use, and fate of polymers (1950 to 2015, in million metric tons) (reprinted with permission from ref. 5 Copyright 2017 AAAS), (b) the mass production and recycled percentage of plastics with different resin identification codes (RICs).6 | ||
Plastics are primarily composed of hydrocarbons, which contain a large amount of chemical energy that may be recovered and utilized.9 It was estimated that the recycling of all global plastic wastes could replace the energy obtained from 3.5 billion barrels of oil per year.10,11 Plastic combustion seems to be a feasible valorization route, but it is hindered by the emissions produced.12 Mechanical recycling turns waste plastic into other useful applications.13 However, the mechanical approach has its limitations, and the quantities of recycled plastics vary geographically.14 Amongst recycling routes, chemical recycling (combustion, chemolysis, pyrolysis, gasification, etc.) provides sustainable solutions to recycle a wider variety of plastic wastes, including mixed, colored, and multilayer-material plastic wastes.15,16 However, chemical recycling still requires further development to overcome obstacles, which include the handling of contaminants such as alkali metals, chlorine, and sulfur, feeding issues from the sticky nature of plastics, and the development of economical and sustainable technology.17
Currently, fossil fuels are used to produce 96% of hydrogen globally, primarily from coal, oil, and natural gas (via the process of gasification and steam methane reforming).18 To meet the growing demand for hydrogen, it is vital to develop technologies that can produce hydrogen efficiently, with low carbon emissions, and at a low cost. Gasification is one of the thermochemical recycling processes in which carbonaceous materials are thermally treated at a high temperature (600–900 °C) in a gasifier to produce gaseous products.7,8,19,20 The gaseous products constitute mainly H2, CnHm, CO, and CO2. The yield and composition of gas vary significantly based on the feedstock composition, atmosphere, temperature, and catalysts.
Low-carbon hydrogen is hydrogen produced from non-renewable sources, with emissions that are at least 70% lower than traditional fossil fuels.21 For low-carbon hydrogen to play a significant role in upcoming energy systems, its production must have minimum carbon emissions and be economically feasible. Unfortunately, many low-carbon hydrogen production methods are not yet fully developed, mainly due to high costs and low efficiency. Fig. 2 illustrates the various routes for producing low-carbon hydrogen, with a focus on the feedstock and conversion processes. Fossil fuel-derived plastic waste and renewable biomass can be transformed into low carbon hydrogen using thermochemical processes such as gasification and steam gasification, by incorporating carbon capture and storage. Current biological and electrolytic techniques, although efficient in generating low-carbon hydrogen, are not effective at processing plastic waste. The gasification process can effectively deal with a larger range of plastic waste and other carbonaceous feedstocks, making it a viable method for converting complex plastic waste or mixtures containing plastic into valuable hydrogen.22 Gasification and reforming technologies are crucial for cost-effective, low-carbon hydrogen production. Integration of carbon capture, utilization, and storage (CCUS) allows for the use of diverse feedstocks such as coal waste, biomass, and waste plastics, resulting in decreased carbon emissions.23 Implementing pre-combustion CO2 capture guarantees zero or even negative carbon emissions from hydrogen creation. CCUS methods in the power and industrial sectors, in addition to direct air capture, play a vital role in sustainable hydrogen production. All thermochemical techniques release carbon dioxide, either through energy input or as a by-product.23,24 Carbon capture and storage are necessary for these methods to contribute to a low-carbon hydrogen economy. The key hurdle to thermochemical production is access to carbon capture and storage for decarbonization. New thermal techniques, like microwave heating and converting fossil fuels underground, are also being developed to reduce carbon dioxide emissions. Biomass, coal waste and plastic waste gasification and co-gasification with carbon capture are also promising options to reduce carbon emissions. Producing hydrogen from plastic waste promotes sustainability; when waste-produced hydrogen is used to fuel vehicles, carbon emissions from the transportation sector can be eliminated.
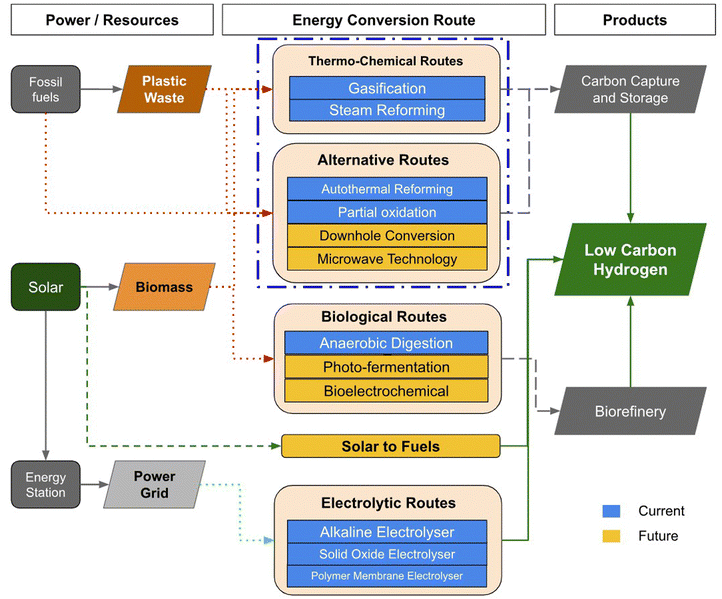 | ||
| Fig. 2 Potential routes of the low-carbon hydrogen production (adapted from ref. 25 Reprinted with permission from the royal society (CC-BY 4)). | ||
This work intends to provide researchers, policymakers, governmental institutions, and commercial producers with a review of the hydrogen/syngas production from plastic waste. The objectives of this review are three-fold: (1) to examine the mechanisms of plastics gasification including the plastics depolymerization, reforming reaction, tar and char formation, and gasification; (2) to discuss the effects of feedstocks and operating conditions on H2 production including plastic-type, temperature, steam/carbon ratio, equivalence ratio, and catalysts; and (3) to study the Integration of carbon capture, utilization, and storage (CCUS) and alternative recovery processes in plastic gasification for low carbon hydrogen. We believe that this review will not merely summarize the reported works but will also guide the way for the development of plastic gasification.
2. Mechanism of plastic gasification
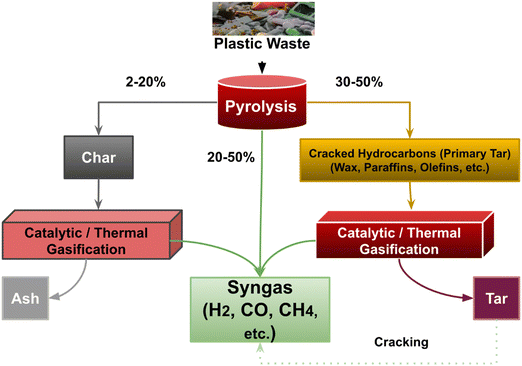
Before gasification, raw plastics are pretreated through several steps including rinsing, milling, and drying. The milling enhances heat and mass transfer during gasification and improves the contact between plastic particles and catalysts. The moisture content in plastics is relatively low, and most of the moisture stays on the external surface, which can be easily removed without diffusivity limitations.26,27 The drying process for plastic waste is not as important as it is for other feedstocks, such as biomass in gasification. During plastic gasification to syngas, plastics undergo several steps, including pyrolysis, reforming, and secondary gasification (Fig. 3). Cracked hydrocarbons or primary tar are the intermediates after pyrolysis. The pyrolysis of plastic waste results in the formation of solid char (2–20% by weight), liquid hydrocarbons (30–50% by weight), and a gas phase consisting of CO, CH4, CO2, H2, and other volatile compounds (20–50% by weight).22.1 Polymer depolymerization to primary tar
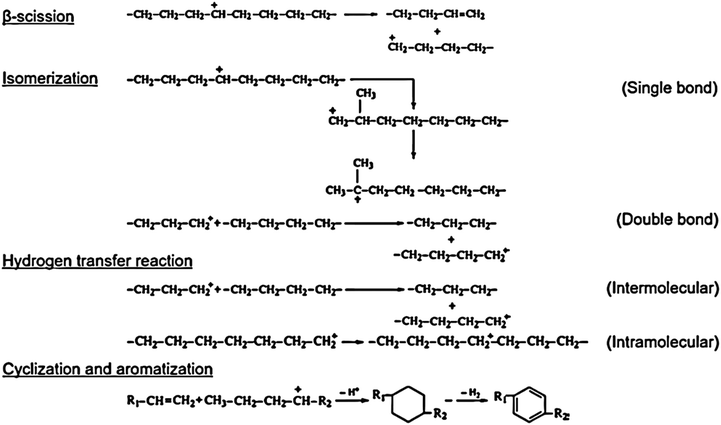
The plastic (polymer) depolymerization plays a significant role in the composition of the volatiles formed and their subsequent reforming reactions in the gaseous phase.28 Plastic (polymer) depolymerization is generally initiated by free-radical formation.29,30 Upon heating, the weak sigma bonds (C–C bonds) in the macromolecular polymer become unstable, leading to the formation of free radicals. These free radicals initiate a series of reactions, including random scission (RS), backbiting (BB), and unzipping (UZ). The RS pathway involves intermolecular hydrogen transfer followed by mid-chain β-scission reactions, producing low molecular weight products (LMWPs). The BB pathway involves specific intramolecular hydrogen transfer reactions followed by mid-chain β-scission reactions, while the UZ pathway entails a series of end-chain β-scission reactions. These processes break the polymer chain into smaller compounds, releasing hydrogen atoms and forming various free radicals.31When end chain β-scission reactions happen, the liquid fraction in pyrolytic oil (C6–C34) is formed and can be recombined to olefins, followed by intramolecular hydrogen shift.32 When the depolymerization temperature goes higher (∼400 °C), the α-scission mechanism becomes dominant and results in producing heavier hydrocarbons in the pyrolytic oil. The breaking of the σ bond (sp2 carbon) generally occurs due to the dissociation energy of α-scission (83–94 kcal mol−1) is more than β-scission (61.5–63 kcal mol−1).33 For low-temperature pyrolysis, the scission reaction typically occurs at the beta position of the polymer chain, breaking it into smaller hydrocarbons. The mechanism model results indicate that the RS pathway is the most dominant, particularly in the early stages of degradation, releasing hydrogen atoms and creates free radicals.31,33 The degradation followed by end-chain beta scission compared to the random chain requires a lot less energy to break down, producing more gaseous hydrocarbons. The liquid fraction in pyrolytic oil (C6–C34) is formed due to both random and end chain beta scission reactions. Radical recombination (depolymerization) followed by intramolecular hydrogen shift results in the formation of olefins. At a higher temperature, the degradation of polymers is caused by side chain elimination followed by the alpha scission mechanism producing heavier hydrocarbon fractions in the pyrolytic oil which can be confirmed by the presence of wax components at higher temperatures. The breaking of the sigma bond (sp2 bond) generally occurs due to alpha scission which utilizes a high amount of dissociation energy compared to beta scission.31,33
Below is the detailed depolymerization process for common polyolefins plastics. Linear or straight-chain polymers (such as PP and PE), undergo β-scission to form monomers via a random chain scission mechanism and then stabilized by intramolecular or intermolecular hydrogen transfer. Inter and intramolecular hydrogen further produce paraffin.34,35Fig. 4 shows the primary reactions in PE depolymerization. In the case of polyethylene, degradation occurs via a random chain scission mechanism forming free-radical fragments. Followed by the hydrogen chain transfer reaction, these free radical fragments are converted to saturated and unsaturated straight molecules such as alkanes and alkenes. Previous research found that the same PE samples produced long-chain hydrocarbons, independent of reaction geometry.
 | ||
| Fig. 4 Scheme of primary reactions in PE depolymerization (reprinted from ref. 32 Elsevier Science Press, Copyright 2005). | ||
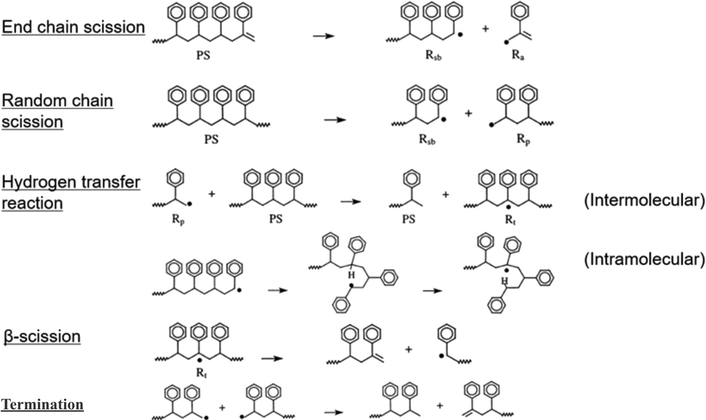
In case of polystyrene (PS), degradation is generally initiated by both end chain scission and random scission,36 followed by beta scission, since it degrades at a lower temperature compared to PE.37 Side elimination results in a higher concentration of styrene monomers in the degradation products. The initiation step for PS can be described by two mechanisms, which are chain end scission and random scission. Random chain dissociates the polymer chain into a primary (Rp) and a secondary benzyl radical (Rsb), while the end chain mechanism dissociates the polymer chain into a secondary benzyl radical (Rsb) and resonantly stabilized allyl benzene radical (Ra), as shown in Fig. 5. The interaction of the propagation beta scission reaction results in the formation of low molecular weight and unsaturated species, which relates to the end chain scission causes high concentration of styrene monomer in the products. Then intramolecular hydrogen transfer reaction produces Rsb and Rp structures while intermolecular hydrogen transfer reaction mechanism. The propagation step is followed by hydrogen abstraction (intermolecular and intramolecular) and beta decomposition.
 | ||
| Fig. 5 Scheme of primary reactions in PS depolymerization (reprinted with permission from ref. 36 Copyright 2001 Elsevier). | ||
Intermolecular hydrogen transfer forms a tertiary carbon radical (Rt). This radical undergoes β-scission, breaking down into saturated and unsaturated monomers. In contrast, intramolecular hydrogen transfer, also known as backbiting, transfers numbered ring structures from secondary (Rsb) and primary (Rp) radicals. Further, Rt undergoes scission at the beta position to form unsaturated end. The unzipping reactions are beta decomposition held with the formation of Rsb and a monomer. The termination step uses the disproportionation reaction which produces unsaturated ends. Fig. 5 shows the primary reactions in PS depolymerization.36
Polyolefins, in contrast to PS, polyethylene terephthalate (PET), or polyvinyl chloride (PVC), can be almost completely converted into volatiles when the pyrolysis is carried out under suitable conditions.38,39 Lopez et al. summarized product composition obtained from recent works on the thermal pyrolysis of polyolefins.40 In general, polyolefins can be converted to waxes, BTX (benzene, toluene, and xylene), or light olefins depending on different pyrolysis temperatures and residence times. Waxes are made up of linear and branched saturated and unsaturated hydrocarbons.41 Waxes are solid at room temperature, which hinders their handling, especially in the condensation equipment of pyrolysis units. Many studies have proven that pyrolysis of polyolefins tend to produce wax at a temperature range between 500 and 650 °C with a short residence time.42–45 When pyrolysis temperature reaches 600–800 °C, the yield of BTX is enhanced with a suitable residence time.46–48 Aromatics are formed in the reaction environment by Diels–Alder condensation of olefins and dehydrogenation reactions.49 At the higher temperatures range (>800 °C), end chain scission including direct scission, 1,5-radical transfer scission, and multiple step-radical transfer scissions prevail in the polymer depolymerization.50 Multiple step-radical transfer scissions lead to produce light olefins.30 Furthermore, the degradation of polyolefins via this latter mechanism produces mainly light olefins. Herein, high-temperature cracking coupled with short residence times is a feasible way to the selective production of light olefins from polyolefins.51 However, the physical and chemical characteristics of plastic wastes may hinder the purity of light olefins. The slow-heated and sticky plastics reduce the thermal degradation kinetics, especially in a gasifier when the reactor cannot provide good heat transfer and high heat transfer rates to avoid the agglomeration.
2.2 Gasification reactions
The intermediate cracked hydrocarbons from the pyrolysis process are further reformed to H2 and CO. This includes a wide variety of reactions, mainly depending on the gasifying agent, feed ratio (equivalence ratio (ER) in the case of air/O2 or steam/carbon (molar) ratio (S/C) in the case of steam), and operating temperature.52 Air or O2 gasification of plastics would produce gas via combustion and partial oxidation reactions. Effect of the agents will be further discussed in Section 3.3. Gasification/reduction reactions are as follows:Steam reforming
| CnHm + nH2O → (n + m/2)H2 + nCO ΔH > 0 | (1) |
Methane reforming
| CH4 + H2O ⇌ 3H2 + CO ΔH = 206 kJ mol−1 | (2) |
Char steam gasification
| C + H2O ⇌ H2 + CO ΔH = 131 kJ mol−1 | (3) |
Dry reforming
| CnHm + nCO2 → (m/2)H2 + 2nCO ΔH > 0 | (4) |
Boudouard reaction
| C + CO2 ⇌ 2CO ΔH = 172 kJ mol−1 | (5) |
Water–gas shift reaction
| CO + H2O ⇌ H2 + CO2 ΔH = −41 kJ mol−1 | (6) |
Apart from eqn (6), all reactions are endothermic, which means additional energy is required to proceed with the reactions. Char gasification only happened when the temperature is higher than 730 °C, which brings a thermodynamic restriction in the water–gas shift reaction.53 The utilization of reforming catalysts could be a common strategy to accelerate the reactions and lower the temperature requirements.34,35 CO2-assisted gasification can be illustrated in following reactions:7–12
| PP + CO2 → Side chains + H2O + H2 + CO + CnHm + Tar ΔH > 0 | (7) |
| Side chains + CO2 → H2 + CO + CnHm + Tar ΔH > 0 | (8) |
| Tar(CxHy) + CO2 → CO + H2 + CnHm (x > m; y > n) ΔH > 0 | (9) |
| CnHm + mCO2 → 2mCO + (n/2)H2 ΔH = 980–3112 kJ mol−1 | (10) |
| CO2 + H2 ⇌ CO + H2O ΔH = 41 kJ mol−1 | (11) |
| C + CO2 ⇌ 2CO ΔH = 172 kJ mol−1 | (12) |
Reaction (7) represents global gasification of PP using CO2 as the gasifying agent, while reaction (8) refers to the side-chains reaction; reaction (9) describes the cracking of tar with CO2 at high temperature; reaction (10) explains volatile hydrocarbons reactions with CO2; reaction (11) expresses reversed WGS reaction; and reaction (12) defines the Boudouard reaction. The difference between non-catalytic and catalytic gasification generally occurred after reaction (7). Reactions (9)–(11) mainly occurred in catalytic gasification leading to high H2 and lower CnHm yields.
For non-catalytic gasification, volatile compounds vaporized directly and left softened PP in the reactor, while reactions (8) and (9) are the main reactions. Without catalysts, reactions (9)–(11) are insignificant during the gasification process. For in situ catalytic gasification, side chains and tar were decomposed by catalyst into H2 and CnHm. However, because of the mass transfer barrier by intimate mixing of PP and catalyst, more carbon residues, i.e., carbon black, were formed, which led to a higher CO yield according to Boudouard reaction (12). For quasi-in situ catalytic gasification, volatiles and tar immediately decomposed when passing through the catalyst bed with CO2 flow and produced more H2 and CnHm than the other two methods.54
Density functional theory (DFT) models of steam gasification of PE are shown in Fig. 6(a)–(d). Fig. 6(f) shows the evolution of the main product H2 in steam gasification systems. The steam gasification mechanism (Fig. 6e) of polyethylene (PE) is comprised of two overlapping stages: thermal depolymerization and steam reforming. During the depolymerization process, C–C bonds are broken either at the ends or within the polymer chain. Throughout this process, multiple R˙ radicals and ˙R˙ double radicals are produced and break down into smaller hydrocarbon fragments, resulting in the production of C1–C4 compounds. The energy barrier for steam reforming reactions is greatly reduced by the free H˙ radicals produced during dehydrogenation and H-transfer reactions in the depolymerization phase. The formation of hydrogen (H2) is attributed to the reforming between H2O vapors and these free H˙ radicals. Additionally, the reactive ˙OH radicals formed during this stage actively contribute to the generation of carbon monoxide (CO). Increasing temperature and water vapor content accelerate the gasification process, improving H2 and CO yields; raising the temperature from 2500 K to 3500 K boosts H2 yield by 20%. Additionally, the steam-to-plastic ratio (S/P) of 1.5 enhances both CO and H2 yields, with water vapor increasing H2 yield by 2.5 times compared to the inert process.54
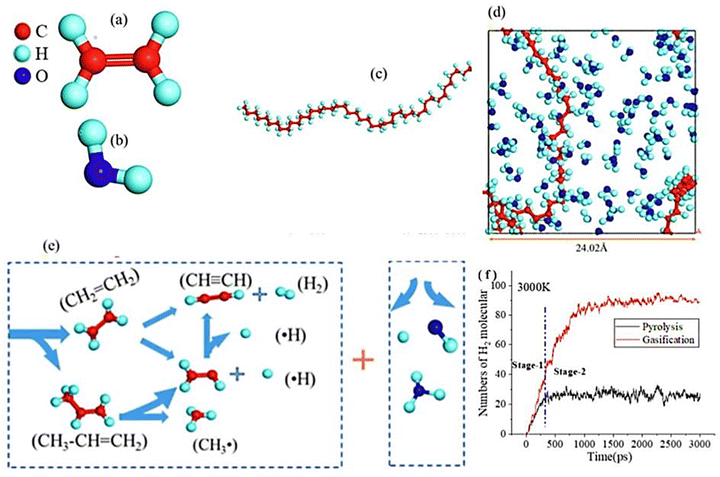 | ||
| Fig. 6 Steam gasification DFT method: (a) ethylene molecule; (b) H2O molecule; (c) the chain of optimized PE; (d) gasification system; (e) depolymerization and steam reforming reaction; (f) distribution of H2 in gasification (adapted with permission from ref. 54 Copyright 2022 Elsevier). | ||
For H2 production, there is no consensus on the best intermediate hydrocarbon for the subsequent reforming reaction. One reason is the direct plastic gasification couple's plastic depolymerization with reforming reaction, which usually operates over 800 °C. The cracked hydrocarbons with a wide product distribution directly react with H2O or CO2 to produce syngas. Moreover, researchers observed that light olefins play a critical role in tar formation.32,55 Therefore, the high tar yield in plastic gasification is one of the reasons for the higher contents of hydrocarbons in the gas product.
2.3 Tar formation
Tar formation during plastic gasification is characterized by the production of a complex mixture of condensable hydrocarbons. This mixture comprises predominantly single to 5-ring aromatic compounds, alongside other organic molecules containing oxygen, sulfur, and nitrogen.56 As mentioned above, the intermediates of hydrocarbons contribute to tar formation, which is a major technical challenge in plastic gasification compared to gasification of coal and biomass. It caused several issues in downstream operations such as blocking and fouling of engines and turbines, filter-pores, and deactivation of catalysts.57The mechanism of tar formation and evolution depends on the plastic composition.16 For example, primary tars of alkanes and alkenes are mainly derived from polyolefin degradation, while primary tars of an aromatic nature are mainly produced from the degradation of polymers with aromatic rings in their structure (i.e. PS and PET58). At high gasification temperatures, primary tars are rapidly cracked into lighter hydrocarbons (i.e. secondary tars and tertiary tars, showed in Fig. 7). Among these light hydrocarbons, light olefins, as tar precursors, plays a critical role in tar formation. As described in a presumable tar formation route, C2–C4 olefins, especially acetylene, are involved in: (i) hydrogen abstraction and acetylene addition and (ii) dehydrogenation and Diels–Alder condensation reactions. Therefore, high tar formation found in plastic gasification was a consequence of the high content of light hydrocarbons during tar formation and evolution. Due to their low thermal stability, linear hydrocarbons were negligible in the tar produced in polyolefins gasification.59,60 Secondary and tertiary tars only crack at operating temperature above 1250 °C and residence times below 0.5 s.61 The suppression of tar during plastics gasification has been studied. For instance, Toledo et al. found that a longer residence time and higher temperature would crack the tar.62 Besides, reactor optimization and the catalyst used would improve the tar elimination efficiency.
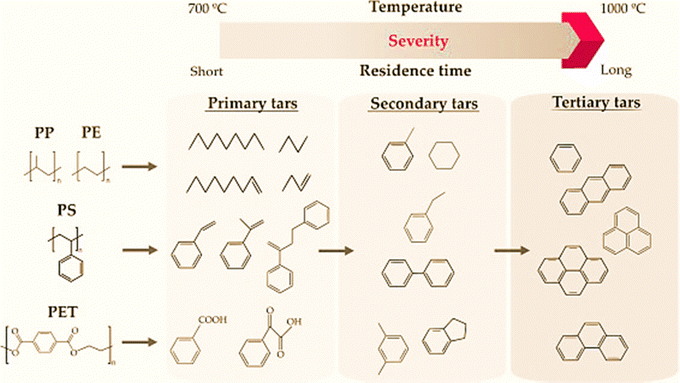 | ||
| Fig. 7 Tar formation and evolution pathways in the gasification of plastics of different nature (reprinted with permission from ref. 3 Copyright 2018 Elsevier). | ||
2.4 Char formation and cracking
Char is the solid residue after gases and tar have been generated from a carbonaceous material during devolatilization or pyrolysis. Many researchers observed a low char yield in the gasifier could catalytically improve reforming reactions for H2 production and may reduce the formation of tar from plastic degradation, due to common plastics which can be almost completely converted into volatiles.63,64 In contrast, a considerable char yield can get in complex plastic wastes or plastic co-gasification with other feedstocks (biomass, coal, etc.).65,66 Therefore, char composition may still be a controlling step when the plastic ratio in the feedstock is low.67,68According to the reactions in Section 3.2, the presence of H2O or CO2 can convert char to H2 and CO, improving the gas yield. Wang et al. investigated CO2-assisted gasification of polyethylene terephthalate (PET).69Fig. 8(a)–(d) showed the morphology of solid residues (i.e. char) collected after reactions at different temperatures. It was observed that during the devolatilization in a CO2 environment, pore structure developed with increasing temperature.
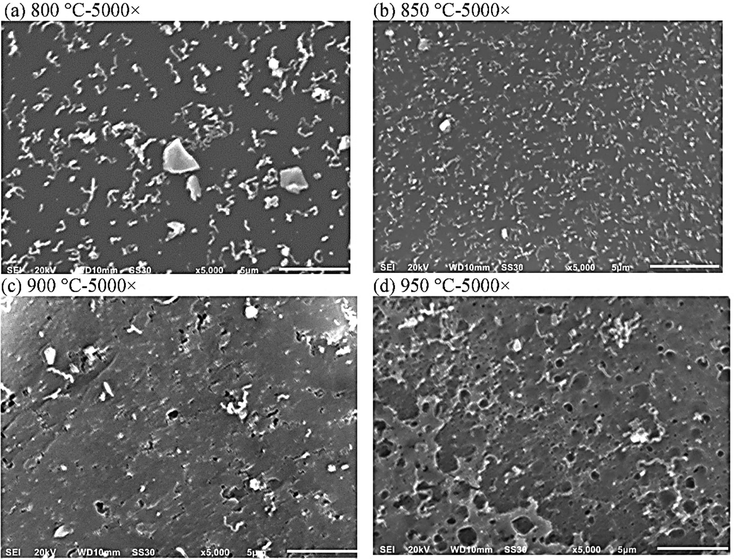 | ||
| Fig. 8 The morphologies of solid residues of PET gasification with CO2 at different temperatures: (a) 880 °C, (b) 850 °C, (c) 900 °C, and (d) 950 °C (reprinted with permission from ref. 69 Copyright 2020 Elsevier). | ||
Moreover, the development of pores on char surface increased the contact with CO2 and subsequently enhanced the Boudouard reaction for CO production. Comparable fine and cavity structures of char were also reported from the CO2/char gasification reactions at high temperatures.70–72 The micropore morphology of chars was found to be critical to the kinetics of gasification reactions. CO2 diffusion through micropores dominated the gasification rate of char.73 It was found that the ratio of the gasification rate to the surface area remained constant with conversion, indicating that the gasification process was controlled by micropores, which contributed to the majority of the BET surface area.74 Therefore, the gasification activity of char also increased with BET surface area.75 As shown in Fig. 9, the surface area (BET) and total pore volume of char increased significantly when gasification temperature increased from 800 °C to 950 °C.76 However, the yield of char decreased along with an increasing gasification temperature because of the consumption of char in the Boudouard reaction.
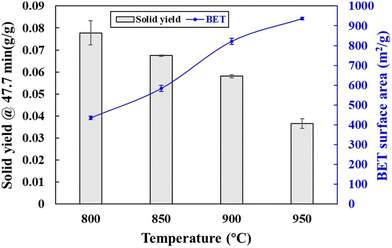 | ||
| Fig. 9 Yield and surface area of char from PET gasification with CO2 at different reaction temperatures (reprinted with permission from ref. 69 Copyright 2020 Elsevier). | ||
In addition, in the presence of CO2, gasification reactions were improved because carbon atoms inside micropores of char reacted with CO2, as described by the Boudouard reaction, resulting in the further development of micropores.77 The char made from PET has a high density, good conductivity, and little ash content, which makes PET suitable for co-gasification with coals.78
3. Effects of operating conditions on H2 production
Taking account of the mechanisms discussed above, process parameters such as plastic type, temperature, equivalence ratio (ER), and catalyst types, are very important in plastics gasification since they directly affect the product yield and gasification performance. The section below discusses the effect of general operating conditions on H2 or syngas production.3.1 Plastic composition and characteristics
Understanding the composition and characteristics of plastics is essential for optimizing gasification parameters and product yields. Table 1 shows the proximate and ultimate analysis of conventional plastics. All plastic polymers have high volatile and low moisture contents.| Plastics | Proximate analysis, % | Ultimate analysis, % | Ref. | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Moisture | Volatile | Ash | Fixed carbon | C | H | N | S | O | ||
| PP | 0.18 | 96.7 | 2 | 1.3 | 85 | 13.5 | 0.06 | 0.03 | 0.2 | 79 |
| PVC | 0.17 | 96.4 | 0 | 3.42 | 38.2 | 4.94 | — | — | — | 79 and 80 |
| PET | 0.61 | 91 | 0.02 | 13 | 65 | 5 | 0.05 | 0.01 | 32 | 80 |
| PS | 0.30 | 99 | 0 | 1 | 90 | 9 | 0.07 | 0.01 | 0 | 79 and 80 |
| LDPE | 0.3 | 99 | 0.4 | 0 | 81 | 13 | 0.07 | 0.02 | 0.2 | 80 |
| PU | — | 83.5 | 6.2 | 10.6 | 62.3 | 6.3 | 6.4 | 0.6 | 24 | 79 |
| HDPE | 0 | 99 | 1.4 | 0.03 | 84 | 14 | 0.08 | 0.02 | 0.3 | 79 and 80 |
Fig. 10(b) shows degradation analysis of conventional plastic waste and mixed plastic waste at a heating rate of 20 °C min−1.30 The peak degradation rate of PS, PET, PP, HDPE, and mixture is approximately at 420, 450, 470, 480, and 460 °C, respectively. The mixed plastic waste degrades at a lower temperature than some of the individual plastic (Fig. 10(a)), plastics due to the synergistic effect.30 Honus et al. assessed the fuel characteristics of pyrolysis gases generated from 8 common plastics (PET, PP, PE, PVC, PS, and 3 mix plastics).81 They found PVC generated the highest content of H2 in the gas (53.6 vol% on average) at 900 °C.82 Williams et al. investigated the pyrolysis of HDPE, LDPE, PP, PS, PVC, and PET individually in a fixed-bed reactor heated at 25 °C min−1 to a final temperature of 700 °C. The results showed that three poly-alkene plastics, HDPE, LDPE, and PP, behave quite similarly in the main gas products and PET generated the highest H2 in the syngas. The authors found the main content of the gas is HCl during PVC pyrolysis.83
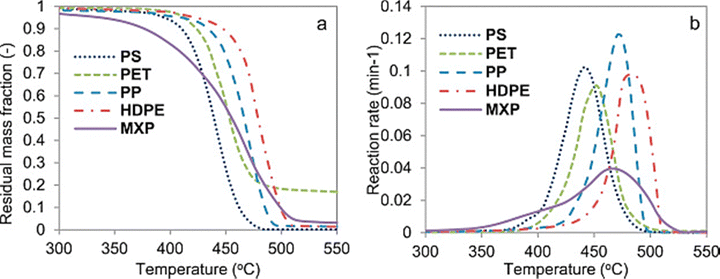 | ||
| Fig. 10 (a) the plot of residual mass fraction vs. temperature curve and (b) the plot of reaction rate vs. temperature curve at 20 °C min−1 for individual and mixed plastic waste (reprinted with permission from ref. 30 Copyright 2019 Elsevier). | ||
The properties of different plastics also influence the product distribution during co-pyrolysis. Özsin et al. investigated co-pyrolysis of biomass with different types of plastics and found PET and PS caused a synergetic effect with biomass to increase yields substantially.84 Chen et al. investigated co-pyrolysis of microalgae Dunaliella salina (DS) with four plastics (PP, PS, PET, PVC).85 They found the solid residue of DS–PP, DS–PS, and DS–PET blends were reduced, due to the hydrogenation reaction between the unsaturated products generated from biomass and the products from plastics. While the solid residue of DS–PVC was increased by 1.36 wt%. In addition, the decomposition of PET and PVC in the blends was accelerated in the co-pyrolysis process, but PP and PS in the blends showed the opposite trend. The presence of polyurethane (PU), PP, PE, PP, and PS increases liquid product yield, while PVC increases the solid product yield.
3.2 Operating temperature
Reaction temperature plays an important role in gasification because plastic gasification is an endothermic process. According to Le Chatelier's Equilibrium Law, high temperature favors endothermic reactions (see the formulas in Section 2.2. Eqn (1)–(5)) and promotes steam gasification of hydrocarbon/char, methane reforming, and the Boudouard reaction. Moreover, the water–gas shift reaction will be suppressed. Shang et al.86 investigated the co-gasification of biomass and plastics with different catalysts and found similar trends in the gas composition regardless of which catalyst was used. As shown in Fig. 11, the contents of H2 and CO increased gradually with the increase of reaction temperature while the contents of CH4 and CO2 decreased. At the same time, in case of no catalyst, CO increased from 26.32 vol% to 34.34 vol%, a 30.47% relative increase. The primary reason for this phenomenon is that the cracking of cycloalkanes and the breaking of macromolecules gradually increased with the increase of temperature. The same trend of this temperature effect was found by Yang et al.,87 and was attributed to higher temperatures providing more favorable conditions for thermal cracking of hydrocarbons.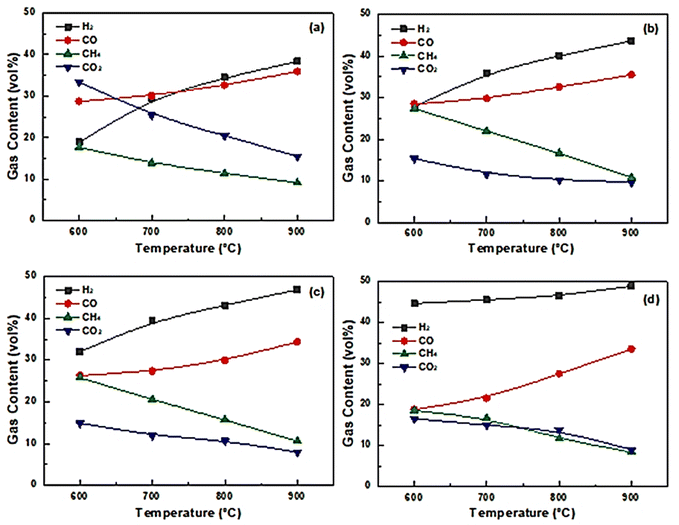 | ||
| Fig. 11 Influence of reactor temperature on gas composition: (a) no catalyst, (b) Ni/CSC, (c) NiFe/CSC, and (d) Ni–Fe–La/CSC (reprinted with permission from ref. 86 the bioresources (CC-BY 4)). | ||
The temperature effect on gas composition led to similar trends, though different values, as indicated in Fig. 12. The increase in temperature favored the production of hydrogen and further cracking of hydrocarbons, as indicated by a reduction in CnHm contents in gas products. A slight decrease in CO2 with a rise of temperature was also found, which may be due to the consumption of CO2 by the dry reforming reaction of CH4, light hydrocarbons, tars, and/or biomass.88 Although the water–gas shift (WGS) reaction produced CO2, Gil et al.89 indicated that CO2-consuming reactions would be more effective than shift reactions when temperature increased.
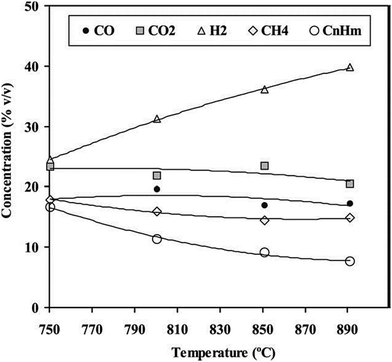 | ||
| Fig. 12 Effect of bed temperature on gas composition for co-gasification of 60% (w/w) of coal mixed with 20% (w/w) of pine and 20% (w/w) of PE wastes. Other operational conditions: steam feeding rate-5 kg h−1, feedstock feeding rate-5 kg daf per h, air feeding rate-4.4 kg h−1 (reprinted with permission from ref. 90 Copyright 2003 Elsevier). | ||
3.3 Gasifying agents
The gasifying agent is a critical factor that affects the efficiency of the gasification process (composition of syngas and the yield of hydrogen). Selection of an optimal gasifying agent is important, as each introduces distinctive chemical reactions that affect the gasification process by influencing syngas yield, tar formation, and energy efficiency.Air gasification of plastic waste produces syngas with varying compositions and hydrogen yields depending on the plastic type and process conditions. For polyolefins like polyethylene and polypropylene, hydrogen yields can reach up to 26 vol%. Plastics containing oxygen in their structure increase CO production, while those with aromatic rings generate more char and tar.91 Mixed plastic waste typically yields lower hydrogen (92.81–122.6 mmol g−1 plastic) compared to single polyolefin plastics. Overall, air gasification of plastics can produce hydrogen yields ranging from 15.33 to 284.40 N m3 ton−1 feed.92,93 Kaewpengkrow et al. (2012) studied a fixed (packed) bed reactor at 700–900 °C with an equivalence ratio (ER) of 0.4, yielding gas compositions of 0.2–4% CO, 0–2% H2, 21–20% CH4, and 5–7% CO2, and a tar yield of 12–18 g m−3. Lee et al. (2013) examined a moving grate reactor fueled by pure O2 at similar temperatures (700–900 °C) and ER (0.15–0.6), resulting in more favorable gas compositions: 22–33% CO, 41–29% H2, 4.3–10% CH4, and 8.2–22% CO2. The gas yield was 1.2–1.5 m3 kg−1. Further research continues to refine these parameters for more consistent results and cleaner syngas production.94,95 Steam gasification of plastics involves two main stages: thermal depolymerization of plastics followed by steam reforming reactions. The steam gasification of PE, PP, PC, and PET revealed that higher steam-to-plastic ratios (SPR) and temperatures enhanced hydrogen production, with PE and PP yielding significantly more H2 than PC and PET. CO production increased as SPR decreased and with higher temperatures and moisture content. Notably, SPR was the most influential factor on H2 production, while temperature and plastic type also played significant roles.96
The effect of the gasifying agent on the co-gasification of rice husk blended with polyethylene (PE) waste at 850 °C is shown in Fig. 13(a) and (b).97 In Fig. 13(a), the highest gas yield is observed with air-steam mixtures, especially at ER = 0.3, while pure steam results in the lowest gas yield. Tar formation decreases with higher ER and the addition of oxygen, but the gas HHV increases with oxygen and steam mixtures due to reduced nitrogen dilution. In Fig. 13(b), the comparison between air and steam as gasification agents reveals distinct differences in gas composition. Using only air favors partial oxidation reactions, leading to higher concentrations of CO and CO2 due to the availability of oxygen, which enhances carbon oxidation. Conversely, using only steam promotes steam reforming reactions, resulting in a higher H2 concentration, as water vapor contributes to hydrogen production. The reduction in CH4 and CnHm concentrations under steam gasification indicates a shift toward hydrogen-rich syngas, whereas air gasification produces CO-rich syngas with lower H2 yields.
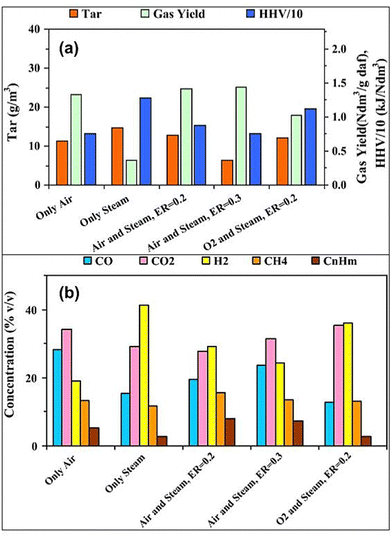 | ||
| Fig. 13 Effect of co-gasification conditions on the co-gasification of rice husk blended with 20% (w/w) polyethylene (PE): (a) tar, gas HHV and syngas yield (b) syngas composition (reprinted with permission from ref. 97 Copyright 2016 Elsevier). | ||
Oxygen gasification of plastics, compared to air gasification, produces syngas with significantly higher hydrogen (H2) and carbon monoxide (CO) content due to the absence of nitrogen dilution, which is present in air gasification. In oxygen gasification, polyethylene (PE) produces higher methane (CH4) concentrations due to the fast cracking of plastics. Gasification with pure O2 is an alternative to air and steam that combines the benefits of both gasifying agents.
Gasification of plastic waste using CO2 has emerged as an effective method for both waste management and CO2 utilization. Studies show that CO2 can act as an efficient gasifying agent for plastics like polyethylene and polypropylene. This process generates syngas with a high CO content, and the use of CO2 and steam mixtures enables control over the H2/CO ratio. Operating conditions such as CO2-feed ratio, residence time, and temperature significantly influence syngas yield and quality. CO2 gasification of plastic waste shows promise for syngas production and waste management.98 Wang et al. (2020) reported increased CO yields (0.5–0.9 g g−1) from PET gasification at 800–950 °C, while simultaneously converting CO2 to valuable syngas.69 Saad and Williams (2016) investigated catalytic dry reforming of mixed plastic wastes, reporting the highest syngas yield (153.67 mmol g−1) from agricultural plastic waste using Ni/Al2O3 catalysts. These studies highlight the potential of CO2 gasification for efficient energy production from plastic wastes while utilizing CO2.99
Recently, several groups investigated the technology of CO2-assisted gasification of plastics.36,40,100–102 Wang et al. studied pathways for PP gasification on a Ni/Al2O3 catalyst at different positions in the presence of CO2 (Fig. 14).
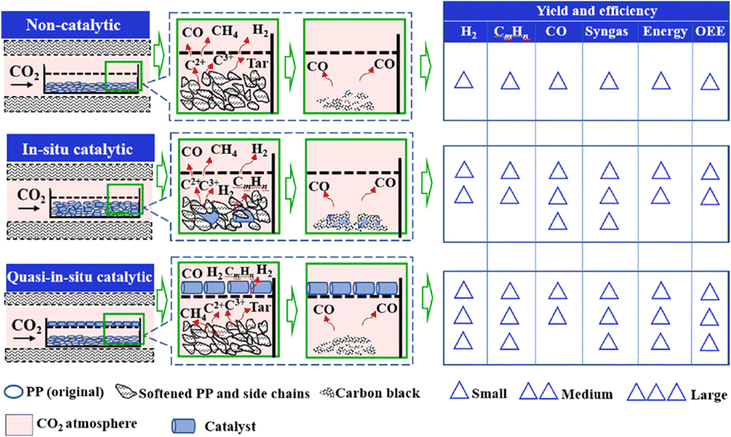 | ||
| Fig. 14 Comparison of yield and efficiency of CO2-assisted gasification of PP among the different catalytic methods (reprinted with permission from ref. 103 Copyright 2021 Elsevier). | ||
Despite these advantages, CO2 gasification typically requires higher temperatures and external energy input, like steam gasification. Moreover, it produces a lower hydrogen yield than steam gasification, making it less favorable for hydrogen-centric applications. Supercritical water gasification (SCWG), a novel process with strong potential for generating hydrogen-rich syngas from biomass and plastics. SCWG operates under supercritical conditions, with temperatures above 374 °C and pressures exceeding 22 MPa, enabling the efficient breakdown of polymers into hydrogen without the need for drying. As a result, SCWG is particularly effective for processing feedstocks with high moisture content, such as sewage sludge and municipal solid waste.104 While SCWG produces high hydrogen yields, its commercial application is still limited due to the high equipment costs associated with operating under supercritical conditions. The method is still in the developmental stage but holds promise for future large-scale hydrogen production (Table 2).
| Gasifying agent | Advantages | Disadvantages |
|---|---|---|
| Air | • Low cost | • Low hydrogen yield |
| • Supports partial oxidation reactions | • Low H2/CO ratio | |
| • HHV (4–6 MJ N−1 m−3) | • Coking reactions can lead to operational issues | |
| Oxygen | • Promotes partial oxidation reactions | • Low H2/CO ratio |
| • Reduced tar formation | • Higher operational costs due to oxygen production | |
| • HHV (10–12 MJ N−1 m−3) | ||
| Steam | • Facilitates steam reforming reactions | • Requires external energy |
| • Highest H2/CO ratio (∼2.0>) | • High operational costs due to steam generation | |
| • HHV (12–18 MJ N−1 m−3) | ||
| CO2 (carbon dioxide) | • Utilizes CO2 recycling | • Low H2/CO ratio |
| • Supports Boudouard reactions | • Requires external energy | |
| • HHV (7–11 MJ N−1 m−3) | • Requires high temperatures (typically >800 °C) | |
3.4 Effect of steam/carbon (molar) ratio (S/C)
The presence of steam increased the production of hydrogen and reduced the tar concentration, promoting both the hydrocarbons reforming and the water gas shift reactions. Many researchers found an increase in the S/C ratio favored both reforming and WGS reactions for H2 production when S/C ratios are less than 4.105–108 The increase of S/C promotes the reforming reaction conversion at high temperatures. Besides, a high S/C ratio would promote tar and coke cracking, especially in a fixed-bed reactor.109 When plastic gasification happens in a fluidized bed, coking was not observed for S/C ratios higher than 3. Further increase in the S/C ratio would not lead to a higher H2 yield, because an excessive amount of H2O cannot improve the reforming reaction.110 The influence of S/C ratio on H2 yield is also applied to the co-gasification of plastics with other feedstocks.110,111 Pinto et al. studied the S/C ratio effect on the co-gasification study of plastic wastes with biomass.112 They controlled the steam flow rate to ensure a constant residence time in all tests. With S/C increasing from 0.5 to 0.8, an increase of the H2 concentration occurs reaching a maximum at S/C = 0.75, CO, CH4, and CnHm slightly decrease, and CO2 slightly increases. They found that the influence of the S/C ratio was less pronounced than that of temperature in the cases, which is also been proved by other studies.113 In general, the presence of steam increased the production of hydrogen and reduced the tar production, promoting both the hydrocarbon reforming and the water–gas shift reactions. The effect of steam/carbon (S/C, molar) ratio on H2 production is shown in Fig. 15. As observed, an increase in the S/C ratio favored both reforming and WGS reactions for H2 production, but this effect decreased at high S/C ratios.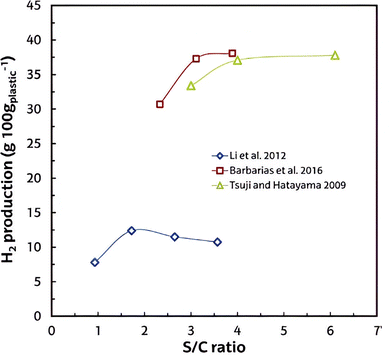 | ||
| Fig. 15 Effect of steam/carbon (molar) ratio on the hydrogen production in plastic wastes reforming process (reprinted with permission from ref. 2 Frontiers 2023 (CC-BY 4)). | ||
3.5 Equivalence ratio (ER)
Air gasification is a good alternative to produce a gas stream suitable for different energy applications.22,114 The ER is the ratio between the actual fuel/air to the stoichiometric fuel/air ratio. It is more important in gasification, especially in air gasification.71 Due to the air deficiency environment and its fraction of the total stoichiometric air in contrast to conventional combustion processes, ER is a key parameter to determine syngas quality and the performance of gasification systems.According to the previous studies, ER is required to be in the range of 0.2 and 0.4 for better yields.37,74 A higher ER value will promote the tar cracking by O2, increase gasifier temperature, and increase syngas yields.115 However, it also increases the opportunities for contact between O2 and H2 and causes H2 combustion, which may reduce the H2 ratio in the final product. Additionally, the ratio of N2 also increases, which reduces the heating value of the syngas product and increases the energy requirement for H2 purification. Aznar et al. studied the gasification of different types of plastic by varying the ER ratio from 0.3 to 0.48.116 They found all the gas components (H2, CO, CO2, CH4, C2Hn) slightly decreased (where H2 ratio reduced from 15.3 vol% to 14.8 vol%), due to a dilution effect by the increase of N2 content. However, the char composition in the product distribution significantly dropped from 345 g kg−1 to 150 g kg−1. Xiao et al.77 analyzed the effect of ER in the air gasification of PP in a bubbling fluidized bed gasifier. The increase in ER from 0.2 to 0.45 caused a significant increase in the gasifier temperature from 703 to 915 °C and the gas yield increased from 76.1 to 94.4 wt%. Similar trends of gas yield and gasifier temperature are also found by other researchers.78,117 Kim et al. studied the ER effect on gas production in the gasification of mixed plastic using activated carbon and dolomite as a catalyst. The H2 ratio decreased from 14.5 to 14 vol% with the increase of ER from 0.21 to 0.61.39 Furthermore, some research found that the H2 yield can be enhanced by adjusting ER in an optimal range. Ruoppolo et al. studied biomass/plastic co-gasification for H2 production and found the H2 decreased from 33 vol% to 18 vol%, and CO2 increased from 16 vol% to 18 vol% with the ER increases from 0.12 to 0.19.39 However, the CO2 amount decreased and H2 amount increased when ER increased from 0.19 to 0.3. Cho et al. performed the gasification of mixed plastics at ER from 0.21 to 0.41.118 The H2 ratio did not change significantly when ER increased from 0.21 to 0.29 and then sharply decreased when further increased ER to 0.41. Considering the complexity of plastic types and composition in the plastic wastes, ER should be carefully adjusted based on the actual H2 yield in each case.
3.6 Catalysts
Catalysts are widely used to enhance the efficiency of plastic gasification.119 Based on the above-mentioned mechanisms of plastic gasification (see Section 2), the roles of catalysts in plastic gasification can be attributed to two aspects: (a) tar cracking and (b) gas reforming. Catalysts can be either directly mixed with plastics for tar cracking (primary catalysts) or placed in a secondary reactor downstream from the gasifier for gas reforming (secondary catalysts).120,121 Considering the function of catalysts, two types of catalyst are widely used in the plastics gasification process – (a) mineral catalysts like dolomite, limestone, etc., and (b) transition metal catalysts such as Ni and supported-Ni materials.Dolomite, a magnesium ore with the general formula MgCO3·CaCO3, is used in the Pidgeon process for the manufacture of magnesium by thermal reduction.122,123 The chemical composition of dolomite varies from source to source, but it generally contains 30 wt% CaO, 21 wt% MgO and 45 wt% CO2; it also contains the trace minerals SiO2, Fe2O3, and Al2O3. Dolomite is a suitable catalyst that can significantly reduce the tar content and increase gas yields from a gasifier.124,125 Therefore, the main function of dolomite is to act as a guard bed for the removal of heavy hydrocarbons before the reforming of the lighter hydrocarbons to produce a product gas of syngas quality. Dolomite activity can be directly related to the pore size and distribution.126 Higher activity is also observed when iron oxide is present in significant amounts.127 The catalyst is most active if calcined and placed downstream of the gasifier in a fluidized bed at temperatures above 800 °C.128
Dolomite is a cheap, disposable catalyst that can significantly reduce the tar content of the product gas from a gasifier. It may be used as a primary catalyst, dry-mixed with the biomass, or more commonly, in a downstream reactor, in which case it is often referred to as a guard bed. Dolomites increase gas yields at the expense of liquid products. With suitable ratios of biomass feed to oxidant, almost 100% elimination of tars can be achieved. The dolomite catalyst deactivates due to carbon deposition and attrition; however, dolomite is inexpensive and easily replaced. The reforming reaction of tars over dolomite occurs at a higher rate with carbon dioxide than steam. Dolomite activity can be directly related to pore size and distribution. Higher activity is also observed when iron oxide is present in significant amounts. Dolomites are not active for reforming the methane present in the product gas and hence they are not suitable catalysts if syngas is required. Biochar and refuse-derived fuel (RDF) char were tested as catalysts for steam-reforming plastic waste volatiles, resulting in hydrogen-rich syngas. The study discovered that RDF char has a larger hydrogen potential than biochar at 1000 °C due to its higher presence of catalytic inorganic metals. RDF char also has a high catalytic activity for reforming oxygen molecules and aromatic rings in PET and PS pyrolysis volatiles. Polyolefin polymers (HDPE, LDPE, and PP) have hydrogen potentials ranging from 60–62%, whereas polystyrene had 53% and PET had 38%.129
Tar reforming mechanisms help understand how tar breaks down during gasification. Fig. 16 illustrates the tar reforming mechanisms during co-gasification over biochar, including adsorption, dehydrogenation, soot production, and gasification. The process involves tar molecules being adsorbed onto biochar, and undergoing dehydrogenation to form soot, which is then gasified to produce additional gases like CO and H2.130
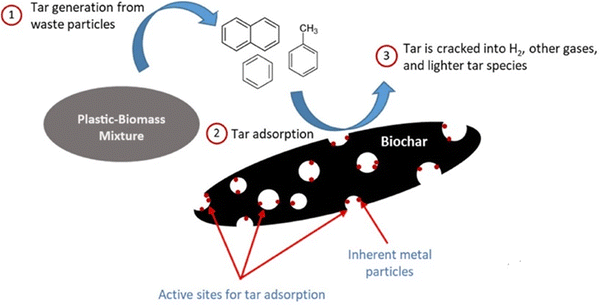 | ||
| Fig. 16 Mechanisms of tar reforming on biochar during gasification (reprinted with permission from ref. 130 Elsevier 2020 (CC-BY 4)). | ||
Nickel-based catalysts have been widely investigated in plastic gasification for promoting H2 production.131,132 The major function of nickel-based catalysts is the adjustment of the gas composition after raw gas cleaning by a dolomite or alkali catalyst. Nickel can also be coupled with alkali metal materials or dolomites as multifunctional catalysts. To maximize the H2 content in the final gas products and reduce the hydrocarbon and methane content, these catalysts are used at a temperature higher than 780 °C. Alipour et al. performed the steam co-gasification of HDPE and coconut shells using Ni as a primary catalyst and CaO as a secondary catalyst. Higher H2 production was obtained at about 50 vol% even at a lower temperature of 650 °C.133 H2 fraction can be further increased to 81.6 vol% at a temperature of 800 °C. The higher H2 yield, even at low temperatures, was due to the use of the Ni catalyst in the steam methane reforming reaction.134 He et al. studied PE gasification of PE with 0.3 kg h−1 feed rate in a fixed bed reactor between 700 and 900 °C using Ni–Al2O3 catalyst. The results show the gas yield reaching 2.04 m3 kg−1 at 900 °C and H2 concentration is about 37 wt%.135 Friengfung et al. studied plastics gasification using dolomite and Ni/dolomite catalysts.136 The experimental results showed the tar yields being higher than 80 wt% without a catalyst. The tar yields of gasification reduced to 50 wt% when using dolomite as catalysts. The tar yields can further decrease to below 10 wt% when using Ni/dolomite catalysts. Similar performance was also observed in recent findings.137 Farooq et al. studied the effect of catalysts on selectivity and yields of gas products of LDPE gasification.138 As shown in Fig. 17, a remarkable increase in the selectivity and yield of H2 was observed at 800 °C for Ni/CeO2–ZrO2 over non-catalytic gasification. Moreover, the low yield of CO indicated a near-complete water gas shift reaction. The oxygen-deficient sites in Ce1−x–ZrxO2−δ were likely to be active sites for the WGS reaction. In addition, as described in steam reforming, the activation of the O–H bond of water and the subsequent reaction of water with CO generated in the gasification reaction led to more hydrogen production. Better dispersion of Ni in these supported catalysts improved the selectivity toward hydrogen products. No coke residue was reported for all catalysts, indicating the enhancement of carbon into CO/CO2/C1−4 during the catalytic gasification process.
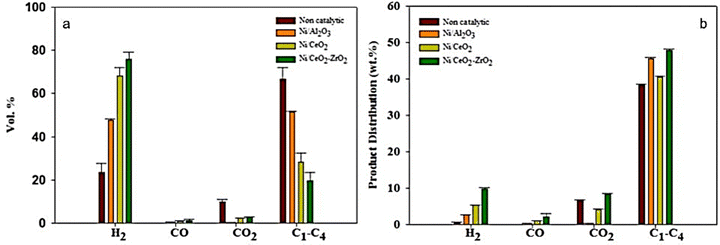 | ||
| Fig. 17 Effect of Ni/CeO2–ZrO2 catalyst on (a) gas selectivity (vol%) and (b) product distribution (wt%) (reprinted with permission from ref. 138 Copyright 2021 Elsevier). | ||
Chai et al. investigated the gas production and yield by changing Ni loading from 0 to 20 wt%.139 As shown in Fig. 18, in the non-catalytic case, H2 composition and yield were only 35.73 mol% and 3.93 mmol g−1, respectively. By using a supported Ni (5 wt%) catalyst, H2 production increased to 85.68 mol%, but H2 yield was still at a relatively low level of 29.35 mmol g−1. The highest H2 content and yield were both achieved with a catalyst of 10 wt% of Ni. However, the continuous increase of Ni loading failed to promote H2 production and yield.
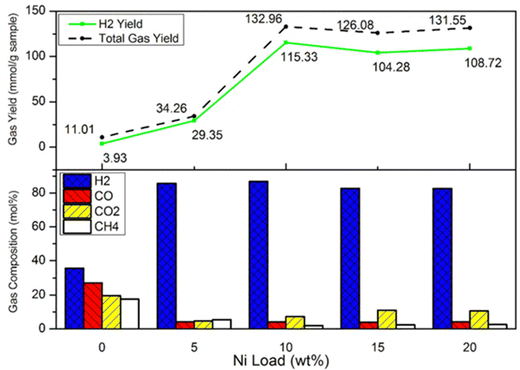 | ||
Fig. 18 Gas compositions and yields when changing Ni load from 0 wt% to 20 wt% (when Ni load from 5 to 20 wt%: with all CaO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C = 5 C = 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, biomass 5, biomass![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) plastic = 5 plastic = 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, pyrolysis T: 700 °C, reforming T: 600 °C, water: 5 mL h−1) *total gas yield is the sum of H2, CO2, CO and C (reprinted with permission from ref. 140 Copyright 2020 Elsevier). 5, pyrolysis T: 700 °C, reforming T: 600 °C, water: 5 mL h−1) *total gas yield is the sum of H2, CO2, CO and C (reprinted with permission from ref. 140 Copyright 2020 Elsevier). | ||
The Ni-based catalyst suffered deactivation due to carbon deposition and nickel particle growth. The deactivation can be reduced by the introduction of a guard bed of dolomite.141 Besides, the addition of dopants such as lanthanum can also reduce carbon deposition.142,143
Bimetallic and non-nickel-based catalysts have received attention in recent studies due to their potential catalytic performance in pyrolysis and steam reforming. While nickel has traditionally been preferred for its high activity and cost efficiency, transition metals such as Fe, Co, and Cu, and noble metals such as Rh, Pt, Pd, and Ru have shown significant effectiveness in these processes. The introduction of these metals into bimetallic systems can improve catalytic activity and coking resistance, resulting in more effective conversion of plastic waste. Table 3 presents a comparison of recent advancements and performance metrics for nickel, non-nickel, and bimetallic catalysts in plastic waste gasification.
| Catalyst | Feed | Reactor configuration | Operating conditions (°C) | H2 concentration (vol%) | H2 production (wt%) | Ref. |
|---|---|---|---|---|---|---|
| Note: TP = temperature of pyrolysis; TR = temperature of reforming. | ||||||
| Ni/Al2O3 | PP | Fluidized/fluidized | TP = 600, TR = 850 | 70 | 34 | 144 |
| 11Ni/Al2O3 | HDPE | Spouted/fixed | TP = 500, TR = 700 | 71 | 34.5 | 145 |
| 11NiAl2O3 | HDPE | Spouted/fluidized | TP = 500, TR = 700 | 72.7 | 38.1 | 108 |
| 4.4Ru/Al2O3 | PS | Fixed/fixed | TP = 400–600, TR = 580–680 | 68.2 | 33 | 146 |
| 0.5Ru/Al2O3 | PP | Fixed/fixed | TP = 400–550, TR = 630 | 54 | 4.5 | 147 |
| 5Ru/Al2O3 | PP | Fixed/fixed | TP = 400–600, TR = 580–680 | 69.8 | 36.5 | 147 |
NiCuAl 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
PP | Fixed/fixed | TP = 500, TR = 800 | 61.1 | 18.9 | 148 |
| NiCuMgAl | PP | Fixed/fixed | TP = 500, TR = 800 | 62.2 | 20.4 | 148 |
| 20Fe/ZrO2 | PS | Fixed/fixed | TP = 500 | 81.5 | 2.6 | 149 |
Zhou et al. investigated the steam reforming of PS volatiles using Ni–Fe bimetallic catalysts supported on ZrO2 in a fixed-bed reactor. Their results indicated that the bimetallic Ni–Fe/ZrO2 catalyst produced 8.6 wt% hydrogen for the 15Ni5Fe/ZrO2 configuration, significantly outperforming the monometallic Fe/ZrO2 catalyst, which achieved a hydrogen production of only 2.6 wt%.149 Park et al. studied the two-step pyrolysis-reforming process of polypropylene (PP) using a fixed-bed reactor and Ru/Al2O3 catalyst (0.5 and 5 wt%). The 5Ru/Al2O3 catalyst achieved a maximum hydrogen production of 36.5 wt% during pyrolysis at 400 °C and reforming at 680 °C, with a steam-to-carbon ratio of 3.7. When polystyrene (PS) was used as a feedstock, the hydrogen production reduced to 33.0 wt%.147
4. Potential routes for low-carbon hydrogen production via gasification
Potential strategies for generating low-carbon hydrogen via gasification include the integration of CCUS techniques with conventional hydrogen production reliant on fossil fuels. CCUS systems effectively capture CO2 produced during the gasification process, decreasing their environmental impact. Co-gasification of plastics and biomass is another sustainable way to use waste materials while lowering reliance on fossil fuels. These strategies have the potential to significantly improve low-carbon hydrogen production and contribute in the transition to a more sustainable energy system.4.1 Integration of carbon capture, utilization and storage
Carbon capture involves collecting CO2 from industrial production to minimize greenhouse gas emissions. Captured CO2 can be stored for a long time, known as carbon capture and storage (CCS). Carbon capture methods include adsorption, absorption, membrane separation, and cryogenic separation. Captured CO2 can also be used in industries through carbon capture and utilization (CCU), either directly in applications like enhanced oil recovery or indirectly by converting it into other chemicals using processes like electrolysis, as CO2 can be transformed into methanol, a valuable industrial chemical. Integrating plastic waste conversion to hydrogen production with CCS provides an economical advantage in reducing carbon emissions. This approach not only provides a way for producing low-carbon hydrogen but also helps mitigate climate change by capturing and storing CO2.139,150 For the development and use of plastic recycling technologies on a large scale, it is critical to fully understand the environmental impact, economic viability, and regulatory incentives of this strategy.In a recent study, an Aspen model incorporates both mixed and single-use plastics and covers five major components of the hydrogen production plant: feedstock handling, gasification, hydrogen purification, combined heat, and power (CHP) generation, and utilities. The analysis finds that the minimal hydrogen selling price of the modeled plant is competitive with fossil fuel hydrogen and existing electrolysis hydrogen. Furthermore, the life cycle study shows that hydrogen from mixed plastic waste has a lesser environmental effect than single-stream plastics.151 The combination of pyrolysis/gasification with CCU to recycle CO2, results in increased gas output and lower CO2 emissions. The use of CCU in pyrolysis/gasification reduces CO2 generation while increasing CO output, the CO2 compositions in the gas products decrease from 24.41 mol% to 13.15 mol% after 90% CO2 is captured.152 Chari et al. developed the Aspen model to analyze the performance of gasifying non-recyclable mixed plastic waste (MPW) to produce hydrogen, with CCS to achieve low-carbon hydrogen production. It identifies hydrogen production from plastic waste as a key in moving towards economical low-carbon hydrogen production and reaching net-zero goals.153 The environmental sustainability assessment study of plastic waste-to-hydrogen production, coupled with CCS, concluded that this process can reduce the climate change impact compared to fossil-based and most electrolytic routes of hydrogen production.154 Xu studied the gasification of mixed plastic waste (MPW) in a conventional integrated gasification combined cycle (IGCC) system, focusing on three different designs. Modifications in design, such as using vacuum pressure swing adsorption (VPSA) for oxygen production and incorporating a calcium-looping (CaL) reactions, improve energy efficiency and carbon capture abilities. The use of VPSA decreases energy usage for oxygen production by 42.14% and boosts net power output by 15.99% compared to traditional methods. Additionally, including the CaL process in Design 3 leads to a carbon capture rate of 84.43% and a high carbon gasification efficiency (CGE) of 50.53%, highlighting the potential for sustainable conversion of plastic waste and reduction of emissions.155 Rosha et al. studied a municipal solid waste (MSW) gasification system with CCS and identified the temperature, pressure, and equivalency ratio are important variables in determining syngas composition. A maximum hydrogen percentage of 42.1% reached by the simulation model, suggesting that MSW can produce hydrogen. Monoethanolamine (MEA) utilized as a solvent for CO2 capture, confirming its effectiveness in the process. The PR-BM thermodynamic model was used to improve the efficiency of the CO2 capture process.156
The US energy association report emphasizes the critical role of CCUS in recycling plastics and reducing their carbon footprint, highlighting the application of carbon capture to gasification processes. It also outlines the potential for commercial-scale implementation of plastic waste gasification and coal co-utilization, aiming to generate interest in more sustainable end-of-life solutions for plastic waste.157 Ma Y. et al. explore a plasma gasification process for converting end-of-life tires (ELTs) into syngas and hydrogen, offering a sustainable disposal method. Results show that using steam and air as gasification agents can achieve high carbon conversion rates, energy recovery rates, and exergy efficiencies, reaching up to 99.12%, 93.67%, and 80.04%, respectively. Additionally, the study compares carbon capture methods, finding that monoethanolamine absorption has a better CO2 capture rate than Rectisol but lower exergy efficiency. The integrated process with steam and air as gasification agents and Rectisol for carbon capture achieves a total exergy efficiency of 36.45%, providing valuable insights for efficient hydrogen production from ELTs.158 Ravi K. et al. study proposes a CCUS-coupled co-gasification process for plastics as a potential negative emission recycling technology, offering a roadmap for commercial implementation, and highlighting the value proposition of producing hydrogen and synthesis gas products from mixed plastic waste. The synergistic approach of integrating gasification and CCUS could significantly reduce greenhouse gas emissions while supporting a circular plastics economy.159 Nhuchhen R. studied waste gasification from molten carbonate fuel in the integrated gasification carbon capture plant (IGCCP) system for the cement industry. This helps increase overall efficiency by providing a sustainable fuel source, resulting in a CO2 capture rate of over 93%, achieving a net power generation efficiency of 31.1%, and providing economic benefits. The IGCCP system transforms waste into energy, decreasing carbon emissions and reliance on non-renewable resources, while tackling waste management issues, offering a cost-efficient, eco-friendly option for decarbonizing heavy industries.160 The integration of absorptive CO2 capture methods into biorefineries to produce liquid fuels through the gasification of woody biomass has been the focus of several studies. These studies emphasize the potential for such technology to reduce CO2 emissions from large-scale power facilities. Nonetheless, further study is needed to fully evaluate the techno-economic and environmental aspects of absorptive CO2 collection methods in small-scale experimental plants.
The cost of producing low-carbon hydrogen is determined by several factors, including feedstock cost, capital plant cost, process scale, and operating expenses. However, existing data is limited and characterized by wide ranges and uncertainties. Fig. 19 shows the estimated costs of hydrogen technologies with added carbon capture and storage expenses. Biomass gasification with carbon capture and storage could cost between $3 to $5 per kilowatt-hour of hydrogen, without factoring in potential benefits from negative emissions, despite limited large-scale demonstrations.
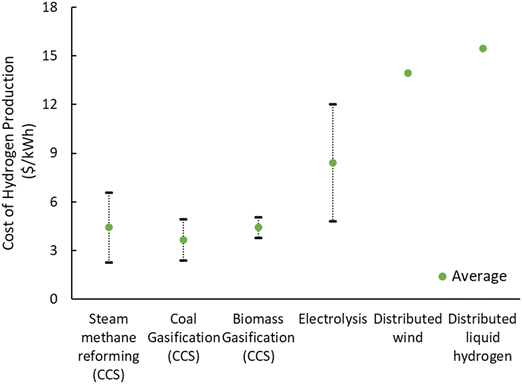 | ||
| Fig. 19 The cost of hydrogen produced from low-carbon hydrogen technologies (adapted from ref. 25 Reprinted with permission from the royal society (CC-BY 4)). | ||
4.2 Co-gasification of plastics/biomass
Biomass is renewable organic material that comes from any organic matter, that is derived from plant and animal materials, such as plants from forests, crops, seaweed, and organic industrial, human, and animal wastes. Similar to plastics, the gasification process can convert biomass into syngas, biochar, and condensable liquid compounds.161 It is considered one of the most efficient methods of converting biomass energy and handling biomass embedded in biomass, and it is becoming one of the best routes for the reuse of waste. The advantages of co-gasification of biomass with plastics have been mentioned in several studies.121,132 Biomass resources are not considered an appropriate feedstock for H2 production because of their low H/C ratio, which usually varies between 0 and 0.3. Conversely, plastic mainly consists of polyolefins, which have a higher H/C ratio than biomass. The co-gasification of biomass and plastics can balance the C, H, and O content, which promotes product quality and H2 yield.162 Additionally, polymer cracking creates numerous free radicals, which promote the decomposition of biomass and suppress the formation of long-chain hydrocarbon compounds.162Table 4 summarizes the recent studies on plastic-biomass co-gasification.| Plastics (ratio) | Biomass (ratio) | Gasifying agent | Catalysts/bed material | Reactor configuration | Temperature (°C) | S/Fc or ERb | Gas yield (m3 kg−1) | Gas compositiona (vol%) | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| a Without balance gases. b Equivalence ratio (ER). c Steam/fuel wt. ratio (S/F). | |||||||||
| PE (0.32) | Wood (0.58) | Steam/air | γ-Al2O3 | Fluidized bed | 900 | S/F: 0.42, ER: 0.14 | — | H 2 : 32, CO: 23, CO2: 23, CnHm: 15 | 163 |
| PET (0.5) | Pinewood (0.5) | N2 | — | Fixed bed | 800 | — | — | H 2 : 1.2, CO: 75.6, CO2: —, CnHm: 23.1 | 164 |
| HDPE (0.5) | Pinewood (0.5) | CO2/N2 | — | Fixed bed | 800 | — | — | H 2 : 1.8, CO: 49.6, CO2:, CnHm: 48.5 | 164 |
| PE (0.5) | Rice husk (0.5) | Water injection | Ni/γ-Al2O3 | Fixed bed | 800 | — | 1.1 | H 2 : 44, CO: 31.3, CO2: 8.1, CnHm: 16.1 | 132 |
| PE (0.2) | Wood (0.8) | Air | — | Fixed bed | 772 | ER 0.32 | — | H 2 : 38.3, CO: 34.1, CO2: 22.8, CnHm: 4.8 | 165 |
| PET (0.5) | Wood (0.5) | Air | Olivine | Fluidized bed | 725–875 | ER: 0.19–0.31 | — | H 2 : 11.5, CO: 34.9, CO2: 45.6, CnHm: 8 | 166 |
| PE (0.3) | Wood pellets (0.7) | Steam | Olivine | Fluidized bed | 850 | S/F: 1.6 | 1.9 | H 2 : 43.6, CO: 24.5, CO2: 17, CnHm: 14.9 | 167 |
| HDPE (0.4) | Pine sawdust (0.6) | Water injection | Ni–CaO–C | Fixed bed | 700 | — | — | H 2 : 52.8, CO: 23.8, CO2: 10.6, CnHm: 12.9 | 139 |
| PP (0.4) | Pine sawdust (0.6) | Water injection | Ni–CaO–C | Fixed bed | 800 | — | — | H 2 : 66.3, CO: 11.6, CO2: 17.4, CnHm: 4.7 | 139 |
| PS (0.3) | Pine sawdust (0.7) | Water injection | Ni–CaO–C | Fixed bed | 800 | — | — | H 2 : 68.1, CO: 8.8, CO2: 17.7, CnHm: 5.3 | 140 |
| PE (0.5) | Beech-wood (0.5) | N2 | Silica sand | Fluidized bed | 850 | — | — | H 2 : 32, CO: 22.9, CO2: 42, CnHm: 3.1 | 111 |
| PE (0.5) | Beech-wood (0.5) | N2 | Olivine | Fluidized bed | 850 | — | — | H 2 : 31.1, CO: 18.8, CO2: 41.5, CnHm: 8.7 | 111 |
| PE (0.5) | Beech-wood (0.5) | N2 | Na–Y zeolite | Fluidized bed | 850 | — | — | H 2 : 27.5, CO: 31.5, CO2: 33.6, CnHm: 7.4 | 111 |
| PE (0.5) | Beech-wood (0.5) | N2 | ZSM-5 zeolite | Fluidized bed | 850 | — | — | H 2 : 37.1, CO: 28.5, CO2: 29.8 CnHm: 4.7 | 111 |
| PE (0.1) | Pine wood (0.9) | Steam | — | Fluidized bed | 740–880 | S/F: 0.8 | 0.63–1.28 | H 2 : 47, CO: 33, CO2: 9, CnHm: 10 | 112 |
| PE (0.2) | Coal (0.6)/pine wood (0.2) | Steam/air | — | Fluidized bed | 740–880 | S/F: 1, air/F: 1.14 | 0.6–1.35 | H 2 : 43, CO: 18, CO2: 22, CnHm: 16 | 112 |
| HDPE (0.67) | Pine sawdust (0.33) | N2 | Ni–Fe@CNF/PCs | Fixed bed | 700 | — | — | H 2 : 59.3, CO: 10.2, CO2: 27.1 CnHm: 3.4 | 168 |
| PE (0.75) | Pinewood (0.25) | N2 | CaO/Fe2O3 | Fixed bed | 850 | — | 2.5 L g−1 | H 2 : 56.1, CO: 31.8, CO2: 4.7 CnHm: 7.5 | 110 |
| PE (0.2) | Rice husk (0.8) | Steam | — | Fluidized bed | 850 | S/F: 1 | 0.35 | H 2 : 45, CO: 16.5, CO2: 26.4, CnHm: 12.1 | 97 |
| PE (0.2) | Rice husk (0.8) | Air | — | Fluidized bed | 850 | ER: 0.2 | 1.3 | H 2 : 21.3, CO: 27, CO2: 37.1, CnHm: 14.6 | 97 |
| PE (0.2) | Rice husk (0.8) | Oxygen | — | Fluidized bed | 850 | ER: 0.2 | 1 | H 2 : 38, CO: 12, CO2: 37, CnHm: 12 | 169 |
| PS (0.3) | Palm kernel shell (0.7) | Air | — | Fixed bed | 800 | — | — | H 2 : 11.6, CO: 27.5, CO2: 30.4, CnHm: 30.4 | 170 |
Brachi et al. studied the co-gasification of olive husk with PET in a pre-pilot fluidized bed gasifier.171 The results showed that H2 production increased from 24.4 to 40.4 vol% with an increase in temperature from 650 to 860 °C. However, CH4 was reduced from 7.8 to 5.3 vol% due to the increased activity of the reforming reaction at the higher temperature.172 Cao et al. investigated the co-gasification of soda lignin with 4 plastics.173 They observed that higher temperature, longer reaction time, and lower concentration improved the gasification efficiency and hydrogen production from the co-gasification of PE/soda lignin (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). The H2 yield increased over 5 times to 57.0 mol kg−1 with an increase in temperature from 500 °C to 750 °C, and the highest H2 yield of 63.3 mol kg−1 was obtained at 700 °C when the concentration was reduced to 5 wt%. Pinto et al. also reported the similar effect of gasification temperature: the rise in gasification temperature promoted H2 production and decreased contents of tars and hydrocarbons.90
1). The H2 yield increased over 5 times to 57.0 mol kg−1 with an increase in temperature from 500 °C to 750 °C, and the highest H2 yield of 63.3 mol kg−1 was obtained at 700 °C when the concentration was reduced to 5 wt%. Pinto et al. also reported the similar effect of gasification temperature: the rise in gasification temperature promoted H2 production and decreased contents of tars and hydrocarbons.90
Cortazar et al. explored the pyrolysis and in-line oxidative steam reforming of various waste polymers and blends, along with biomass/HDPE blends, to generate hydrogen. It was found that operating under autothermal conditions can produce up to 25 wt% of hydrogen. The simulation emphasized the importance of temperature, steam/plastic ratio, and equivalency ratio in maximizing H2 production. Co-feeding oxygen into the reforming process was identified as a promising solution for addressing energy needs and catalyst deactivation challenges.174 The plastics ratio in the feedstocks has a decisive influence on the H2 production of co-gasification. Pinto and Alvarez reported that below 20 wt% of feed, the increasing plastic content could promote the H2 yield.175,176 Ahmed et al. studied co-gasification of PE and wood chips in a fixed-bed reactor at 900 °C.12 The authors found the peak values of hydrogen yield were obtained at PE percentage of 60–80%. It indicates small amounts of biomass to the PE can result in higher energy yield than that obtained from the 100% PE sample. However, other studies found that too much plastic would decrease H2 yield.177–179 Lopez investigated the content of HDPE (0–100 wt%) in the co-pyrolysis/gasification of biomass under a steam atmosphere. H2 production decreased when the HDPE content was higher than 50 wt%.87 Burra and Gupta observed a similar decrease in H2 production when the plastic content was greater than 60 wt% for three different plastics (PP, PET, and PC) in co-pyrolysis/gasification with biomass.173 Chai et al. found the optimized content of plastics was 30 wt% for PS, and 40 wt% for HDPE and PP, respectively.139
Abdelouahed et al. also studied the synergistic effect between biomass and plastics on H2 products.180 During pyrolysis/gasification, H radicals from plastics produce H2 and also promote the cracking of complicated hydrocarbons (e.g. aromatics), lighter hydrocarbons, and the formation of CO. Furthermore, H2O, as another H resource, is reformed with generated lighter hydrocarbons and CO to promote total gas yield (including H2 yield). This is consistent with the higher total gas yield and H2 yield when more biomass is in the feedstock. Chai et al. studied increasing HDPE content can promote the H2 yield due to increasing H/C. However, when greater than 40 wt% in feed, excessive HDPE starts to prevent the release of radicals from biomass, which further hinders reforming reactions to produce more H2.139
The plastic type also influences the product distribution and yield of biomass-plastics co-gasification. Cao et al. investigated the co-gasification of soda lignin with four plastics173 and concluded that the order of the efficiencies of 4 plastics was distinct; the order was: PE > PC ≈ PP > acrylonitrile–butadiene–styrene (ABS) and the addition of soda lignin improved all the gasification efficiencies. The alkali salts in soda lignin also catalyzed the reforming of CH4 and C2H6 that was generated from the decomposition of plastics, especially PE and PP, and improved hydrogen production. Wilk et al. investigated the steam co-gasification of four types of plastic with soft wood pellets. They found the H2 ratio in the syngas initially decreased and then increased with an increase in the plastic ratio in the mixture of wood and plastic from the shredder light fraction (SLF). Conversely, the H2 ratio in the gas increased with the increase of PE in the wood-PE mixture. The authors believe there are nonlinear effects that occur during the co-gasification of biomass and plastics.167
Except for co-gasification with biomass and coal, plastics are co-gasified with industrial biowastes, such as paper waste and rice waste. Until now, the co-gasification of plastics with wastes has not been fully investigated, and a handful of works are listed in Table 5. Déparrois et al. studied the CO2 co-gasification of paper and PS using a laboratory-scale tube reactor.181 When the PS ratio of 10–30% in the feedstocks, the H2, CO, CO2, product gas, and energy yield provided the highest synergy during co-pyrolysis. It ascribes to the volatiles from paper decomposition enhanced the decomposition of polystyrene and also slowed the reaction rate and thus yielded lower char contribution from polystyrene. The energy yield and product gas yield are highest at 20% plastic addition. The reason can be ascribed to the high char yields from the paper which increased the decomposition rate of PS to increase the product gas yield and energy efficiency. Ouadi et al. investigated the gasification of wastes generated from secondary fiber paper mills. The brown paper mill's rejects consist of 20 wt% mixed plastics and 80 wt% paper fibers. The producer gas composition of the reject with 20 wt% wood chips was 16.24% H2, 23.34% CO, 12.71% CO2 5.21% CH4, and 42.49% N2 (v/v%) with a higher heating value of 7.3 MJ N−1 m−3. They found that the presence of plastics in waste may agglomerate and block the gasifier.182 Considering the wastes generated in agricultural production, Pinto et al. studied the co-gasification of PE and rice production wastes for syngas.183 The results showed the samples of 20% PE/80% rice husk can generate more H2 than that of 20% PE 80% rice straw. Besides, pure oxygen and steam as the gasifying agents are good options, due to the lack of nitrogen diluting effect.
| Plastics ratio | Wastes ratio | Gasifying agent | Catalysts/bed material | Reactor configuration | Temperature (°C) | S/F or ER | Gas yield | Gas composition (vol%) | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| PS(0.2) | Paper waste (0.8) | 25% N2/75% CO2 | — | Fixed bed | 900 | 5.9g g−1 | H 2 : 11, CO: 48 | 181 | |
| Plastic waste (0.16) | Paper fibers (0.64)/wood chips (0.2) | Air | — | Fixed bed | 800–1000 | ER: 0.2 | H 2 : 16.2, CO: 23.3, CO2: 12.7, CnHm: 5.2 | 184 | |
| PE (0.2) | Rice wastes (0.8) | CO2 | — | Fixed bed | 850 | 1.3 m3 kg−1 | H 2 : 33, CO: 12, CO2: 34, CnHm: 14 | 185 |
There are far more wastes that could be used as feedstocks and be transferred to syngas. Akkache et al. investigated wastewater sludge, waste wood, reeds, olive pomace, solid recovered fuel, paper labels, and plastic labels using a fixed bed reactor.185 The results showed that all feedstocks are recoverable by gasification. The major concerns regarding the wastewater sludge were the pollutant precursors release (NH3, H2S…) and the ash slagging and fouling. The authors believe plastic wastes can be used in co-gasification with wastewater sludge without any restriction according to the study criteria. Sewage sludge is a major disposal and environmental issue. However, sewage sludge can be utilized for energy production through a thermochemical conversion process due to its high energy content of 24 MJ kg−1 on a dry basis.186
4.3 Co-Gasification of plastics/other wastes
Coal production in the US was topped at ∼1.2 billion tons in 2007–2008, and still maintained at ∼0.7 billion tons in 2019.106 Coal has been primarily used as an affordable fuel for generating heat and electricity. Hydrogen and syngas can be produced from coal gasification technology.106 In general, there are three major types (or “ranks”) of coal. The physicochemical properties of the three ranks of coal are summarized in Table 6.| Coal | Heat value, MJ kg−1 | Ash content, wt% | Fixed carbon content, wt% | Moisture content, wt% | Sulfur content, wt% | Formula |
|---|---|---|---|---|---|---|
| Anthracite | 30–35 | 9.7–20.2 | 85–98 | <15 | 0.6–0.77 | C240H90O4NS |
| Bituminous | 25–35 | 3.3–11.7 | 44.9–85 | 2.2–15.9 | 0.7–4.0 | C137H97O9NS |
| Lignite | 0.9–19 | 10–50 | 25–35 | 30–60 | 0.4–1.0 | — |
The inclusion of plastic waste in coal gasification as a feedstock contributes to a low-carbon hydrogen production process through carbon capture and utilization mechanisms. This process allows for the sequestration of carbon from plastic waste, reducing overall emissions. Converting waste into energy and using less coal adds to the sustainability of the process. Combining plastic waste with coal in gasification can boost efficiency and hydrogen output, cutting the process's carbon footprint. Various studies of coal/plastic gasification found that plastic components can increase the gas yield and the fraction of light hydrocarbons. Table 7 summarizes the studies on coal-plastic co-gasification. Aznar et al. studied the ternary co-gasification of coal, biomass, and plastic wastes in a fluidized bed reactor.115 They observed that plastics in the mixture increased the hydrocarbon fraction and reduced that of CO, CO2, and H2.
| Plastics ratio | Wastes ratio | Gasifying agent | Catalysts/bed material | Reactor configuration | Temperature (°C) | S/F or ER | Gas yield | Gas composition (vol%) | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| PE (0.1) | Bituminous (0.9) | Steam/air | — | Fluidized bed | 850 | S/F: 0.85, ER: 0.2 | 1.3 | H 2 : 40, CO: 17, CO2: 16, CnHm: 17 | 187 |
| PET (0.23) | Coal (0.77) | N2 | — | Fluidized bed | 877 | — | — | H 2 : 16, CO: 28, CO2: 13, CnHm: 2.5 | 188 |
| PE | Brown coal (0.5) wood (0.2) | Air | — | Fluidized bed | 868 | — | — | H 2 : 10.55, CO: 10.62, CO2: 11.71, CnHm: 7.98 | 189 |
| PE & PP (0.3) | German brown coal (0.5) & wood (0.2) | Air/35% O2 | Quartz sand | Fluidized bed | 850 | ER = 0.25 | 1.7–2 | H 2 : 13.98, CO: 18.9, CO2: 16.49, CnHm: 10 | 190 |
| Mixed plastics (0.15) | Bituminous coal (0.85) | H2O | — | Fixed bed (two-steps) | 1200 | — | — | H 2 : 79.24, CO: 11.94, CO2: 0.52, CnHm: 4.90 | 191 |
| Mixed plastics (0.2) | Bituminous coal (0.8) | H2O | — | Fixed bed (two-steps) | 1200 | — | — | H 2 : 81.66, CO: 11.43, CO2: 0.31, CnHm: 4.14 | 192 |
| Mixed plastics (0.5) | Low-quality coal (0.5) | Steam | Mg | Fixed bed | 1000 | — | — | H 2 : 29.2, CO: 24.3, CO2: 0.9, CnHm: 29.3 | 193 |
| Mixed plastics (0.5) | Low-quality coal (0.5) | Steam | NiO | Fixed bed | 1000 | — | — | H 2 : 27.5, CO: 22.2, CO2: 0.9, CnHm: 31 | 193 |
| Mixed plastics (0.5) | Low-quality coal (0.5) | Steam | Ni | Fixed bed | 1000 | — | — | H 2 : 22.4, CO: 18.3, CO2: 2, CnHm: 34.2 | 193 |
| PET (0.23) | Brown coal (0.77) | 10% O2/N2 | Silica sand | Fluidized bed | 905 | — | — | H 2 : 14.02, CO: 15.3, CO2: 13.5, CnHm: 1.7 | 181 |
Coal gasification is considered one of the dominant technologies for the production of H2 and syngas (containing H2 and CO).111 They found a lower H2 fraction and an increase in tar formation when plastics were presented. Zaccariello et al. investigated the co-gasification in a fluidized bed reactor fed with plastic waste, wood, and coal.189 The results indicated that the plastic component induces light hydrocarbon generation, which reduces the hydrogen content in the syngas. Coal addition to the blends does not seem to influence gas production and yield, compared with the tests without coal. Other works also didn’t see obvious synergistic effects between coal and plastic.192 In contrast, Kriz et al. found a synergistic effect between coal and plastic in a two-stage co-gasification.191 By incorporating a thermal-degradation module, the hydrogen content reached 80 vol% in the produced syngas. The results indicated that plastic addition has a very important influence on the increase of the overall hydrogen content in the pyrolytic gas during two-stage pyrolysis. The hydrogen bound in polymer chains almost quantitatively converts into gaseous hydrogen. Du et al. investigated the co-gasification of coal and PET in a fluidized bed reactor.188 The results showed CO (28 vol%) and H2 (16 vol%) are the main components in the syngas, which was generated from the gasification reaction of char. The species of syngas are mainly generated from the devolatilization reaction. Besides, they found the generation of syngas from the 5 mm sample was noticed to be delayed for about 0.1 s as compared to that of the 1.5 mm sample because of particle inner temperature gradient.
Coal char and ash in gasifiers could catalytically enhance reforming reactions for H2 production and may reduce the tar content of the gas phase product.95,114 The addition of coal to plastic gasification could be a useful method to improve the plastic gasification process. For example, the addition of coal may solve the feeding issue of plastics due to their sticky plastic nature. The production of hydrogen from coal/plastics (landfill wastes) enhances the sustainable usage of plastic waste and offers significant environmental benefits.
Considerable strides are being made in the field of industrial-scale gasification of plastic waste, with companies like Enerkem, Synova, and Brightlands Chemelot leading efforts to develop cutting-edge large-scale technologies. The Varkaus Corenso Plant in Finland utilizes plastic waste as feedstock in its 50 MWth bubbling fluidized bed (BFB) gasifier to produce syngas for industrial fuel, with additional potential for hydrogen recovery. These developments highlight the transformative capability of gasification to transform waste and clean energy initiatives.194
4.4 Alternative heating processes
Several new technologies and methods have been thoroughly investigated aimed at producing syngas with a high hydrogen content from different feedstocks. Recently, an alternative flash Joule heating process was developed for clean hydrogen and high-purity graphene production in the catalyst-free conversion of plastic waste. The process involves the instant breaking down of polyolefins, leading to the production of hydrogen without releasing carbon dioxide. By utilizing flash Joule heating (FJH), plastic is rapidly deconstructed into hydrogen and graphene, resulting in a negative cost for hydrogen production due to the sale of the valuable graphene byproduct. The life-cycle assessment of this process demonstrates a significant reduction in emissions compared to other hydrogen production methods, contributing towards achieving net-zero emissions.195 Ismail et al. investigated an integrated gasification system that efficiently converts plastic waste into hydrogen, electricity, heating, and fresh water. Energy and exergy analyses demonstrate high system efficiency, with energy and exergy efficiencies of 66.24% and 48.10%, respectively. Geothermal energy systems can be a viable option for waste-to-energy conversion and CO2 reduction because they can also produce hydrogen and fresh water, demonstrating their adaptability and environmental sustainability.196Microwave dielectric heating offers a way to evenly distribute electromagnetic wave energy to the absorbing medium, in contrast to traditional methods that only heat from the outer layer to the interior.197 This method, employed since the 1970s and further developed in the 1990s, has played a key role in different material treatments and chemical processes, such as microwave-enhanced pyrolysis of plastic wastes, rubber, biomass, and municipal solid waste.198 Microwaves have a high energy utilization and temperature elevation rate, as well as even heating and compact reactor designs, making them ideal for improving hydrogen production and providing a convenient and efficient method for storing and releasing hydrogen.199,200 Plastics can be a useful resource for producing hydrogen with the help of microwave-assisted catalytic upgrading, providing both economic benefits and environmental advantages.4,201Fig. 20 shows how the electric field interacts with molecules during microwave heating through dipolar rotation and ionic conduction. Dipolar rotation involves molecules rotating continuously to align their dipole with the electric field, producing heat due to friction. Iron, cobalt, and nickel catalysts are often used because they can activate carbon–hydrogen bonds effectively during microwave heating. These catalysts are essential in selectively disrupting carbon–hydrogen bonds to generate hydrogen. Their magnetic properties make them highly responsive to microwaves, facilitating rapid conversion into heat.202 Co-coated Fe–Al catalyst for LDPE dehydrogenation, demonstrating significantly higher hydrogen yield under microwave irradiation compared to conventional heating methods. Fe/Ni–CeO2@CNTs substrates efficiently transform HDPE plastics into pure hydrogen and carbon nanotubes using microwave radiation. Recent research findings emphasize the possibility of using microwave-assisted catalytic upgrading to produce hydrogen efficiently from plastic waste.203,204 Catalysts containing iron (Fe) and nickel (Ni) are commonly chosen for the dehydrogenation of plastics because of their ability to activate carbon–hydrogen bonds. Table 8 summarizes the recent studies on Microwave catalytic gasification of plastic.
 | ||
| Fig. 20 Mechanisms of microwave heating: dipolar rotation and ionic conduction (reprinted from ref. 205 accessed 2024). | ||
| Feedstock | Catalyst | Catalytic temperature (°C) | Oxidizing agent | H2 (mmol g−1) | Ref. |
|---|---|---|---|---|---|
| LDPE | Fe–Co–Al | 600–900 | — | 61.39 | 206 |
| HDPE | Fe–FeAl2O4 | 300–450 | — | 47.3 | 207 |
| Plastic mixture | Ni–Fe | 800 | — | 42.3 | 208 |
| PP | Fe, Ni | 800 | H2O | 25.14 | 209 |
| Plastic mixture | FeAlOx | 10–20 | — | 55.6 | 210 |
| Plastic + corn stover | Silicon carbide | 700 | Air | 30.5 | 211 |
Thermal plasma can be created using a DC or AC electrical discharge, RF induction, or MW discharge. DC arc discharge causes high energy density and temperature at the electrodes, resulting in a jet plasma. When high voltage is applied between electrodes in the gas phase, a breakdown occurs, resulting in the creation of positive ions and electrons, which causes gas discharge. Plasma technology also emerges as a highly favorable choice for plastic waste gasification. In the early twentieth century, plasma was adopted by the chemical industry for producing acetylene from natural gas. Since the 1980s, plasma technology has become increasingly popular for managing hazardous solid waste and has shown effectiveness in treating hazardous substances like asbestos, turning radioactive waste into glass, and handling different chemicals.212–214 Like traditional thermal processes, Fig. 21 depicts the heat transfer between particles and plasma. A thermal plasma, at thermodynamic equilibrium, acts similarly to a fluid with distinct thermodynamic and transport characteristics, allowing for heat transfer via convection, conduction, and radiation. Heat transfer rates are very high in thermal plasma due to its high enthalpy, thermal conductivity, and radiation intensity. In plasma gasification steam water is generally preferred over air or oxygen because it produces the desired reactions including the steam reforming reaction and increases the H2 ratio in the syngas. However, the steam reforming reaction is highly endothermic and needs high temperature (1100–1700 K). Specific reactors are designed to separate the plasma gasification from the combustion. The high temperature obtained in the reactor without using the combustion process allows for the production of syngas with high purity.215 Ma et al. studied tire gasification using steam and air as gasification agents in plasma gasification. The research showed that the carbon conversion efficiency was 99.12%, with an energy recovery rate of 93.67% and an exergy efficiency of 36.45%. The research emphasizes the positive impact of utilizing plasma gasification for the sustainable disposal of used tires and the effective creation of hydrogen fuel.158 The initial composition of the waste processed influences the quantities of H2 and CO generated, resulting in irregularities ranging from 49.4 to 64.4 vol% for H2 and 24.8 to 36 vol% for CO under uniform experiment conditions, depending on the type of waste.214 Plasma gasification provides a promising route for efficiently transforming plastic waste into hydrogen production. Plasma can handle a wide range of waste materials, such as plastics, biomass, and hazardous waste. In terms of the environment specifically for hazardous waste, plasma gasification proves advantageous, with minimal environmental impact compared to conventional waste treatment methods. Despite challenges such as initial costs and waste sorting requirements, continuous progress and refining of processes are predicted to enhance the efficiency of plasma gasification and hydrogen production. Table 9 summarizes the recent studies on thermal plasma gasification of plastic.
 | ||
| Fig. 21 Basic heat transfer mechanisms for plasma-assisted heating (reprinted with permission from ref. 216 Copyright 2020 Elsevier). | ||
5. Prospective
To achieve sustainable low-carbon hydrogen production, it is crucial to continue advancing technologies such as coupling carbon capture and storage utilization with conventional gasification for hydrogen production and plastic waste gasification via co-gasification with biomass and other waste. These technologies could transform the energy industry by allowing for the creation of clean hydrogen from a range of sources, aiding the shift towards a greener energy system.• The advancement of low-carbon hydrogen production using gasification offers a promising pathway for sustainable energy systems. The integration of CCUS techniques with conventional hydrogen production allows us to generate low-carbon hydrogen. CCUS systems effectively capture carbon dioxide emissions produced during the gasification process, decreasing their environmental impact. Co-gasification of plastics and biomass also offers a sustainable way to use waste materials while lowering reliance on fossil fuels. Ongoing research focuses on enhancing gasification efficiency and cost-effectiveness through innovative materials, components, and systems, including plasma, catalytic gasification, and microwave technologies. CCUS-integrated steam methane and auto-thermal reforming technologies are also key areas of interest, aiming to produce hydrogen while minimizing CO2 emissions. These technologies can transform waste gaseous hydrocarbons into hydrogen, which helps in valorizing waste and decreasing greenhouse gas emissions.
• Further research into the mechanisms of plastic mixture gasification, particularly focusing on char and tar reforming reactions, promises to deepen our understanding of these complex reactions at the fundamental level. Gasification of plastics with other feedstocks shows potential for effective plastic waste management. While there are challenges associated with feeding, melting, and clogging the reactor, research is ongoing to address these issues through the pre-treatment of plastics with other feedstocks, such as pre-grinding and palletization. There is still a need to optimize the selectivity and yield of H2 and syngas, which can be studied by adjusting operating parameters such as, but not limited to, plastic types and mixtures of plastics; plastics blended with other materials, such as coal waste and biomass, along with the blend ratio; gasification agents (steam and CO2); and gasification temperature. Additionally, the use of char and ash as potential catalysts needs further investigation. Other promising heterogeneous catalysts for hydrogenation and reforming of hydrocarbons should also be considered for the co-gasification of coal waste/biomass and plastic wastes. The synergistic effects between the plastic and biomass co-gasification, have been studied in much more detail. In contrast, the co-gasification of coal waste/plastics or plastics with other wastes is still not thoroughly studied. Their synergistic effect needs to be better understood.
• Another hurdle in these gasification processes is impurities, such as toxic chlorine compounds from PVC and sulfur and toxic metals from coal, in the raw syngas makes the cleanup processes more complicated. Contaminants, especially strongly acidic gases like H2S and HCl, could poison downstream catalysts, gradually deteriorate process equipment, and foul CO2 sorbent materials in carbon capture operations. These issues could be addressed through the pre-treatment of plastic wastes and coal to remove the impurities and need further investigation.
• Exploring new electrified decarbonization technologies, such as microwave dielectric heating and plasma technology, at pilot scale shows potential for effectively producing syngas from plastic waste and biomass in the future. These developments bring the possibility of creating low carbon hydrogen and managing waste efficiently, which helps support a circular economy and reduce environmental footprint.
Acronym of selected common plastics
| ABS | Acrylonitrile-butadiene-styrene |
| BET | Brunauer, Emmett and Teller |
| BTX | Benzene, toluene, and xylene |
| ER | Equivalence ratio |
| HIPS | High impact polystyrene = TPS |
| PA | Polyamide (nylon) |
| PBT | Polybutylene terephthalate |
| PC | Polycarbonate |
| PE/LDPE/HDPE | Polyethylene, low/high density |
| PET (PETE) | Polyethylene terephthalate |
| PP | Polypropylene |
| PS | Polystyrene or styrofoam |
| PU/PUR | Polyurethane |
| PVC | Polyvinyl chloride |
Author contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript. M. A. B.: writing – original draft, review & editing, visualization, investigation, formal analysis, data curation, conceptualization. J. T.: writing – original draft, visualization, investigation, formal analysis, data curation, conceptualization. J. W.: writing – review & editing, investigation, formal analysis. Y. S.: writing – review & editing, investigation, formal analysis. M. G.: supervision, resources, project administration, funding acquisition. F. S.: writing – original draft, review & editing, methodology, investigation, formal analysis, data curation, conceptualization, supervision, resources, project administration, funding acquisition. P. W.: writing – review & editing, methodology, investigation, formal analysis, data curation, conceptualization, supervision, resources, project administration, funding acquisition.Data availability
The review paper is based on an analysis of existing literature and does not involve original data collection. No new data or software were used; only existing research was analyzed in the review process. The references used in this review paper are publicly available and can be accessed through online databases.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work acknowledges the financial support provided by the National Energy Technology Laboratory (NETL) and the U.S. Department of Energy (DOE)’s Fossil Energy and Carbon Management’s Gasification Program. Neither the United States Government nor any agency thereof, nor any of its employees, nor the support contractor, nor any of their employees, makes any warranty, expressor implied, or assumes any legal liability or responsibility for the accuracy, completeness, or usefulness of any information, apparatus, product, or process disclosed, or represents that its use would not infringe privately owned rights. Reference herein to any specific commercial product, process, or service by trade name, trademark, manufacturer, or otherwise does not necessarily constitute or imply its endorsement, recommendation, or favoring by the United States Government or any agency thereof. The views and opinions of authors expressed herein do not necessarily state or reflect those of the United States Government or any agency thereof.References
- R. C. Thompson, C. J. Moore, F. S. V. Saal and S. H. Swan, Plastics, the environment and human health: current consensus and future trends, Philos. Trans. R. Soc., B, 2009, 364(1526), 2153–2166 CrossRef CAS PubMed.
- H. H. Shah, M. Amin, A. Iqbal, I. Nadeem, M. Kalin and A. M. Soomar, et al., A review on gasification and pyrolysis of waste plastics, Front. Chem., 2023, 10, 960894 CrossRef PubMed.
- G. Lopez, M. Artetxe, M. Amutio, J. Alvarez, J. Bilbao and M. Olazar, Recent advances in the gasification of waste plastics. A critical overview, Renewable Sustainable Energy Rev., 2018, 82, 576–596 CrossRef CAS.
- S. S. Sharma and V. S. Batra, Production of hydrogen and carbon nanotubes via catalytic thermo-chemical conversion of plastic waste: review, J. Chem. Technol. Biotechnol., 2020, 95(1), 11–19 CrossRef CAS.
- R. Geyer, J. R. Jambeck and K. L. Law, Production, use, and fate of all plastics ever made, Sci. Adv., 2017, 3(7), e1700782 CrossRef PubMed.
- S. Lemonick, Chemical solutions for a chemical problem, C&EN Global Enterprise, 2018, 96(25), 26–29 Search PubMed.
- R. G. Hunt, J. D. Sellers and W. E. Franklin, Resource and environmental profile analysis: a life cycle environmental assessment for products and procedures, Environ. Impact Assess. Rev., 1992, 12(3), 245–269 CrossRef.
- Y. Chen, A. K. Awasthi, F. Wei, Q. Tan and J. Li, Single-use plastics: production, usage, disposal, and adverse impacts, Sci. Total Environ., 2021, 752, 141772 CrossRef CAS PubMed.
- A. Demirbas, Pyrolysis of municipal plastic wastes for recovery of gasoline-range hydrocarbons, J. Anal. Appl. Pyrolysis, 2004, 72(1), 97–102 CrossRef CAS.
- M. G. Kibria, N. I. Masuk, R. Safayet, H. Q. Nguyen and M. Mourshed, Plastic Waste: Challenges and Opportunities to Mitigate Pollution and Effective Management, Int. J. Environ. Res., 2023, 17(1), 20 CrossRef CAS PubMed.
- R. K. Singh and B. Ruj, Time and temperature depended fuel gas generation from pyrolysis of real world municipal plastic waste, Fuel, 2016, 174, 164–171 CrossRef CAS.
- I. I. Ahmed, N. Nipattummakul and A. K. Gupta, Characteristics of syngas from co-gasification of polyethylene and woodchips, Appl. Energy, 2011, 88(1), 165–174 CrossRef CAS.
- A. Saravanan and P. Senthil Kumar, Environmental Footprints of Recycled Polyester. Textile Science and Clothing Technology, in Advancements in Recycled Polyesters, ed. S. Muthu, Springer, Singapore, 2020.
- J. Hopewell, R. Dvorak and E. Kosior, Plastics recycling: challenges and opportunities, Philos. Trans. R. Soc., B, 2009, 364(1526), 2115–2126 CrossRef CAS PubMed.
- M. Solis and S. Silveira, Technologies for chemical recycling of household plastics – A technical review and TRL assessment, Waste Manage., 2020, 105, 128–138 CrossRef CAS PubMed.
- A. Lee and M. S. Liew, Tertiary recycling of plastics waste: an analysis of feedstock, chemical and biological degradation methods, J. Mater. Cycles Waste Manage., 2021, 23(1), 32–43 CrossRef.
- D. Munir, M. F. Irfan and M. R. Usman, Hydrocracking of virgin and waste plastics: a detailed review, Renewable Sustainable Energy Rev., 2018, 90, 490–515 CrossRef CAS.
- C. M. Kalamaras and A. M. Efstathiou, Hydrogen Production Technologies: Current State and Future Developments, Conference Papers in Energy, 2013, 690627, DOI:10.1155/2013/690627 (Accessed: Jan 14, 2025).
- F. Ardolino, C. Lodato, T. F. Astrup and U. Arena, Energy recovery from plastic and biomass waste by means of fluidized bed gasification: a life cycle inventory model, Energy, 2018, 165, 299–314 CrossRef CAS.
- S. D. Anuar Sharuddin, F. Abnisa, W. M. A. Wan Daud and M. K. Aroua, Energy recovery from pyrolysis of plastic waste: study on non-recycled plastics (NRP) data as the real measure of plastic waste, Energy Convers. Manage., 2017, 148, 925–934 CrossRef CAS.
- S. Rocio Gonzalez, Clean Air Task Force. Assessing hydrogen emissions across the entire lifecycle. available from, 2022, https://www.catf.us/2022/10/hydrogen-lca-emissions-across-life-cycle (Accessed: Jan. 14, 2025).
- U. Arena, Process and technological aspects of municipal solid waste gasification. A review, Waste Manage., 2012, 32(4), 625–639 CrossRef CAS PubMed.
- J. Speirs, P. Balcombe, E. Johnson, J. Martin, N. Brandon and A. Hawkes, A greener gas grid: What are the options, Energy Policy, 2018, 118, 291–297 CrossRef.
- C. Wu and P. T. Williams, Hydrogen from waste plastics by way of pyrolysis–gasification, Proceedings of the ICE: Waste and Resource Management, 2015, 167(1), 35–46 Search PubMed.
- Royal Society, Options for producing low-carbon hydrogen at scale: policy briefing, 2018, https://royalsociety.org/-/media/policy/projects/hydrogen-production/energy-briefing-green-hydrogen.pdf (Accessed: Jan14 2025).
- S. H. Gebre, M. G. Sendeku and M. Bahri, Recent Trends in the Pyrolysis of Non-Degradable Waste Plastics, ChemistryOpen, 2021, 10(12), 1202–1226 CrossRef CAS PubMed.
- J. I. Gug, D. Cacciola and M. J. Sobkowicz, Processing and properties of a solid energy fuel from municipal solid waste (MSW) and recycled plastics, Waste Manage., 2015, 35, 283–292 CrossRef CAS PubMed.
- S. R. Horton, J. Woeckener, R. Mohr, Y. Zhang, F. Petrocelli and M. T. Klein, Molecular-Level Kinetic Modeling of the Gasification of Common Plastics, Energy Fuels, 2015, 30(3), 1662–1674 CrossRef.
- P. Dave and A. K. Joshi, Plasma pyrolysis and gasification of plastics waste – a review, J. Sci. Ind. Res., 2010, 69, 177–179 CAS.
- R. K. Singh, B. Ruj, A. K. Sadhukhan and P. Gupta, Thermal degradation of waste plastics under non-sweeping atmosphere: part 1: effect of temperature, product optimization, and degradation mechanism, J. Environ. Manage., 2019, 239, 395–406 CrossRef CAS PubMed.
- S. E. Levine and L. J. Broadbelt, Detailed mechanistic modeling of high-density polyethylene pyrolysis: low molecular weight product evolution, Polym. Degrad. Stab., 2009, 94(5), 810–822 CrossRef CAS.
- S. Moldoveanu, Analytical pyrolysis of synthetic organic polymers, Elsevier, 2005 Search PubMed.
- Y. R. Luo, Handbook of bond dissociation energies in organic compounds. Handbook of Bond Dissociation Energies in Organic Compounds, CRC Press, 2002 Search PubMed.
- Y. Sakata, M. A. Uddin, K. Koizumi and K. Murata, Thermal degradation of polyethylene mixed with poly(vinyl chloride) and poly(ethyleneterephthalate), Polym. Degrad. Stab., 1996, 53(1), 111–117 CrossRef CAS.
- C. Vasile, H. Pakdel, B. Mihai, P. Onu, H. Darie and S. Ciocâlteu, Thermal and catalytic decomposition of mixed plastics, J. Anal. Appl. Pyrolysis, 2001, 57(2), 287–303 CrossRef CAS.
- T. Faravelli, M. Pinciroli, F. Pisano, G. Bozzano, M. Dente and E. Ranzi, Thermal degradation of polystyrene, J. Anal. Appl. Pyrolysis, 2001, 60(1), 103–121 CrossRef CAS.
- U. Arena, L. Zaccariello and M. L. Mastellone, Tar removal during the fluidized bed gasification of plastic waste, Waste Manage., 2009, 29(2), 783–791 CrossRef CAS PubMed.
- M. L. Mastellone and U. Arena, Olivine as a tar removal catalyst during fluidized bed gasification of plastic waste, AIChE J., 2008, 54(6), 1656–1667 CrossRef CAS.
- G. Ruoppolo, P. Ammendola, R. Chirone and F. Miccio, H2-rich syngas production by fluidized bed gasification of biomass and plastic fuel, Waste Manage., 2012, 32(4), 724–732 CrossRef CAS PubMed.
- G. Lopez, M. Artetxe, M. Amutio, J. Bilbao and M. Olazar, Thermochemical routes for the valorization of waste polyolefinic plastics to produce fuels and chemicals. A review, Renewable Sustainable Energy Rev., 2017, 73, 346–368 CrossRef CAS.
- M. Predel and W. Kaminsky, Pyrolysis of mixed polyolefins in a fluidised-bed reactor and on a pyro-GC/MS to yield aliphatic waxes, Polym. Degrad. Stab., 2000, 70(3), 373–385 CrossRef CAS.
- B. Ciuffi, D. Chiaramonti, A. M. Rizzo, M. Frediani and L. Rosi, A Critical Review of SCWG in the Context of Available Gasification Technologies for Plastic Waste, Appl. Sci., 2020, 10(18), 6307 CrossRef CAS.
- T. Rajabloo, W. De Ceuninck, L. Van Wortswinkel, M. Rezakazemi and T. Aminabhavi, Environmental management of industrial decarbonization with focus on chemical sectors: a review, J. Environ. Manage., 2022, 302, 114055 CrossRef CAS PubMed.
- F. Rahim Malik, H. B. Yuan, J. C. Moran and N. Tippayawong, Overview of hydrogen production technologies for fuel cell utilization, Eng. Sci. Technol. Int. J., 2023, 43, 101452 Search PubMed.
- M. El-Shafie, S. Kambara, Y. Hayakawa, M. El-Shafie, S. Kambara and Y. Hayakawa, Hydrogen Production Technologies Overview, J. Power Energy Eng., 2019, 7(1), 107–154 CrossRef.
- J. A. Onwudili, C. Muhammad and P. T. Williams, Influence of catalyst bed temperature and properties of zeolite catalysts on pyrolysis-catalysis of a simulated mixed plastics sample for the production of upgraded fuels and chemicals, J. Energy Inst., 2019, 92(5), 1337–1347 CrossRef CAS.
- S. H. Jung, M. H. Cho, B. S. Kang and J. S. Kim, Pyrolysis of a fraction of waste polypropylene and polyethylene for the recovery of BTX aromatics using a fluidized bed reactor, Fuel Process. Technol., 2010, 91(3), 277–284 CrossRef CAS.
- M. H. Cho, S. H. Jung and J. S. Kim, Pyrolysis of Mixed Plastic Wastes for the Recovery of Benzene, Toluene, and Xylene (BTX) Aromatics in a Fluidized Bed and Chlorine Removal by Applying Various Additives, Energy Fuels, 2009, 24(2), 1389–1395 CrossRef.
- C. M. Simon and W. Kaminsky, Chemical recycling of polytetrafluoroethylene by pyrolysis, Polym. Degrad. Stab., 1998, 62(1), 1–7 CrossRef CAS.
- T. Ueno, E. Nakashima and K. Takeda, Quantitative analysis of random scission and chain-end scission in the thermal degradation of polyethylene, Polym. Degrad. Stab., 2010, 95(9), 1862–1869 CrossRef CAS.
- M. Artetxe, G. Lopez, G. Elordi, M. Amutio, J. Bilbao and M. Olazar, Production of Light Olefins from Polyethylene in a Two-Step Process: Pyrolysis in a Conical Spouted Bed and Downstream High-Temperature Thermal Cracking, Ind. Eng. Chem. Res., 2012, 51(43), 13915–13923 CrossRef CAS.
- A. Abdulrasheed, A. A. Jalil, Y. Gambo, M. Ibrahim, H. U. Hambali and M. Y. Shahul Hamid, A review on catalyst development for dry reforming of methane to syngas: Recent advances, Renewable Sustainable Energy Rev., 2019, 108, 175–193 CrossRef CAS.
- C. Di Blasi, Combustion and gasification rates of lignocellulosic chars, Prog. Energy Combust. Sci., 2009, 35(2), 121–140 CrossRef CAS.
- W. Xuan, H. Wang, S. Yan and D. Xia, Exploration on the steam gasification mechanism of waste PE plastics based on ReaxFF-MD and DFT methods, Fuel, 2022, 315, 123121 CrossRef CAS.
- Z. Fu, Q. Sun, S. Yang, F. Hua, Y. Ji and Y. Cheng, Molecular-level kinetic modelling for the pyrolysis of high-density polyethylene in a two-stage process, J. Anal. Appl. Pyrolysis, 2024, 178, 106428 CrossRef CAS.
- C. Li and K. Suzuki, Tar property, analysis, reforming mechanism and model for biomass gasification—An overview, Renewable Sustainable Energy Rev., 2009, 13(3), 594–604 CrossRef CAS.
- L. Dai, N. Zhou, Y. Lv, K. Cobb, Y. Cheng and Y. Wang, et al., Pyrolysis-catalysis for waste polyolefin conversion into low aromatic naphtha, Energy Convers. Manage., 2021, 245, 114578 CrossRef CAS.
- I. Çit, A. Sinaǧ, T. Yumak, S. Uçar, Z. Misirlioǧlu and M. Canel, Comparative pyrolysis of polyolefins (PP and LDPE) and PET, Polym. Bull., 2010, 64(8), 817–834 CrossRef.
- S. M. Al-Salem and A. Dutta, Wax Recovery from the Pyrolysis of Virgin and Waste Plastics, Ind. Eng. Chem. Res., 2021, 60(22), 8301–8309 CrossRef CAS.
- V. Yadav, M. A. Sherly, P. Ranjan, R. O. Tinoco, A. Boldrin and A. Damgaard, et al., Framework for quantifying environmental losses of plastics from landfills, Resour., Conserv. Recycl., 2020, 161, 104914 CrossRef.
- C. Font Palma, Modelling of tar formation and evolution for biomass gasification: a review, Appl. Energy, 2013, 111, 129–141 CrossRef CAS.
- J. A. Sancho, M. P. Aznar and J. M. Toledo, Catalytic Air Gasification of Plastic Waste (Polypropylene) in Fluidized Bed. Part I: Use of in-Gasifier Bed Additives, Ind. Eng. Chem. Res., 2008, 47(4), 1005–1010 CrossRef CAS.
- S. D. Anuar Sharuddin, F. Abnisa, W. M. A. Wan Daud and M. K. Aroua, A review on pyrolysis of plastic wastes, Energy Convers. Manage., 2016, 115, 308–326 CrossRef CAS.
- M. Artetxe, G. Lopez, M. Amutio, I. Barbarias, A. Arregi and R. Aguado, et al., Styrene recovery from polystyrene by flash pyrolysis in a conical spouted bed reactor, Waste Manage., 2015, 45, 126–133 CrossRef CAS PubMed.
- V. Esmaeili, J. Ajalli, A. Faramarzi, M. Abdi and M. Gholizadeh, Gasification of wastes: the impact of the feedstock type and co-gasification on the formation of volatiles and char, Int. J. Energy Res., 2020, 44(5), 3587–3606 CrossRef CAS.
- S. Salavati, C. T. Zhang, S. Zhang, Q. Liu, M. Gholizadeh and X. Hu, Cross-interaction during Co-gasification of wood, weed, plastic, tire and carton, J. Environ. Manage., 2019, 250, 109467 CrossRef CAS PubMed.
- J. Li, K. G. Burra, Z. Wang, X. Liu and A. K. Gupta, Syngas evolution and energy efficiency in CO2 assisted gasification of ion-exchanged pine wood, Fuel, 2022, 317, 123549 CrossRef CAS.
- H. Fatehi and X. S. Bai, Structural evolution of biomass char and its effect on the gasification rate, Appl. Energy, 2017, 185, 998–1006 CrossRef CAS.
- Z. Wang, K. G. Burra, X. Li, M. Zhang, X. He and T. Lei, et al., CO2-assisted gasification of polyethylene terephthalate with focus on syngas evolution and solid yield, Appl. Energy, 2020, 276, 115508 CrossRef CAS.
- S. Q. Li, Q. Yao, S. E. Wen, Y. Chi and J. H. Yan, Properties of Pyrolytic Chars and Activated Carbons Derived from Pilot-Scale Pyrolysis of Used Tires, J. Air Waste Manage. Assoc., 2005, 55(9), 1315–1326 CrossRef CAS PubMed.
- J. Eshun, L. Wang, E. Ansah, A. Shahbazi, K. Schimmel and V. Kabadi, et al., Characterization of the physicochemical and structural evolution of biomass particles during combined pyrolysis and CO2 gasification, J. Energy Inst., 2019, 92(1), 82–93 CrossRef CAS.
- T. S. Farrow, C. Sun and C. E. Snape, Impact of CO2 on biomass pyrolysis, nitrogen partitioning, and char combustion in a drop tube furnace, J. Anal. Appl. Pyrolysis, 2015, 113, 323–331 CrossRef CAS.
- S. A. Scott, J. F. Davidson, J. S. Dennis, P. S. Fennell and A. N. Hayhurst, The rate of gasification by CO2 of chars from waste, Proc. Combust. Inst., 2005, 30(2), 2151–2159 CrossRef.
- M. V. Gil, J. Fermoso, C. Pevida, J. J. Pis and F. Rubiera, Intrinsic char reactivity of plastic waste (PET) during CO2 gasification, Fuel Process. Technol., 2010, 91(11), 1776–1781 CAS.
- G. Duman, M. A. Uddin and J. Yanik, The effect of char properties on gasification reactivity, Fuel Process. Technol., 2014, 118, 75–81 CrossRef CAS.
- J. B. Parra, C. O. Ania, A. Arenillas, F. Rubiera, J. M. Palacios and J. J. Pis, Textural development and hydrogen adsorption of carbon materials from PET waste, J. Alloys Compd., 2004, 379(1–2), 280–289 CrossRef CAS.
- D. Vamvuka, E. Karouki, S. Sfakiotakis and P. Salatino, Gasification of Waste Biomass Chars by Carbon Dioxide via Thermogravimetry—Effect of Catalysts, Combust. Sci. Technol., 2012, 184(1), 64–77 CAS.
- A. Esfandiari, T. Kaghazchi and M. Soleimani, Preparation and evaluation of activated carbons obtained by physical activation of polyethyleneterephthalate (PET) wastes, J. Taiwan Inst. Chem. Eng., 2012, 43(4), 631–637 CrossRef CAS.
- S. L. Wong, N. Ngadi, T. A. T. Abdullah and I. M. Inuwa, Current state and future prospects of plastic waste as source of fuel: a review, Renewable Sustainable Energy Rev., 2015, 50, 1167–1180 CrossRef CAS.
- V. Sharma, A. Kalam Hossain, G. Griffiths, G. Duraisamy, A. Krishnasamy and V. Ravikrishnan, et al., Plastic waste to liquid fuel: a review of technologies, applications, and challenges, Sustainable Energy Technol. Assess., 2022, 53, 102651 CrossRef.
- S. Honus, S. Kumagai, V. Molnár, G. Fedorko and T. Yoshioka, Pyrolysis gases produced from individual and mixed PE, PP, PS, PVC, and PET—Part II: fuel characteristics, Fuel, 2018, 221, 361–373 CrossRef CAS.
- W. Torres, S. S. Pansare and J. G. Goodwin, Hot Gas Removal of Tars, Ammonia, and Hydrogen Sulfide from Biomass Gasification Gas, Catal. Rev., 2007, 49(4), 407–456 CrossRef CAS.
- P. T. Williams and E. A. Williams, Interaction of Plastics in Mixed-Plastics Pyrolysis, Energy Fuels, 1998, 13(1), 188–196 CrossRef.
- G. Özsin and A. E. Pütün, A comparative study on co-pyrolysis of lignocellulosic biomass with polyethylene terephthalate, polystyrene, and polyvinyl chloride: synergistic effects and product characteristics, J. Cleaner Prod., 2018, 205, 1127–1138 CrossRef.
- R. Chen, S. Zhang, X. Yang, G. Li, H. Zhou and Q. Li, et al., Thermal behaviour and kinetic study of co-pyrolysis of microalgae with different plastics, Waste Manage., 2021, 126, 331–339 CrossRef CAS PubMed.
- S. Shang, C. Guo, K. Lan, Z. Li, W. He and Z. Qin, et al., Hydrogen-rich Syngas Production via Catalytic Gasification of Sewage Sludge and Wheat Straw Using Corn Stalk Char-supported Catalysts, BioResources, 2020, 15, 4294–4313 CAS.
- Y. Yang, J. Zhu, G. Zhu, L. Yang and Y. Zhu, The effect of high temperature on syngas production by immediate pyrolysis of wet sewage sludge with sawdust, J. Therm. Anal. Calorim., 2018, 132(3), 1783–1794 CrossRef CAS.
- H. Hofbauer and R. Rauch, Stoichiometric Water Consumption of Steam Gasification by the FICFB-Gasification Process, Prog. Thermochem. Biomass Convers., 2008, 199–208 Search PubMed.
- J. Gil, M. P. Aznar, M. A. Caballero, E. Francés and J. Corella, Biomass Gasification in Fluidized Bed at Pilot Scale with Steam–Oxygen Mixtures. Product Distribution for Very Different Operating Conditions, Energy Fuels, 1997, 11(6), 1109–1118 CrossRef CAS.
- F. Pinto, C. Franco, R. N. André, C. Tavares, M. Dias and I. Gulyurtlu, et al., Effect of experimental conditions on co-gasification of coal, biomass and plastics wastes with air/steam mixtures in a fluidized bed system, Fuel, 2003, 82(15–17), 1967–1976 CrossRef CAS.
- Y. S. Jeong, J. W. Kim, H. W. Ra, M. W. Seo, T. Y. Mun and J. S. Kim, Characteristics of Air Gasification of 10 Different Types of Plastic in a Two-Stage Gasification Process. ACS Sustain, Chem. Eng., 2022, 10(14), 4705–4716 CAS.
- Y. Li, M. A. Nahil and P. T. Williams, Hydrogen/Syngas Production from Different Types of Waste Plastics Using a Sacrificial Tire Char Catalyst via Pyrolysis-Catalytic Steam Reforming, Energy Fuels, 2023, 37(9), 6661–6673 CrossRef CAS.
- D. Gündüz Han, K. Erdem and A. Midilli, Investigation of hydrogen production via waste plastic gasification in a fluidized bed reactor using Aspen Plus, Int. J. Hydrogen Energy, 2023, 48(99), 39315–39329 CrossRef.
- P. Kaewpengkrow, D. Atong and V. Sricharoenchaikul, Pyrolysis and gasification of landfilled plastic wastes with Ni–Mg–La/Al2O3 catalyst, Environ. Technol., 2012, 33(22), 2489–2495 CrossRef CAS PubMed.
- J. W. Lee, T. U. Yu, J. W. Lee, J. H. Moon, H. J. Jeong and S. S. Park, et al., Gasification of Mixed Plastic Wastes in a Moving-Grate Gasifier and Application of the Producer Gas to a Power Generation Engine, Energy Fuels, 2013, 27(4), 2092–2098 CrossRef CAS.
- M. Mojaver, T. Azdast and R. Hasanzadeh, Assessments of key features and Taguchi analysis on hydrogen rich syngas production via gasification of polyethylene, polypropylene, polycarbonate and polyethylene terephthalate wastes, Int. J. Hydrogen Energy, 2021, 46(58), 29846–29857 CrossRef CAS.
- F. Pinto, R. André, M. Miranda, D. Neves, F. Varela and J. Santos, Effect of gasification agent on co-gasification of rice production wastes mixtures, Fuel, 2016, 180, 407–416 CrossRef CAS.
- P. Kannan, G. Lakshmanan, A. Al Shoaibi and C. Srinivasakannan, Equilibrium model analysis of waste plastics gasification using CO2 and steam, Waste Manage. Res., 2017, 35(12), 1247–1253 CrossRef CAS PubMed.
- J. M. Saad and P. T. Williams, Catalytic dry reforming of waste plastics from different waste treatment plants for production of synthesis gases, Waste Manage., 2016, 58, 214–220 CrossRef CAS PubMed.
- T. Maqsood, J. Dai, Y. Zhang, M. Guang and B. Li, Pyrolysis of plastic species: a review of resources and products, J. Anal. Appl. Pyrolysis, 2021, 159, 105295 CrossRef CAS.
- B. Singh and N. Sharma, Mechanistic implications of plastic degradation, Polym. Degrad. Stab., 2008, 93(3), 561–584 CrossRef CAS.
- M. Arabiourrutia, G. Elordi, G. Lopez, E. Borsella, J. Bilbao and M. Olazar, Characterization of the waxes obtained by the pyrolysis of polyolefin plastics in a conical spouted bed reactor, J. Anal. Appl. Pyrolysis, 2012, 94, 230–237 CrossRef CAS.
- Z. Wang, X. Liu, K. G. Burra, J. Li, M. Zhang and T. Lei, et al., Towards enhanced catalytic reactivity in CO2-assisted gasification of polypropylene, Fuel, 2021, 284, 119076 CrossRef CAS.
- J. Chen, L. Fu, M. Tian, S. Kang and E. Jiaqiang, Comparison and synergistic effect analysis on supercritical water gasification of waste thermoplastic plastics based on orthogonal experiments, Energy, 2022, 261, 125104 CrossRef CAS.
- J. Li, S. Liao, W. Dan, K. Jia and X. Zhou, Experimental study on catalytic steam gasification of municipal solid waste for bioenergy production in a combined fixed bed reactor, Biomass Bioenergy, 2012, 46, 174–180 CrossRef CAS.
- Y. Wang and C. M. Kinoshita, Experimental analysis of biomass gasification with steam and oxygen, Sol. Energy, 1992, 49(3), 153–158 CrossRef CAS.
- T. Tsuji and A. Hatayama, Gasification of waste plastics by steam reforming in a fluidized bed, J. Mater. Cycles Waste Manage., 2009, 11(2), 144–147 CrossRef CAS.
- I. Barbarias, G. Lopez, J. Alvarez, M. Artetxe, A. Arregi and J. Bilbao, et al., A sequential process for hydrogen production based on continuous HDPE fast pyrolysis and in-line steam reforming, Chem. Eng. J., 2016, 296, 191–198 CrossRef CAS.
- T. Tsuji, S. Okajima, A. Sasaki and T. Masuda, Steam Reforming of Oils Produced from Waste Plastics, J. Chem. Eng. Jpn., 2005, 38(10), 859–864 CrossRef CAS.
- Q. Liu, C. Hu, B. Peng, C. Liu, Z. Li and K. Wu, et al., High H2/CO ratio syngas production from chemical looping co-gasification of biomass and polyethylene with CaO/Fe2O3 oxygen carrier, Energy Convers. Manage., 2019, 199, 111951 CrossRef CAS.
- H. L. Zhu, Y. S. Zhang, M. Materazzi, G. Aranda, D. J. L. Brett and P. R. Shearing, et al., Co-gasification of beech-wood and polyethylene in a fluidized-bed reactor, Fuel Process. Technol., 2019, 190, 29–37 CrossRef CAS.
- F. Pinto, C. Franco, R. N. André, M. Miranda, I. Gulyurtlu and I. Cabrita, Co-gasification study of biomass mixed with plastic wastes, Fuel, 2002, 81(3), 291–297 CrossRef CAS.
- S. Li, I. Cañete Vela, M. Järvinen and M. Seemann, Polyethylene terephthalate (PET) recycling via steam gasification – The effect of operating conditions on gas and tar composition, Waste Manage., 2021, 130, 117–126 CrossRef CAS PubMed.
- J. G. Speight, Handbook of industrial hydrocarbon processes (Second Edition), Gulf Professional Publishing, 2020 Search PubMed.
- M. P. Aznar, M. A. Caballero, J. A. Sancho and E. Francés, Plastic waste elimination by co-gasification with coal and biomass in fluidized bed with air in pilot plant, Fuel Process. Technol., 2006, 87(5), 409–420 CrossRef CAS.
- R. Xiao, B. Jin, H. Zhou, Z. Zhong and M. Zhang, Air gasification of polypropylene plastic waste in fluidized bed gasifier, Energy Convers. Manage., 2007, 48(3), 778–786 CrossRef CAS.
- J. W. Kim, T. Y. Mun, J. O. Kim and J. S. Kim, Air gasification of mixed plastic wastes using a two-stage gasifier for the production of producer gas with low tar and a high caloric value, Fuel, 2011, 90(6), 2266–2272 CrossRef CAS.
- M. H. Cho, T. Y. Mun and J. S. Kim, Air gasification of mixed plastic wastes using calcined dolomite and activated carbon in a two-stage gasifier to reduce tar, Energy, 2013, 53, 299–305 CrossRef CAS.
- C. Wu and P. T. Williams, Pyrolysis–gasification of plastics, mixed plastics and real-world plastic waste with and without Ni–Mg–Al catalyst, Fuel, 2010, 89(10), 3022–3032 CrossRef CAS.
- I. S. Pieta, W. S. Epling, A. Kazmierczuk, P. Lisowski, R. Nowakowski and E. M. Serwicka, Waste into Fuel—Catalyst and Process Development for MSW Valorisation, Catalysts, 2018, 8(3), 113 CrossRef.
- M. Shahbaz, T. Al-Ansari, M. Inayat, S. A. Sulaiman, P. Parthasarathy and G. McKay, A critical review on the influence of process parameters in catalytic co-gasification: current performance and challenges for a future prospectus, Renewable Sustainable Energy Rev., 2020, 134, 110382 CrossRef CAS.
- V. V. Quyen, V. T. T. Trang, N. D. Nam, T. D. Huy and D. N. Binh, Research on the manufacturing magnesium from thanhhoa dolomite by pidgeon process, EUREKA, Phys. Eng., 2020, 2020(6), 97–107 Search PubMed.
- D. Sutton, B. Kelleher and J. R. H. Ross, Review of literature on catalysts for biomass gasification, Fuel Process. Technol., 2001, 73(3), 155–173 CrossRef CAS.
- S. Gunasekaran and G. Anbalagan, Thermal decomposition of natural dolomite, Bull. Mater. Sci., 2007, 30(4), 339–344 CrossRef CAS.
- M. W. Islam, A review of dolomite catalyst for biomass gasification tar removal, Fuel, 2020, 267, 117095 CrossRef CAS.
- A. Orío, J. Corella and I. Narváez, Characterization and Activity of Different Dolomites for Hot Gas Cleaning in Biomass Gasification, Dev. Thermochem. Biomass Convers., 1997, 1144–1157 Search PubMed.
- A. Soomro, S. Chen, S. Ma and W. Xiang, Catalytic activities of nickel, dolomite, and olivine for tar removal and H2-enriched gas production in biomass gasification process, Energy Environ., 2018, 29(6), 839–867 CrossRef CAS.
- J. Delgado, M. P. Aznar and J. Corella, Calcined Dolomite, Magnesite, and Calcite for Cleaning Hot Gas from a Fluidized Bed Biomass Gasifier with Steam: Life and Usefulness, Ind. Eng. Chem. Res., 1996, 35(10), 3637–3643 CrossRef CAS.
- Y. Li and P. T. Williams, Catalytic Biochar and Refuse-Derived Char for the Steam Reforming of Waste Plastics Pyrolysis Volatiles for Hydrogen-Rich Syngas, Ind. Eng. Chem. Res., 2023, 62(36), 14335–14348 CrossRef CAS.
- I. N. Zaini, Y. Gomez-Rueda, C. García López, D. K. Ratnasari, L. Helsen and T. Pretz, et al., Production of H2-rich syngas from excavated landfill waste through steam co-gasification with biochar, Energy, 2020, 207, 118208 CrossRef CAS.
- C. Wu and P. T. Williams, Hydrogen production by steam gasification of polypropylene with various nickel catalysts, Appl. Catal., B, 2009, 87(3–4), 152–161 CrossRef CAS.
- D. Xu, Y. Xiong, J. Ye, Y. Su, Q. Dong and S. Zhang, Performances of syngas production and deposited coke regulation during co-gasification of biomass and plastic wastes over Ni/γ-Al2O3 catalyst: role of biomass to plastic ratio in feedstock, Chem. Eng. J., 2020, 392, 123728 CrossRef CAS.
- R. Alipour Moghadam Esfahani, L. Osmieri, S. Specchia, S. Yusup, A. Tavasoli and A. Zamaniyan, H2-rich syngas production through mixed residual biomass and HDPE waste via integrated catalytic gasification and tar cracking plus bio-char upgrading, Chem. Eng. J., 2017, 308, 578–587 CrossRef.
- F. L. Chan and A. Tanksale, Review of recent developments in Ni-based catalysts for biomass gasification, Renewable Sustainable Energy Rev., 2014, 38, 428–438 CrossRef CAS.
- M. He, B. Xiao, Z. Hu, S. Liu, X. Guo and S. Luo, Syngas production from catalytic gasification of waste polyethylene: influence of temperature on gas yield and composition, Int. J. Hydrogen Energy, 2009, 34(3), 1342–1348 CrossRef CAS.
- P. Friengfung, E. Jamkrajang, S. Sunphorka, P. Kuchonthara and L. Mekasut, NiO/Dolomite Catalyzed Steam/O2 Gasification of Different Plastics and Their Mixtures, Ind. Eng. Chem. Res., 2014, 53(5), 1909–1915 CrossRef CAS.
- X. Xiao, D. D. Le, L. Li, X. Meng, J. Cao and K. Morishita, et al., Catalytic steam gasification of biomass in fluidized bed at low temperature: conversion from livestock manure compost to hydrogen-rich syngas, Biomass Bioenergy, 2010, 34(10), 1505–1512 CrossRef CAS.
- A. Farooq, S. Moogi, S. H. Jang, H. P. R. Kannapu, S. Valizadeh and A. Ahmed, et al., Linear low-density polyethylene gasification over highly active Ni/CeO2–ZrO2 catalyst for enhanced hydrogen generation, J. Ind. Eng. Chem., 2021, 94, 336–342 CrossRef CAS.
- Y. Chai, N. Gao, M. Wang and C. Wu, H2 production from co-pyrolysis/gasification of waste plastics and biomass under novel catalyst Ni–CaO–C, Chem. Eng. J., 2020,(382), 122947 CrossRef CAS.
- Y. Chai, M. Wang, N. Gao, Y. Duan and J. Li, Experimental study on pyrolysis/gasification of biomass and plastics for H2 production under new dual-support catalyst, Chem. Eng. J., 2020, 396, 125260 CrossRef CAS.
- A. Olivares, M. P. Aznar, M. A. Caballero, J. Gil, E. Francés and J. Corella, Biomass Gasification: Produced Gas Upgrading by In-Bed Use of Dolomite, Ind. Eng. Chem. Res., 1997, 36(12), 5220–5226 CrossRef CAS.
- U. Oemar, Y. Kathiraser, M. L. Ang, K. Hidajat and S. Kawi, Catalytic Biomass Gasification to Syngas Over Highly Dispersed Lanthanum-Doped Nickel on SBA-15, ChemCatChem, 2015, 7(20), 3376–3385 CrossRef CAS.
- Z. Xiao, C. Wu, L. Wang, J. Xu, Q. Zheng and L. Pan, et al., Boosting hydrogen production from steam reforming of ethanol on nickel by lanthanum doped ceria, Appl. Catal., B, 2021, 286, 119884 CrossRef CAS.
- S. Czernik and R. J. French, Production of Hydrogen from Plastics by Pyrolysis and Catalytic Steam Reform, Energy Fuels, 2006, 20(2), 754–758 CrossRef CAS.
- A. Erkiaga, G. Lopez, I. Barbarias, M. Artetxe, M. Amutio and J. Bilbao, et al., HDPE pyrolysis-steam reforming in a tandem spouted bed-fixed bed reactor for H2 production, J. Anal. Appl. Pyrolysis, 2015, 116, 34–41 CrossRef CAS.
- T. Namioka, A. Saito, Y. Inoue, Y. Park, T. J. Min and S. A. Roh, et al., Hydrogen-rich gas production from waste plastics by pyrolysis and low-temperature steam reforming over a ruthenium catalyst, Appl. Energy, 2011, 88(6), 2019–2026 CrossRef CAS.
- Y. Park, T. Namioka, S. Sakamoto, T. J. Min, S. A. Roh and K. Yoshikawa, Optimum operating conditions for a two-stage gasification process fueled by polypropylene by means of continuous reactor over ruthenium catalyst, Fuel Process. Technol., 2010, 91(8), 951–957 CrossRef CAS.
- C. Wu and P. T. Williams, Investigation of Ni–Al, Ni–Mg–Al and Ni–Cu–Al catalyst for hydrogen production from pyrolysis–gasification of polypropylene, Appl. Catal., B, 2009, 90(1–2), 147–156 CrossRef CAS.
- H. Zhou, J. M. Saad, Q. Li and Y. Xu, Steam reforming of polystyrene at a low temperature for high H2/CO gas with bimetallic Ni–Fe/ZrO2 catalyst, Waste Manage., 2020, 104, 42–50 CrossRef CAS PubMed.
- F. Raganati, P. Ammendola, N. Slavu and C. Dinca, Clean Energy from Poplar and Plastic Mix Valorisation in a Gas Turbine with CO2 Capture Process, Processes, 2023, 11(10), 2922 CrossRef.
- K. Lan and Y. Yao, Feasibility of gasifying mixed plastic waste for hydrogen production and carbon capture and storage, Commun. Earth Environ., 2022, 3(1), 1–11 CrossRef.
- Y. Chai, N. Packham and M. Wang, Process improvement analysis of pyrolysis/gasification of biomass and waste plastics with carbon capture and utilisation through process simulation, Fuel, 2022, 324, 124571 CrossRef CAS.
- S. Chari, A. Sebastiani, A. Paulillo and M. Materazzi, The Environmental Performance of Mixed Plastic Waste Gasification with Carbon Capture and Storage to Produce Hydrogen in the UK, ACS Sustainable Chem. Eng., 2023, 11(8), 3248–3259 CrossRef CAS.
- C. Salah, S. Cobo, J. Pérez-Ramírez and G. Guillén-Gosálbez, Environmental Sustainability Assessment of Hydrogen from Waste Polymers, ACS Sustainable Chem. Eng., 2023, 11(8), 3238–3247 CrossRef CAS PubMed.
- H. Xu and B. Shi, Design and System Evaluation of Mixed Waste Plastic Gasification Process Based on Integrated Gasification Combined Cycle System, Processes, 2022, 10(3), 499 CrossRef CAS.
- P. Rosha and H. Ibrahim, Hydrogen production via solid waste gasification with subsequent amine-based carbon dioxide removal using Aspen Plus, Int. J. Hydrogen Energy, 2023, 48(64), 24607–24618 CrossRef CAS.
- N. Gupta, B. W. Senior, R. Leader and M. C. Duffy, United States Energy Association: Co-utilization of Coal and Mixed Scrap Plastics via Syngas Production with Carbon Capture, Utilization, and Storage (CCUS), USEA Report, 2021 Search PubMed , Available from https://usea.org/sites/default/files/event-/USEA_Battelle%20Plastics_Report_FINAL.pdf (Accessed Jan. 14, 2025).
- Y. Ma, H. Qi, J. Zhang, P. Cui, Z. Zhu and Y. Wang, Thermodynamic analysis of a carbon capture hydrogen production process for end-of-life tires using plasma gasification, J. Cleaner Prod., 2023, 384, 135662 CrossRef CAS.
- P. Ravi Ganesh, D. Krishnan Nair and B. Webster, CCUS-Coupled Co-Gasification of Plastics as a Potential Negative Emissions Recycling Technology for Synthesis Gas and Hydrogen Production in the United States of America, Proceedings of the 16th Greenhouse Gas Control Technologies Conference (GHGT-16) 23-24 Oct 2022 DOI:10.2139/ssrn.4281137.
- D. R. Nhuchhen, Integrated gasification carbon capture plant using molten carbonate fuel cell: an application to a cement industry, Energy, 2023, 282, 128614 CrossRef CAS.
- E. Shayan, V. Zare and I. Mirzaee, Hydrogen production from biomass gasification; a theoretical comparison of using different gasification agents, Energy Convers. Manage., 2018, 159, 30–41 CrossRef CAS.
- K. P. Shadangi and K. Mohanty, Co-pyrolysis of Karanja and Niger seeds with waste polystyrene to produce liquid fuel, Fuel, 2015, 153, 492–498 CrossRef CAS.
- J. M. N. Van Kasteren, Co-gasification of wood and polyethylene with the aim of CO and H2 production, J. Mater. Cycles Waste Manage., 2006, 8(2), 95–98 CrossRef CAS.
- Z. Wang, J. Li, K. G. Burra, X. Liu, X. Li and M. Zhang, et al., Synergetic Effect on CO2-Assisted Co-Gasification of Biomass and Plastics, J. Energy Resour. Technol., 2021, 143(3), 031901 CrossRef CAS.
- I. G. Harouna, B. Berger, J. Hervé, O. Sanogo, T. Daho and S. K. Ouiminga, et al., Experimental study of the co-gasification of wood and polyethylene in a two-stage gasifier, Energy Sci. Eng., 2020, 8(7), 2322–2334 CrossRef CAS.
- A. Arregi, M. Amutio, G. Lopez, M. Artetxe, J. Alvarez and J. Bilbao, et al., Hydrogen-rich gas production by continuous pyrolysis and in-line catalytic reforming of pine wood waste and HDPE mixtures, Energy Convers. Manage., 2017, 136, 192–201 CrossRef CAS.
- V. Wilk and H. Hofbauer, Co-gasification of Plastics and Biomass in a Dual Fluidized-Bed Steam Gasifier: Possible Interactions of Fuels, Energy Fuels, 2013, 27(6), 3261–3273 CrossRef CAS.
- S. Zhang, S. Zhu, H. Zhang, X. Liu and Y. Xiong, High quality H2-rich syngas production from pyrolysis-gasification of biomass and plastic wastes by Ni–Fe@Nanofibers/Porous carbon catalyst, Int. J. Hydrogen Energy, 2019, 44(48), 26193–26203 CrossRef CAS.
- F. Pinto, R. André, M. Miranda, D. Neves, F. Varela and J. Santos, Effect of gasification agent on co-gasification of rice production wastes mixtures, Fuel, 2016, 180, 407–416 CrossRef CAS.
- M. H. Basha, S. A. Sulaiman and Y. Uemura, Co-gasification of palm kernel shell and polystyrene plastic: effect of different operating conditions, J. Energy Inst., 2020, 93(3), 1045–1052 CrossRef CAS.
- P. Brachi, R. Chirone, F. Miccio, M. Miccio, A. Picarelli and G. Ruoppolo, Fluidized bed co-gasification of biomass and polymeric wastes for a flexible end-use of the syngas: focus on bio-methanol, Fuel, 2014, 128, 88–98 CrossRef CAS.
- M. Bassyouni, S. W. Ul Hasan, M. H. Abdel-Aziz, S. M. S. Abdel-Hamid, S. Naveed and A. Hussain, et al., Date palm waste gasification in downdraft gasifier and simulation using ASPEN HYSYS, Energy Convers. Manage., 2014, 88, 693–699 CrossRef CAS.
- C. Cao, C. Bian, G. Wang, B. Bai, Y. Xie and H. Jin, Co-gasification of plastic wastes and soda lignin in supercritical water, Chem. Eng. J., 2020, 388, 124277 CrossRef CAS.
- M. Cortazar, N. Gao, C. Quan, M. A. Suarez, G. Lopez and S. Orozco, et al., Analysis of hydrogen production potential from waste plastics by pyrolysis and in line oxidative steam reforming, Fuel Process. Technol., 2022, 225, 107044 CrossRef CAS.
- F. Pinto, C. Franco, R. N. André, M. Miranda, I. Gulyurtlu and I. Cabrita, Co-gasification study of biomass mixed with plastic wastes, Fuel, 2002, 81(3), 291–297 CrossRef CAS.
- J. Alvarez, S. Kumagai, C. Wu, T. Yoshioka, J. Bilbao and M. Olazar, et al., Hydrogen production from biomass and plastic mixtures by pyrolysis-gasification, Int. J. Hydrogen Energy, 2014, 39(21), 10883–10891 CrossRef CAS.
- Y. Chai, M. Wang, N. Gao, Y. Duan and J. Li, Experimental study on pyrolysis/gasification of biomass and plastics for H2 production under new dual-support catalyst, Chem. Eng. J., 2020, 396, 125260 CrossRef CAS.
- K. G. Burra and A. K. Gupta, Synergistic effects in steam gasification of combined biomass and plastic waste mixtures, Appl. Energy, 2018, 211, 230–236 CrossRef CAS.
- Y. Chai, M. Wang, N. Gao, Y. Duan and J. Li, Experimental study on pyrolysis/gasification of biomass and plastics for H2 production under new dual-support catalyst, Chem. Eng. J., 2020, 396, 125260 CrossRef CAS.
- L. Abdelouahed, O. Authier, G. Mauviel, J. P. Corriou, G. Verdier and A. Dufour, Detailed modeling of biomass gasification in dual fluidized bed reactors under aspen plus, Energy Fuels, 2012, 26(6), 3840–3855 CrossRef CAS.
- N. Déparrois, P. Singh, K. G. Burra and A. K. Gupta, Syngas production from co-pyrolysis and co-gasification of polystyrene and paper with CO2, Appl. Energy, 2019, 246, 1–10 CrossRef.
- US EIA A. Coal explained – U.S. Energy Information Administration (EIA). Available from: https://www.eia.gov/energyexplained/coal/.
- F. Pinto, R. André, M. Miranda, D. Neves, F. Varela and J. Santos, Effect of gasification agent on co-gasification of rice production wastes mixtures, Fuel, 2016, 180, 407–416 CrossRef CAS.
- M. Ouadi, J. G. Brammer, M. Kay and A. Hornung, Fixed bed downdraft gasification of paper industry wastes, Appl. Energy, 2013, 103, 692–699 CrossRef CAS.
- S. Akkache, A. B. Hernández, G. Teixeira, F. Gelix, N. Roche and J. H. Ferrasse, Co-gasification of wastewater sludge and different feedstock: feasibility study, Biomass Bioenergy, 2016, 89, 201–209 CrossRef CAS.
- U. Arena, L. Zaccariello and M. L. Mastellone, Fluidized bed gasification of waste-derived fuels, Waste Manage., 2010, 30(7), 1212–1219 CrossRef CAS PubMed.
- F. Pinto, R. N. André, C. Franco, H. Lopes, I. Gulyurtlu and I. Cabrita, Co-gasification of coal and wastes in a pilot-scale installation 1: effect of catalysts in syngas treatment to achieve tar abatement, Fuel, 2009, 88(12), 2392–2402 CrossRef CAS.
- S. Du, S. Yuan and Q. Zhou, Numerical investigation of co-gasification of coal and PET in a fluidized bed reactor. Renew, Energy, 2021, 172, 424–439 CAS.
- L. Zaccariello and M. L. Mastellone, Fluidized-Bed Gasification of Plastic Waste, Wood, and Their Blends with Coal, Energies, 2015, 8(8), 8052–8068 CrossRef CAS.
- M. L. Mastellone, L. Zaccariello, D. Santoro and U. Arena, The O2-enriched air gasification of coal, plastics and wood in a fluidized bed reactor, Waste Manage., 2012, 32(4), 733–742 CrossRef CAS PubMed.
- V. Kříž and O. Bičáková, Hydrogen from the two-stage pyrolysis of bituminous coal/waste plastics mixtures, Int. J. Hydrogen Energy, 2011, 36(15), 9014–9022 CrossRef.
- H. J. Song, J. Lee, A. Gaur, J. J. Park and J. W. Park, Production of gaseous fuel from refuse plastic fuel via co-pyrolysis using low-quality coal and catalytic steam gasification, J. Mater. Cycles Waste Manage., 2010, 12(4), 295–301 CrossRef CAS.
- M. Pohořelý, M. Vosecký, P. Hejdová, M. Punčochář, S. Skoblja and M. Staf, et al., Gasification of coal and PET in fluidized bed reactor, Fuel, 2006, 85(17–18), 2458–2468 CrossRef.
- D. T. Pio and L. A. C. Tarelho, Industrial gasification systems (>3 MWth) for bioenergy in Europe: current status and future perspectives, Renewable Sustainable Energy Rev., 2021, 145, 111108 CrossRef CAS.
- K. M. Wyss, K. J. Silva, K. V. Bets, W. A. Algozeeb, C. Kittrell and C. H. Teng, et al., Synthesis of Clean Hydrogen Gas from Waste Plastic at Zero Net Cost, Adv. Mater., 2023, 35(48), 2306763 CrossRef CAS PubMed.
- M. M. Ismail and I. Dincer, A new renewable energy based integrated gasification system for hydrogen production from plastic wastes, Energy, 2023, 270, 126869 CrossRef CAS.
- R. R. Mishra and A. K. Sharma, Microwave–material interaction phenomena: heating mechanisms, challenges and opportunities in material processing, Composites, Part A, 2016, 81, 78–97 CrossRef CAS.
- J. Zhao, D. Wang, L. Zhang, M. He, W. Ma and S. Zhao, Microwave-enhanced hydrogen production: a review, RSC Adv., 2023, 13(22), 15261–15273 RSC.
- C. O. Kappe, Controlled Microwave Heating in Modern Organic Synthesis, Angew. Chem., Int. Ed., 2004, 43(46), 6250–6284 CrossRef CAS PubMed.
- Z. Kawala and T. Atamańczuk, Microwave-Enhanced Thermal Decontamination of Soil, Environ. Sci. Technol., 1998, 32(17), 2602–2607 CrossRef CAS.
- Y. Yan, H. Zhou, S. M. Xu, J. Yang, P. Hao and X. Cai, et al., Electrocatalytic Upcycling of Biomass and Plastic Wastes to Biodegradable Polymer Monomers and Hydrogen Fuel at High Current Densities, J. Am. Chem. Soc., 2023, 145(11), 6144–6155 CrossRef CAS PubMed.
- G. Lopez and L. Santamaria, Microwaving plastic into hydrogen and carbons, Nat. Catal., 2020, 3(11), 861–862 CrossRef CAS.
- J. C. Acomb, C. Wu and P. T. Williams, The use of different metal catalysts for the simultaneous production of carbon nanotubes and hydrogen from pyrolysis of plastic feedstocks, Appl. Catal., B, 2016, 180, 497–510 CrossRef CAS.
- J. Wang, Y. Pan, J. Song and Q. Huang, A high-quality hydrogen production strategy from waste plastics through microwave-assisted reactions with heterogeneous bimetallic iron/nickel/cerium catalysts, J. Anal. Appl. Pyrolysis, 2022, 166, 105612 CrossRef CAS.
- Microwave Heating – Mechanism and Theory. Available from: https://cem.com/microwave-heating-mechanism-and-theory. (Accessed: Jan. 14, 2025).
- W. Li, K. Qian, Z. Yang, X. Ding, W. Tian and D. Chen, Promotion effect of cobalt doping on microwave-initiated plastic deconstruction for hydrogen production over iron catalysts, Appl. Catal., B, 2023, 327, 122451 CrossRef CAS.
- L. Yao, B. Yi, X. Zhao, W. Wang, Y. Mao and J. Sun, et al., Microwave-assisted decomposition of waste plastic over Fe/FeAl2O4 to produce hydrogen and carbon nanotubes, J. Anal. Appl. Pyrolysis, 2022, 165, 105577 CrossRef CAS.
- D. Yao, Y. Zhang, P. T. Williams, H. Yang and H. Chen, Co-production of hydrogen and carbon nanotubes from real-world waste plastics: influence of catalyst composition and operational parameters, Appl. Catal., B, 2018, 221, 584–597 CrossRef CAS.
- D. Yao and C. H. Wang, Pyrolysis and in-line catalytic decomposition of polypropylene to carbon nanomaterials and hydrogen over Fe- and Ni-based catalysts, Appl. Energy, 2020, 265, 114819 CrossRef CAS.
- X. Jie, W. Li, D. Slocombe, Y. Gao, I. Banerjee and S. Gonzalez-Cortes, et al., Microwave-initiated catalytic deconstruction of plastic waste into hydrogen and high-value carbons, Nat. Catal., 2020, 3(11), 902–912 CrossRef CAS . Available from: https://www.nature.com/articles/s41929-020-00518-5.
- A. Abedin, X. Bai, M. Smith and P. Muley, Microwave-assisted co-gasification of mixed plastics and corn stover: a synergistic approach to produce clean hydrogen, Energy Convers. Manage., 2023, 280, 116774 CrossRef CAS.
- E. Gomez, D. A. Rani, C. R. Cheeseman, D. Deegan, M. Wise and A. R. Boccaccini, Thermal plasma technology for the treatment of wastes: a critical review, J. Hazard. Mater., 2009, 161(2–3), 614–626 CrossRef CAS PubMed.
- A. Faaij, R. Van Ree, L. Waldheim, E. Olsson, A. Oudhuis and A. Van Wijk, et al., Gasification of biomass wastes and residues for electricity production, Biomass Bioenergy, 1997, 12(6), 387–407 CrossRef.
- A. S. An'shakov, V. A. Faleev, A. A. Danilenko, E. K. Urbakh and A. E. Urbakh, Investigation of plasma gasification of carbonaceous technogeneous wastes, Thermophys. Aeromech., 2007, 14(4), 607–616 CrossRef.
- H. Knoef, Handbook biomass gasification, BTG Biomass Technology Group, 2005 Search PubMed.
- V. S. Sikarwar, M. Hrabovský, G. Van Oost, M. Pohořelý and M. Jeremiáš, Progress in waste utilization via thermal plasma, Prog. Energy Combust. Sci., 2020, 81, 100873 CrossRef.
- L. Tang, H. Huang, H. Hao, H. Wang, Y. Wang and C. Zhu, Waste tires disposal by thermal plasma for power generation and carbon black recovery, APPEEC 27-31 March 2009. Available from: https://doi.org/10.1109/APPEEC.2009.4918968 (Accessed: Jan 2025).
- P. Carabin and G. Holcroft, Plasma Resource Recovery Technology: Converting Waste to Energy and Valuable Products, NAWTEC13, 2008 Nov 11. Available from: https://dx.doi.org/10.1115/NAWTEC13-3155 (Accessed: Jan 2025).
- K. P. Willis, S. Osada and K. L. Willerton, Plasma Gasification: Lessons Learned at Eco-Valley WTE Facility, NAWTEC18, 2010 Nov 1, Available from: https://dx.doi.org/10.1115/NAWTEC18-3515 (Accessed: Jan 2025).
- L. Tang, H. Huang, Z. Zhao, C. Z. Wu and Y. Chen, Pyrolysis of polypropylene in a nitrogen plasma reactor, Ind. Eng. Chem. Res., 2003, 42(6), 1145–1150 CrossRef CAS.
Footnote |
| † M. A. B. and T. J. contributed equally. |
| This journal is © The Royal Society of Chemistry 2025 |