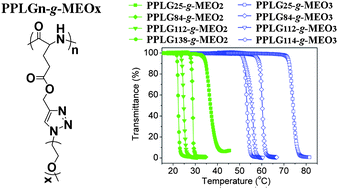
A series of novel temperature-sensitive polypeptides were synthesized by ring opening polymerization (ROP) of γ-propargyl-L-glutamateN-carboxyanhydride (PLG-NCA) and subsequent click reaction between the pendant alkyne groups and 1-(2-methoxyethoxy)-2-azidoethane (MEO2-N3) or 1-(2-(2-methoxyethoxy)ethoxy)-2-azidoethane (MEO3-N3). The efficient click grafting and structure of the resultant copolymers were verified by 1H NMR, 13C NMR and GPC. All the copolymers hold α-helix conformation, and could self-assemble into amphiphilic nanoparticles in aqueous solution with hydrodynamic radii (Rh) of 32.3–62.8 nm. The graft copolymers exhibited sharp temperature-dependent phase transitions, and the LCST could be adjusted from 22.3 to 74.1 °C by varying the molecular weight, the length of the OEG side chain, the polymer concentration and salt concentration. MTT assays revealed that the graft copolymers exhibited no detectable cytotoxicity at all test concentrations up to 1 mg mL−1. In vitrodegradation tests demonstrated that the graft copolymers could be degraded by proteinase K. The drug release behaviors from the PPLG112-g-MEO2 nanoparticles were evaluated at 37 °C and 15 °C using doxorubicin (DOX) as a model drug. The drug release behavior displayed thermosensitivity, and a sustained release profile was observed at physiological temperature. These results suggested that the novel biodegradable and biocompatible polypeptide derivatives with adjustable temperature sensitivity could be a promising material for biomedical applications.