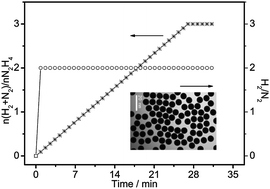
A facile, reliable, and robust synthetic method for the preparation of monodisperse Ni3Fe single-crystalline nanospheres with 182.3 m2 g−1 specific surface area is reported. The results of an investigation of the shape/size distribution, structural characteristics, and composition of these Ni3Fe nanospheres are presented, and a possible mechanism for the formation of the nanospheres at high temperatures in a cetylamine–palmitic acid solution in the presence of tri-iso-butyl thallium and borane trimethylamine complexes is proposed. During the decomposition of hydrazine, when supported on carbon (Ni3Fe/C), the monodisperse Ni3Fe nanospheres exerted 100% H2 selectivity, and showed exceedingly high activity at room temperature. The decomposition reaction is first-order in Ni3Fe concentration, but zero-order in N2H4 concentration; the activation energy was 48.1 ± 2.0 kJ mol−1. Furthermore, the Ni3Fe/C was a stable catalyst for N2H4 hydrolytic dehydrogenation; it provided 15 840 total turnovers in 30 h. An Ni3Fe nanosphere-based catalyst such as this that is efficient under mild reaction conditions represents a promising step toward the practical development of N2H4 as a feasible hydrogen storage medium for fuel cell applications.