Ryota Miyaji, Keisuke Asano and Seijiro Matsubara
Org. Biomol. Chem., 2014,12, 119-122
DOI:
10.1039/C3OB41938J,
Paper
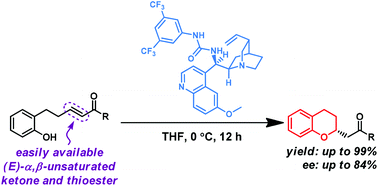
Cinchona-alkaloid-urea-based bifunctional organocatalysts facilitate the catalytic asymmetric synthesis of chroman derivatives via an intramolecular oxy-Michael addition reaction. Phenol derivatives bearing an easily available (E)-α,β-unsaturated ketone or a thioester moiety are useful substrates for the title transformation. This method represents a facile synthesis of various optically active 2-substituted chromans in high yield.