Hyphenated techniques of thermal analysis for dibenz[b,f][1,4]oxazepine
Abstract
As a third-generation tear agent, dibenz[b,f][1,4]oxazepine (CR) has been widely used for anti-terrorism and riot control efforts. To improve the efficiency of CR use and determine the toxicity of its decomposition products, it is necessary to study its thermal stability and thermal decomposition behaviour. The mass loss and thermal behaviour of CR were studied at different heating rates using thermogravimetry (TGA) and differential scanning calorimetry (DSC) techniques. The gas products were analyzed using Fourier transform infrared (FTIR) spectroscopy. The present work also studied the thermal decomposition characteristics of CR for temperatures in the 200–600 °C range using the pyrolysis-gas chromatography/mass spectrometry (PY-GC/MS) technique, and the decomposition products were identified. The results show that CR fuses at approximately 69 °C, and that the heating rate has a relatively strong influence on the extrapolated initial decomposition temperature. In the absence of oxygen, when the heating rate is 2 °C min−1, CR starts to decompose at 172 °C. The mechanism of the thermal decomposition is described by the Zhuralev–Lesokin–Tempelman equation, 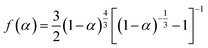 , and the activation energy is approximately 230 kJ mol−1. In the thermal pyrolysis experiment, the first step of thermal decomposition of CR occurs between 200 and 300 °C. Below 600 °C, in an aerobic environment, the pyrolysis reaction occurs to produce 2-aminodiphenyl ether, whereas the oxidizing reaction occurs to produce 10,11-dihydrodibenz[b,f][1,4]oxazepin-11-one, with the products obtained independent of temperature. According to the experimental results, the burning temperature for a mixture of CR and fireworks is suggested to be below 200 °C.
, and the activation energy is approximately 230 kJ mol−1. In the thermal pyrolysis experiment, the first step of thermal decomposition of CR occurs between 200 and 300 °C. Below 600 °C, in an aerobic environment, the pyrolysis reaction occurs to produce 2-aminodiphenyl ether, whereas the oxidizing reaction occurs to produce 10,11-dihydrodibenz[b,f][1,4]oxazepin-11-one, with the products obtained independent of temperature. According to the experimental results, the burning temperature for a mixture of CR and fireworks is suggested to be below 200 °C.
![Graphical abstract: Hyphenated techniques of thermal analysis for dibenz[b,f][1,4]oxazepine](/en/Image/Get?imageInfo.ImageType=GA&imageInfo.ImageIdentifier.ManuscriptID=C5AY03368C&imageInfo.ImageIdentifier.Year=2016)

 Please wait while we load your content...
Please wait while we load your content...