Hydrogen bond cooperativity and anticooperativity within the water hexamer
Abstract
The hydrogen bond (HB), arguably the most important non-covalent interaction in chemistry, is getting renewed attention particularly in materials engineering. We address herein HB non-additive features by examining different structures of the water hexamer (cage, prism, book, bag and ring). To that end, we rely on the interacting quantum atoms (IQA) topological energy partition, an approach that has been successfully used to study similar effects in smaller water clusters (see Chem. – Eur. J., 19, 14304). Our IQA interaction energies, 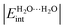 , are used to classify the strength of HBs in terms of the single/double character of the donor and acceptor H2O molecules involved in the interaction. The strongest hydrogen bonds on this new scale entail double donors and acceptors that show larger values of
, are used to classify the strength of HBs in terms of the single/double character of the donor and acceptor H2O molecules involved in the interaction. The strongest hydrogen bonds on this new scale entail double donors and acceptors that show larger values of 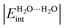 than those observed in homodromic cycles, paradigms of cooperative effects. Importantly, this means that besides the traditional HB anticooperativity ascribed to double acceptors and donors, the occurrence of these species is also related to HB strengthening. Overall, we hope that the results of this research will lead to a further understanding of the HB non-additivity in intramolecular and intermolecular interactions.
than those observed in homodromic cycles, paradigms of cooperative effects. Importantly, this means that besides the traditional HB anticooperativity ascribed to double acceptors and donors, the occurrence of these species is also related to HB strengthening. Overall, we hope that the results of this research will lead to a further understanding of the HB non-additivity in intramolecular and intermolecular interactions.


 Please wait while we load your content...
Please wait while we load your content...