J. K. Molloy, O. Jarjayes, C. Philouze, L. Fedele, D. Imbert and F. Thomas
Chem. Commun., 2017,53, 605-608
DOI:
10.1039/C6CC07942C,
Communication
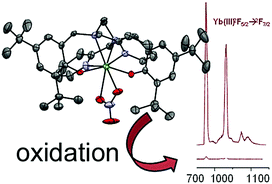
The reversible oxidation of coordinated phenolates into phenoxyl radicals results in a dramatic quenching (>95%) of the luminescence of the f metal ion.