Revisiting binding of plutonium to transferrin by CE-ICP-MS
Abstract
Capillary electrophoresis coupled with an inductively coupled plasma mass spectrometer was applied for the first time to determine the binding constant of human transferrin (Tf) for tetravalent plutonium. The experiments were carried out in a buffer 2-(N-morpholino)ethanesulfonic acid (MES) at pH 6, 0.1 M NaCl and at a temperature of 25 °C. The nitrilotriacetate anion (NTA) used in this study prevents the hydrolysis of plutonium and is an ideal competitor with Tf for Pu, both ligands sharing comparable binding strength. The separation revealed unambiguous two peaks associated with the complex Pu(NTA)2 used as the initial species and with Pu–transferrin. Two series of independent experiments were conducted and gave the first stepwise conditional bicarbonate-free Pu–transferrin binding constant of 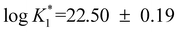 . In the absence of bicarbonate the affinity of transferrin for plutonium at pH 6 is about 104 times stronger than that of iron at pH 6.7
. In the absence of bicarbonate the affinity of transferrin for plutonium at pH 6 is about 104 times stronger than that of iron at pH 6.7 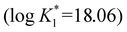 .
.



 Please wait while we load your content...
Please wait while we load your content...