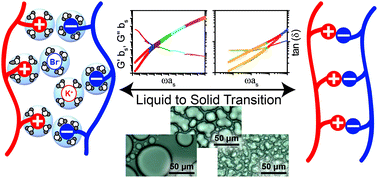
Polyelectrolyte complexation has long been known to result in both liquid and solid complexes. However, the exact nature of the liquid-to-solid transition remains an open question. We have used rheology to explain this phenomenon for the model system of poly(4-styrenesulfonic acid, sodium salt) (PSS) and poly(diallyldimethyl ammonium chloride) (PDADMAC) in the presence of potassium bromide (KBr). The use of a time-salt superposition allows for a detailed analysis of changes in the linear viscoelastic response for both liquid complex coacervates and solid polyelectrolyte complexes as a function of salt concentration, and facilitates unambiguous determination of the mechanism for this phase transition. Decreasing salt concentration, and the commensurate decrease in the water content of PSS/PDADMAC/KBr complexes is shown to lead to the formation of a physical gel due to the development of a network with trapped electrostatic crosslinks that percolates the sample at a critical salt concentration.