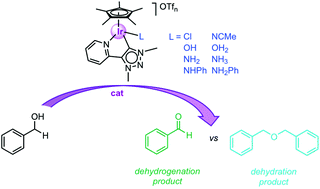
We synthesized a set of triazolylidene iridium(III) complexes [IrCp*(C^N)L]n+ (Cp* = pentamethylcyclopentadienyl, C^N = C,N-bidentate coordinating pyridyl-triazolylidene) containing different neutral or anionic ancillary ligands L and evaluated their impact on the catalytic activity in alcohol conversion. We demonstrate that these ancillary ligands have a strong influence on the catalytic selectivity and direct whether the iridium center preferentially catalyzes either the dehydrogenation or the dehydration of benzyl alcohol. Ligand exchange experiments provide a direct correlation of ligand lability with catalytic activity and selectivity. These results underline the relevance of ancillary ligands and provide a rational approach to tailor the catalytic activity of the iridium center towards aldehyde formation (loss of H2) or etherification (elimination of H2O).