Bond energy, site preferential occupancy and Eu2+/3+ co-doping system induced by Eu3+ self-reduction in Ca10M(PO4)7 (M = Li, Na, K) crystals†
Abstract
Ca10M(PO4)7:Eu (M = Li, Na, K) phosphors have been synthesized via a solid-state reaction process, their phase purity was examined using XRD patterns, and Rietveld refinement confirmed that the Ca10Li(PO4)7, Ca10Na(PO4)7 and Ca10K(PO4)7 are pure phases. The photoluminescence properties of the Ca10M(PO4)7:Eu (M = Li, Na, K) phosphors showed that the self-reduction of Eu3+ to Eu2+ can occur in an air atmosphere. Eu3+ ions can be reduced to Eu2+ ions when doped in Ca10Li(PO4)7, Ca10Na(PO4)7 and Ca10K(PO4)7 crystals, which was detected using photoluminescence spectra. In this work, the bond energy method was used to determine and explain the mechanism of site occupation of Eu entering the host matrix. According to the calculated value of the deviation of bond energy for Eu3+-doped Ca10M(PO4)7 (M = Li, Na, K) crystals, the similar value between 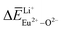 and
and 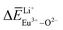 ,
, 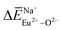 and
and 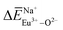 , and
, and 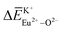 and
and 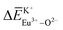 can provide the conditions for the self-reduction of Eu3+ in the Ca10M(PO4)7 (M = Li, Na, K) system. Meanwhile, the smaller deviation values of
can provide the conditions for the self-reduction of Eu3+ in the Ca10M(PO4)7 (M = Li, Na, K) system. Meanwhile, the smaller deviation values of 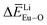 ,
, 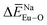 , and
, and 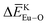 in Ca10Li(PO4)7, Ca10Na(PO4)7, and Ca10K(PO4)7 crystals and
in Ca10Li(PO4)7, Ca10Na(PO4)7, and Ca10K(PO4)7 crystals and 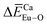 in Ca10K(PO4)7 crystals indicated that the preferential sites of Eu ion occupancy in the Ca10M(PO4)7 (M = Li, Na, K) lattices are Li, Na, K and Ca sites. The conclusions obtained from the calculated results of the bond energy method are consistent with the Rietveld refinement and the photoluminescence spectra of Ca10M(PO4)7 (M = Li, Na, K).
in Ca10K(PO4)7 crystals indicated that the preferential sites of Eu ion occupancy in the Ca10M(PO4)7 (M = Li, Na, K) lattices are Li, Na, K and Ca sites. The conclusions obtained from the calculated results of the bond energy method are consistent with the Rietveld refinement and the photoluminescence spectra of Ca10M(PO4)7 (M = Li, Na, K).



 Please wait while we load your content...
Please wait while we load your content...