Lizheng Yang, Yipin Zhang, Xiaodong Zou, Hongjian Lu and Guigen Li
Green Chem., 2018,20, 1362-1366
DOI:
10.1039/C7GC03392C,
Paper
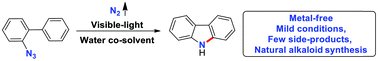
An effective and operationally simple protocol is reported for the synthesis of versatile carbazoles. With water as a co-solvent, visible-light rather than various metals is used to facilitate the conversion of readily available 2-azidobiphenyls under mild conditions. Various functionalized bioactive natural alkaloids, such as glycoborine, clausine C, clausine L, clausine H and clauszoline K, were synthesized efficiently with nitrogen as a sole byproduct. The reaction could be performed in water, acidic or alkaline buffer solutions, showing its potential for applications in biochemistry.