Modeling and prediction of methanol air release from bleached chemi-thermo mechanical pulp board†
Abstract
This paper reports on the modeling, prediction and evaluation approaches of methanol release from bleached chemi-thermo mechanical pulp (BCTMP) board during storage. A pseudo-first order desorption kinetics model of methanol release was established for describing the desorption behavior of methanol from BCTMP, i.e., 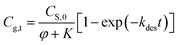 , in which the desorption constant (K) and rate constant (kdes) were well described by van't Hoff and Arrhenius equations. Based on the simulation experiments at various temperatures, the desorption activation energy of methanol and its adsorption enthalpy is calculated and is 53.7 and −86.2 kJ mol−1 K−1, respectively. With the developed model, the risk of methanol release for the storage of BCTMP board can be examined by either the time-dependent kinetics model or a two-step thermodynamic approach using the equilibrium concentration of methanol in indoor air. This paper provides a valuable tool to assess the risk of methanol release for the paper industry and related warehouse departments.
, in which the desorption constant (K) and rate constant (kdes) were well described by van't Hoff and Arrhenius equations. Based on the simulation experiments at various temperatures, the desorption activation energy of methanol and its adsorption enthalpy is calculated and is 53.7 and −86.2 kJ mol−1 K−1, respectively. With the developed model, the risk of methanol release for the storage of BCTMP board can be examined by either the time-dependent kinetics model or a two-step thermodynamic approach using the equilibrium concentration of methanol in indoor air. This paper provides a valuable tool to assess the risk of methanol release for the paper industry and related warehouse departments.



 Please wait while we load your content...
Please wait while we load your content...