Understanding the effects of vicinal carbon substituents and configuration on organofluorine hydrogen-bonding interaction†
Abstract
An investigation of C–F⋯H–O hydrogen bonds in the complexes CHnXCHnF⋯H2O (n = 0, 1, 2; X = H, F, Cl, Br) was performed at the MP2/aug-cc-pVTZ level. We found that the electron-withdrawing halogen substituents on the vicinal carbon cause the fluorine atom, participating in the hydrogen bond formation, to be less negatively charged. Thus, the halogen groups weaken the strength of organofluorine hydrogen bond by inductive effect. The position of the substituents on the vicinal carbon affects the strength of the C–F⋯H–O interaction. Compared with that in other isomers, the electron withdrawing substituent in 1-fluoro-ethane with stagger conformation as well as in 1-fluoro-ethene with trans configuration much weakens the interaction of C–F⋯H–O due to the hyperconjugative interaction between σ(C–F) and 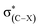 . By analogy, the electron-donating ones could largely strengthen it. We found that there is a good linear relationship between electron density at the BCP of F⋯H and Wiberg bond indexes (WBI) as well as between natural bond-bond polarizability (NBBP) and WBI, which indicates that the magnitude of NBBP and WBI could be a good indicator of the hydrogen bond strength.
. By analogy, the electron-donating ones could largely strengthen it. We found that there is a good linear relationship between electron density at the BCP of F⋯H and Wiberg bond indexes (WBI) as well as between natural bond-bond polarizability (NBBP) and WBI, which indicates that the magnitude of NBBP and WBI could be a good indicator of the hydrogen bond strength.



 Please wait while we load your content...
Please wait while we load your content...