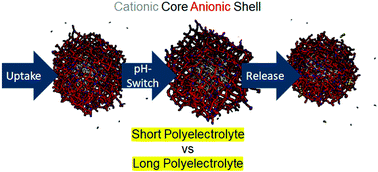
To realize carriers for drug delivery, cationic containers are required for anionic guests. Nevertheless, the toxicity of cationic carriers limits their practical use. In this study, we investigate a model system of polyampholyte N-isopropylacrylamide (NIPAM)-based microgels with a cationic core and an anionic shell to study whether the presence of a negative shell allows the cationic core to be shielded while still enabling the uptake and release of the anionic guest polyelectrolytes. These microgels are loaded with polystyrene sulfonate of different molecular weights to investigate the influence of their chain length on the uptake and release process. By means of small-angle neutron scattering, we evaluate the spatial distribution of polystyrene sulfonate within the microgels. The guest molecules are located in different parts of the core–shell microgels depending on their size. By combining these scattering results with UV-vis spectroscopy, electrophoretic mobility and potentiometric titrations we gain complementary results to investigate the uptake and release process of polyelectrolytes in polyampholyte core–shell microgels. Moreover, Brownian molecular dynamic simulations are performed to compare the experimental and theoretical results of this model. Our findings demonstrate that the presence of a shell still enables efficient uptake of guest molecules into the cationic core. These anionic guest molecules can be released through an anionic shell. Furthermore, the presence of a shell enhances the stability of the microgel–polyelectrolyte complexes with respect to the cationic precursor microgel alone.