Surface chemistry imposes selective reduction of CO2 to CO over Ta3N5/LaTiO2N photocatalyst†
Abstract
Understanding the mechanism underlying photocatalytic product selectivity will provide valuable guidance in developing novel catalysts and reaction pathways. In this article, the role of surface chemistry in product generation is well-demonstrated using LaTiO2N, Ta3N5, and their hybrids with dominant (002) and (020) facets, respectively. Photocatalytic test results show that CO2 could be reduced to CH4 and CO in the presence of LaTiO2N and Ta3N5 photocatalysts, respectively. The detected intermediates suggest that the reaction pathway could possibly be 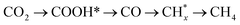 . Product selectivity is dependent on the ability to capture CO onto the photocatalyst surface, which is probably related to the electronegativity of the adsorption sites. Strong adsorption of CO on the LaTiO2N surface favors the subsequent hydrogenation reaction to generate CH4, while weak CO adsorption on Ta3N5 results in CO as the main product of CO2 reduction, which is demonstrated by theoretical calculations as well. Such product selectivity is mainly related to the surface chemistry of the photocatalyst and independent of the surface charge concentration. Our results provide a progressive understanding of selective product formation during photocatalytic CO2 reduction.
. Product selectivity is dependent on the ability to capture CO onto the photocatalyst surface, which is probably related to the electronegativity of the adsorption sites. Strong adsorption of CO on the LaTiO2N surface favors the subsequent hydrogenation reaction to generate CH4, while weak CO adsorption on Ta3N5 results in CO as the main product of CO2 reduction, which is demonstrated by theoretical calculations as well. Such product selectivity is mainly related to the surface chemistry of the photocatalyst and independent of the surface charge concentration. Our results provide a progressive understanding of selective product formation during photocatalytic CO2 reduction.



 Please wait while we load your content...
Please wait while we load your content...