Bing Tang, Fan Lv, Kangkang Chen, Lijuan Jiao, Qingyun Liu, Hua Wang and Erhong Hao
Chem. Commun., 2019,55, 4691-4694
DOI:
10.1039/C9CC01602C,
Communication
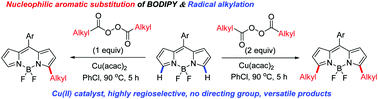
An efficient Cu(II)-catalyzed, C–H alkylation of BODIPY with a variety of alkyl diacyl peroxides has been developed for the first time, providing a late-stage and straightforward method for controllable synthesis of monoalkylated and dialkylated BODIPYs via a radical process that otherwise is difficult to obtain by literature methods. This chemo- and site-selective transformation will allow for the introduction of a variety of functionalities on the BODIPY core for highly versatile tethering to receptors and to other molecules of interest.