Identifying the rate-determining step of the electrocatalytic hydrodechlorination reaction on palladium nanoparticles†
Abstract
Identifying the rate-determining step over the catalysts and clarifying the underlying mechanisms are crucial for maximizing the electrocatalytic hydrodechlorination (EHDC) efficiency for detoxification of the chlorophenol pollutants in water. Here, monodisperse palladium nanoparticles (Pd NPs) separately supported on carbon (C) and titanium nitride (TiN) were synthesized as two model catalysts. The support effects on EHDC efficiency, kinetics and current efficiency towards 2,4-dichlorophenol (2,4-DCP), and the electronic structure of Pd and its binding strengths with 2,4-DCP, phenol and Cl− (the primary EHDC product) were investigated by experimental and density functional theory (DFT) analyses. The low current efficiency (<30%) of both catalysts and the good description of EHDC kinetics by the Langmuir–Hinshelwood model suggest that the 2,4-DCP coverage on Pd, rather than the well-known adsorbed hydrogen 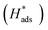 generation, determines EHDC efficiency. Furthermore, the superior EHDC efficiency on TiN–Pd (96.4% vs. 80.9% on C–Pd), coupled with the weakened adsorption of 2,4-DCP and phenol on TiN-supported Pd, demonstrates that the 2,4-DCP coverage is largely influenced by phenol due to its poisoning effect by blocking active sites, and phenol desorption is the rate-determining step of EHDC on the catalyst. The support TiN enables alleviation of the phenol poisoning by modulating the electronic structure of Pd. The d band center of Pd can serve as a potential descriptor of EHDC efficiency, and its optimization for balancing 2,4-DCP and phenol adsorption should be an effective strategy to enhance EHDC.
generation, determines EHDC efficiency. Furthermore, the superior EHDC efficiency on TiN–Pd (96.4% vs. 80.9% on C–Pd), coupled with the weakened adsorption of 2,4-DCP and phenol on TiN-supported Pd, demonstrates that the 2,4-DCP coverage is largely influenced by phenol due to its poisoning effect by blocking active sites, and phenol desorption is the rate-determining step of EHDC on the catalyst. The support TiN enables alleviation of the phenol poisoning by modulating the electronic structure of Pd. The d band center of Pd can serve as a potential descriptor of EHDC efficiency, and its optimization for balancing 2,4-DCP and phenol adsorption should be an effective strategy to enhance EHDC.



 Please wait while we load your content...
Please wait while we load your content...