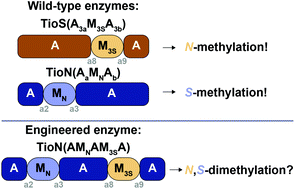
The adenylation (A) domains found in nonribosomal peptide synthetases (NRPSs) exhibit tremendous plasticity. Some A domains have been shown to display the ability to contain within them the catalytic portion of an auxiliary domain, most commonly that of a methyltransferase (M) enzyme. This unique feature of A domains interrupted by M domains allows them to possess bifunctionality, where they can both adenylate and methylate an amino acid substrate. Additionally, these types of inserted M domains are able to selectively carry out either backbone or side chain methylation of amino acids. Interruptions with M domains are naturally found to occur either between the a2–a3 or the a8–a9 of the ten conserved motifs of A domains. Herein, we set out to answer the following question: Can one A domain support two different M domain interruptions occurring in two different locations (a2–a3 and a8–a9) of the A domain and possess the ability to adenylate an amino acid and methylate it on both its side chain and backbone? To answer this question we added a backbone methylating M3S domain from TioS(A3aM3SA3b) between the a8–a9 region of a mono-interrupted A domain, TioN(AaMNAb), that already contained a side chain methylating MN domain between its a2–a3 region. We evaluated the di-interrupted A domain TioN(AMNAM3SA) with a series of radiometric and mass spectrometry assays and found that this engineered enzyme was indeed capable of all three activities. These findings show that production of an active trifunctional di-interrupted A domain is possible and represents an exciting new avenue for future nonribosomal peptide (NRP) derivatization.