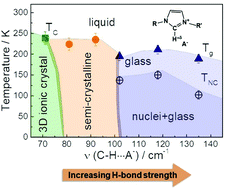
Self-assembled ionic liquid crystals are anisotropic ionic conductors, with potential applications in areas as important as solar cells, battery electrolytes and catalysis. However, many of these applications are still limited by the lack of precise control over the variety of phases that can be formed (nematic, smectic, or semi/fully crystalline), determined by a complex pattern of different intermolecular interactions. Here we report the results of a systematic study of crystallization of several imidazolium salts in which the relative contribution of isotropic coulombic and directional H-bond interactions is carefully tuned. Our results demonstrate that the relative strength of directional H-bonds with respect to the isotropic Coulomb interaction determines the formation of a crystalline, semi-crystalline or glassy phase at low temperature. The possibility of pinpointing H-bonding directionality in ionic liquids make them model systems to study the crystallization of an ionic solid under a perturbed Coulomb potential.