A theoretical investigation on the atmospheric degradation of the
 radical: reactions with NO, NO2,
radical: reactions with NO, NO2,
 and NO3†
and NO3†
Abstract
The 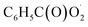 radical is the key intermediate in the atmospheric oxidation of benzaldehyde, and its further chemistry contributes to local air pollution. The reaction mechanisms of the
radical is the key intermediate in the atmospheric oxidation of benzaldehyde, and its further chemistry contributes to local air pollution. The reaction mechanisms of the 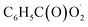 radical with NO, NO2,
radical with NO, NO2, 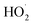 and NO3 were studied by quantum chemistry calculations at the CCSD(T)/CBS//M06-2X/def2-TZVP level of theory. The explicit potential energy curves were provided in order to reveal the atmospheric fate of the
and NO3 were studied by quantum chemistry calculations at the CCSD(T)/CBS//M06-2X/def2-TZVP level of theory. The explicit potential energy curves were provided in order to reveal the atmospheric fate of the 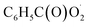 radical comprehensively. The main products of the reaction of
radical comprehensively. The main products of the reaction of 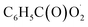 with NO are predicted to be
with NO are predicted to be 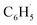 , CO2 and NO2. The reaction of
, CO2 and NO2. The reaction of 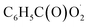 with NO2 is reversible, and its main product would be C6H5C(O)O2NO2 which was predicted to be more stable than PAN (peroxyacetyl nitrate) at room temperature. The decomposition of C6H5C(O)O2NO2 at different ambient temperatures would be a potential long-range transport source of NOx in the atmosphere. The predominant products of the reaction
with NO2 is reversible, and its main product would be C6H5C(O)O2NO2 which was predicted to be more stable than PAN (peroxyacetyl nitrate) at room temperature. The decomposition of C6H5C(O)O2NO2 at different ambient temperatures would be a potential long-range transport source of NOx in the atmosphere. The predominant products of the reaction 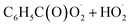 are predicted to be C6H5C(O)O2H, C6H5C(O)OH, O2 and O3, while HO˙ is of minor importance. So, the reaction of
are predicted to be C6H5C(O)O2H, C6H5C(O)OH, O2 and O3, while HO˙ is of minor importance. So, the reaction of 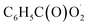 with
with 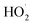 would be an important source of ozone and carboxylic acids in the local atmosphere, and has less contribution to the regeneration of HO˙ radicals. The reaction of
would be an important source of ozone and carboxylic acids in the local atmosphere, and has less contribution to the regeneration of HO˙ radicals. The reaction of 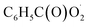 with NO3 should mainly produce
with NO3 should mainly produce 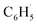 , CO2, O2 and NO2, which might play an important role in atmospheric chemistry of peroxy radicals at night, but has less contribution to the night-time conversion of
, CO2, O2 and NO2, which might play an important role in atmospheric chemistry of peroxy radicals at night, but has less contribution to the night-time conversion of 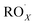 (
(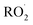 and RO˙) to
and RO˙) to 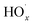 (
(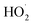 and HO˙) in the local atmosphere. The results above are in good accordance with the reported experimental observations.
and HO˙) in the local atmosphere. The results above are in good accordance with the reported experimental observations.





 Please wait while we load your content...
Please wait while we load your content...
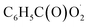 radical: reactions with NO, NO2,
radical: reactions with NO, NO2, 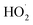 and NO3
and NO3