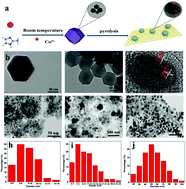
The catalytic hydrogenation of aromatic nitro compounds containing multiple functional groups into amino compounds with high conversion rates, selectivity, and stability under mild conditions is a great challenge. Herein, a well defined catalyst (Co@NC) is prepared through the pyrolysis of the Co-centered metal–organic framework (MOF) at the optimized temperature. The as-synthesized catalyst exhibits a high conversion rate and selectivity for the hydrogenation of 12 aromatic nitro compounds with different competing groups into desired amino compounds with hydrazine hydrate under mild conditions (80 °C, 30 min, and 1 atm). The catalyst also shows excellent stability and can be reused over 20 times without considerably losing its activity. It is found that the Co–Nx site is the main active site for catalytic hydrogenation, and the Mott–Schottky effect between the surface Co NPs and N-doped carbon can further promote the hydrogenation reaction. EXAFS, TEM, XPS, and Raman analyses confirm that cobalt nanoparticles (NPs) are properly encapsulated by the N-doped carbon matrix at the optimized temperature, and the Co species maintain a high spin state after the catalysis, which may be responsible for the high performance of Co@NC. This work demonstrates not only a highly efficient catalyst for hydrogenation under mild conditions, but also provides insight into the active sites in Co-based catalysts for hydrogenation.