Xiaochen Ji, Qiong Liu, Zhongzhen Wang, Pu Wang, Guo-Jun Deng and Huawen Huang
Green Chem., 2020,22, 8233-8237
DOI:
10.1039/D0GC01872D,
Communication
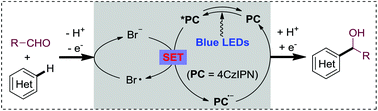
Photoredox-neutral hydroxyalkylations of quinolines with aldehydes, induced by sustainable visible light under mild conditions, are described. Non-toxic and inexpensive LiBr is found to be the key for the success of the atom-economical Minisci method. Combined with a highly oxidative photocatalyst and visible light irradiation, the bromide additive mediates the H abstraction/acyl radical formation directly from aldehydes. The present mild photoredox neutral protocol provides an important alternative, especially for the challenging Minisci hydroalkylations, as well as a promising approach for atom-economical Minisci reactions with broader N-heterocycle spectra.