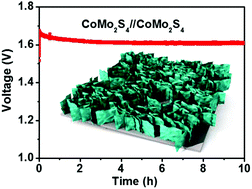
In view of the large-scale production of hydrogen (H2) by means of water electrolysis, it is of utmost importance to develop high-efficiency, stable and low-cost electrocatalysts. We report here on the synthesis and electrocatalytic properties of interconnected porous nanoflakes of the bimetallic catalyst CoMo2S4. These as-grown CoMo2S4 electrodes show higher electrocatalytic ability as compared to the reference CoMoO6 material, requiring a low OER/HER overpotential of 306/162 mV to reach 10 mA cm−2. We ascribe the excellent catalytic efficiency of our novel nanostructured CoMo2S4 catalyst to the combination of an increased number of active sites, enhanced charge transport properties, and a better contact at the CoMo2S4/electrolyte interface. When interconnected CoMo2S4 is used as a bifunctional catalyst in two-electrode electrolyzer cells, it maintains a voltage of 1.65 V to achieve 10 mA cm−2 for at least 10 hours, thus opening a new route for the design of efficient, stable and low-cost catalysts.