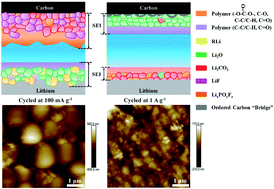
Porous carbon materials have been studied as anodes in Li-ion batteries for several decades. Interestingly, there is a phenomenon that is still not explicit, in which the specific capacity gradually increases during cycling, especially at a large current rate. Herein, this work demonstrates that the mechanism of increasing capacity is related to the doped transition metal in carbon materials and conducted current rate for the first time. Specifically, the transition-metal-free carbon shows a general electrochemical cycling performance of a gradually fading capacity at a large current rate. However, the capacity of transition-metal-doped carbon is dramatically increased after an initial slow decrease. Also, for the transition-metal-doped materials cycled at a low current rate, a loose and thick solid electrolyte interphase grows, resulting in severe decomposition of electrolyte, sluggish Li-ion diffusion, and a fading capacity during extensive cycling. With a large current rate, the solid electrolyte interphase is more compact, flat, and thin, which significantly decreases the diffusion length of Li ions and alleviates the decomposition of electrolyte on electrode surfaces. Moreover, the components and morphologies of solid electrolyte interphases after cycling at both small and large current rates are investigated through atomic force microscopy and X-ray photoelectron spectroscopy. The mechanisms behind the increased capacity are revealed. These results present a comprehensive understanding of the increased-capacity phenomenon and guidelines for designing high-performing carbon materials.