The effect of explicit polarity on the conformational behavior of a single polyelectrolyte chain
Abstract
In this work using dissipative particle dynamics simulations with explicit treatment of polar species we demonstrate that the molecular nature of dielectric media has a significant impact on swelling and collapse of a polyelectrolyte chain in a dilute solution. We show that the small-scale effects related to the presence of polar species lead to the intensification of the electrostatic interactions when the charges are close to each other and/or their density is high enough. As a result, the electrostatic strength 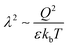 , usually regarded as the main parameter governing the polyelectrolyte chain collapse, does not have a universal meaning: the value of λ at which the coil-to-globule transition occurs is found to be dependent on the specific fixed value of the solvent bulk permittivity ε while varying the monomer unit charge Q and vice versa. This effect is observed even when the backbone and the counterions have the same polarity as the solvent beads, i.e. no dielectric mismatch is present. The reason for such behavior is rationalized in terms of the “effective” dielectric permittivity εeff which depends on the volume fraction φ of charged units inside the polymer chain volume; using εeff instead of ε collapses all data onto one master curve describing the chain shrinking with λ. Furthermore, it is shown that a polar chain adopts less swollen conformations in the polyelectrolyte regime and collapses more easily compared to a non-polar chain.
, usually regarded as the main parameter governing the polyelectrolyte chain collapse, does not have a universal meaning: the value of λ at which the coil-to-globule transition occurs is found to be dependent on the specific fixed value of the solvent bulk permittivity ε while varying the monomer unit charge Q and vice versa. This effect is observed even when the backbone and the counterions have the same polarity as the solvent beads, i.e. no dielectric mismatch is present. The reason for such behavior is rationalized in terms of the “effective” dielectric permittivity εeff which depends on the volume fraction φ of charged units inside the polymer chain volume; using εeff instead of ε collapses all data onto one master curve describing the chain shrinking with λ. Furthermore, it is shown that a polar chain adopts less swollen conformations in the polyelectrolyte regime and collapses more easily compared to a non-polar chain.



 Please wait while we load your content...
Please wait while we load your content...