Steric effects in the hydrogen evolution reaction based on the TMX4 active center: Fe–BHT as a case study†
Abstract
In this work, Fe–BHT is identified as the most efficient catalyst for the hydrogen evolution reaction (HER) among the TM–BHTs (TM = Sc, Ti, V, Cr, Mn, Fe, Co, and Ni), with an overpotential as low as 0.09 V. It is found that Fe dz2 orbitals do not participate in the bonding with surrounding S/N atoms in the FeX4 active center but are bonding states for hydrogen adsorption. In accordance with our results, a steric effect determined energy gap 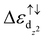 acts as an efficient descriptor for the HER activity, which has never been discussed in previous studies. In addition, strain engineering proves the proposed steric effects, which also highlights the importance of the point group symmetry of active centers.
acts as an efficient descriptor for the HER activity, which has never been discussed in previous studies. In addition, strain engineering proves the proposed steric effects, which also highlights the importance of the point group symmetry of active centers.



 Please wait while we load your content...
Please wait while we load your content...