An unprecedented quinoid–donor–acceptor strategy to boost the carrier mobilities of semiconducting polymers for organic field-effect transistors†
Abstract
Quinoidal–aromatic conjugated polymers hold great application potential in organic field-effect transistors (OFETs). However, the development of high mobility quinoidal–aromatic conjugated polymers still lags behind the more popular donor–acceptor (D–A) conjugated polymers, mainly owing to the lack of a rational design strategy and efficient building block. Herein, a novel quinoid–donor–acceptor (Q–D–A) strategy is demonstrated to modulate the energy-level and boost the charge carrier transport mobility of conjugated polymers as opposed to the D–A system. On the basis of this strategy, a quinoidal–aromatic conjugated polymer, namely PAQM-BT, is designed and synthesized. With the combined use of quinoid, donor and acceptor units in the backbone, the resulting Q–D–A polymer PAQM-BT displays the narrowest bandgap with the deepest-lying lowest unoccupied molecular orbital (LUMO) energy level, highest backbone coplanarity, enhanced thin-film crystallinity and smallest effective hole mass 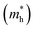 in comparison with the corresponding D–A polymer PT3B1 and quinoid–donor (Q–D) polymer PAQM-3T. Benefitting from the more effective intra- and inter-chain charge transport, as corroborated by experiment and theoretical calculations, OFET devices based on PAQM-BT exhibit a highly boosted hole mobility of up to 5.10 cm2 V−1 s−1, which is one and four orders of magnitude higher than that of PAQM-3T and PT3B1, respectively, and is among the highest for quinoidal–aromatic conjugated polymers. The potent Q–D–A strategy not only allows the energy level to be modulated but also leads to effective charge carrier transport, opening up possibilities to the development of high mobility quinoidal–aromatic conjugated polymers based on a variety of quinoids, donors, and acceptors.
in comparison with the corresponding D–A polymer PT3B1 and quinoid–donor (Q–D) polymer PAQM-3T. Benefitting from the more effective intra- and inter-chain charge transport, as corroborated by experiment and theoretical calculations, OFET devices based on PAQM-BT exhibit a highly boosted hole mobility of up to 5.10 cm2 V−1 s−1, which is one and four orders of magnitude higher than that of PAQM-3T and PT3B1, respectively, and is among the highest for quinoidal–aromatic conjugated polymers. The potent Q–D–A strategy not only allows the energy level to be modulated but also leads to effective charge carrier transport, opening up possibilities to the development of high mobility quinoidal–aromatic conjugated polymers based on a variety of quinoids, donors, and acceptors.

- This article is part of the themed collection: Journal of Materials Chemistry A HOT Papers


 Please wait while we load your content...
Please wait while we load your content...
