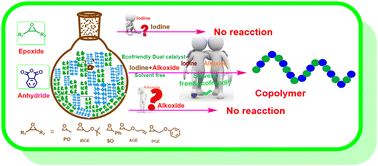
A new environmentally benign synthesis of polyesters involves ring-opening alternating copolymerization (ROAC) of phthalic anhydride (PA) with various epoxides, utilizing molecular iodine and alkali metal alkoxides as novel dual catalytic systems under solvent-free conditions. With a suitable combination of iodine and alkali metal alkoxides, the reaction can be performed to a level of >99% conversion with good regioselectivity and respectable TOF values of 16 to 50 h−1. We examined a series of commercial alkali metal alkoxides (MeOLi/Na/K, iPrOLi/Na/K, and tBuOLi/Na/K) in combination with molecular iodine for the copolymerization of epoxides with PA. The results indicate that the sodium and potassium alkoxides in combination with iodine showed higher activity. Particularly, I2/tBuOK showed markedly higher activity exhibiting respectable TOF values in the range of 16 to 50 h−1 towards the ROAC of PA with epoxides, which is comparable to the activity of metal-based complex catalysts. In addition, this methodology was employed for the copolymerization of PA with various epoxides such as CHO, PO, AGE, PGE, SO and tBGE. Each product exhibits a perfectly alternating sequence distribution, controlled molar mass (Mn up to 37.14 kDa), and low dispersity (Đ up to 1.04). Therefore, in the present work a novel and simple catalytic system was developed for regioselective fabrication of polyesters with diverse structures.