Synthesis, crystal structures and spectroscopic properties of pure YSb2O4Br and YSb2O4Cl as well as Eu3+- and Tb3+-doped samples†
Abstract
The quaternary halide-containing yttrium(III) oxidoantimonates(III) YSb2O4Cl and YSb2O4Br were synthesised through solid-state reactions from the binary components (Y2O3, Sb2O3 and YX3, X = Cl and Br) at 750 °C in evacuated fused silica ampoules with eutectic mixtures of NaX and CsX (X = Cl and Br) as fluxing agents. YSb2O4Cl crystallizes tetragonally in the non-centrosymmetric space group P4212 with unit-cell parameters of a = 773.56(4) pm and c = 878.91(6) pm, whereas YSb2O4Br is monoclinic (space group: P21/c) with a = 896.54(6) pm, b = 780.23(5) pm, c = 779.61(5) pm and β = 91.398(3)°, both for Z = 4. The two new YSb2O4X compounds contain [YO8]13− polyhedra, which are connected via four common edges to form 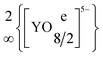 layers (d(Y3+–O2−) = 225–254 pm) without any Y3+⋯X− bonds (d(Y3+⋯X−) > 400 pm). Moreover, all oxygen atoms belong to ψ1-tetrahedral [SbO3]3− units, which are either connected to four-membered rings [Sb4O8]4− in the chloride (Y2[Sb4O8]Cl2 for Z = 2) or endless chains in the bromide (Y1/2(SbO2)Br1/2 for Z = 8) by common vertices. With distances of 307 pm in YSb2O4Cl and 326 pm in YSb2O4Br there are not even substantial bonding Sb3+⋯X− (X = Cl and Br) interactions at work. Luminescence spectroscopy on samples doped with trivalent europium and terbium showed an energy transfer from the oxidoantimonate(III) moieties as the sensitizer in the host structure onto the lanthanoid activators.
layers (d(Y3+–O2−) = 225–254 pm) without any Y3+⋯X− bonds (d(Y3+⋯X−) > 400 pm). Moreover, all oxygen atoms belong to ψ1-tetrahedral [SbO3]3− units, which are either connected to four-membered rings [Sb4O8]4− in the chloride (Y2[Sb4O8]Cl2 for Z = 2) or endless chains in the bromide (Y1/2(SbO2)Br1/2 for Z = 8) by common vertices. With distances of 307 pm in YSb2O4Cl and 326 pm in YSb2O4Br there are not even substantial bonding Sb3+⋯X− (X = Cl and Br) interactions at work. Luminescence spectroscopy on samples doped with trivalent europium and terbium showed an energy transfer from the oxidoantimonate(III) moieties as the sensitizer in the host structure onto the lanthanoid activators.



 Please wait while we load your content...
Please wait while we load your content...